Abstract
Wheat/soybean rotation is an important double-cropping system in the Huang-Huai-Hai plain of China. Continuous soybean cropping could cause soil quality deterioration and plant growth inhibition. However, the effects of continuous wheat/soybean cropping on soybean rhizosphere microbes remain largely unknown. In this study, we compared the soybean yield and rhizosphere soil microbial community between continuous winter wheat/summer soybean (W/S) with two harvests in one year and winter wheat/summer soybean-winter wheat/summer maize (W/S-W/M) with four harvests in two years. The results showed that the soybean yield in the W/S group significantly (p < 0.05) declined within the first two years. The W/S-W/M showed higher soybean yield and soil fertility index than the W/S group. The sequencing results revealed that cropping rotation had a higher impact on the fungal community than the bacterial community. The W/S group showed 22.08–23.01% higher alpha diversity of the fungal community, but the alpha diversity of the bacterial group did not vary significantly in this group. The fungal community composition in the W/S and W/S-W/M groups differed significantly. In the W/S-W/M group, a higher relative abundance of plant growth-promoting fungi (e.g., Mortierella), nematophagous fungi (e.g., Plectosphaerella), and biological control fungi (e.g., Coniothyrium) was observed. In the W/S group, a higher relative abundance of lignocellulose-degrading fungi (e.g., Trechispora, Myceliophthora, Botryotrichum, and Coniochaeta) and pathogenic fungi (e.g., Pyrenochaetopsis and Cyphellophora) was observed. LEfSe analysis demonstrated that Mortierella, Myceliophthora, and Trechispora could serve as crucial biomarkers. Mortierella was positively associated with available P levels and negatively associated with NO3−-N levels and pH while Trechispora showed the opposite trend. The findings of this study could enhance the current understanding of the mechanisms associated with the continuous wheat/soybean cropping obstacles and ensure the sustainability of agricultural production.
1. Introduction
Soybean is one of the most important protein sources worldwide. It is also an important nitrogen-fixing oil crop, which is the primary source of food protein, feed protein, and edible oils for humans and animals [1]. The wheat/soybean system is an important double-cropping system for grains and beans in the Huang-Huai-Hai region, which ensures the security of national food supplies. However, in our previous studies, we have observed that long-term continuous cropping of wheat/soybean rotation induced stunted growth of soybean plants and underdeveloped root systems, resulting in decreased crop yield. Soybean is a typical continuous cropping-sensitive crop, which aggravates soil-borne diseases and depletes soil nutrients, impacting crop yield and quality [2]. Previous studies have shown that an approximately 10–30% reduction in the crop yield could be the result of a continuous soybean cropping system for over 1–3 years [3]. Additionally, short-term continuous cropping of soybean conducted for less than three years increased soybean root rot diseases and cyst nematode populations significantly compared with the crop rotation system. Therefore, continuous soybean cropping has become an important limiting factor for the high and stable yield of soybean. Crop rotation is an alternative approach to maintaining soil quality and crop productivity [4]. However, the effects of continuous wheat/soybean cropping on soybean production in a double cropping system demand further investigation.
Rhizosphere microbiota is one of the main indicators of soil environmental quality, and influence crop health and yield significantly [5,6]. Rhizosphere microbial communities could explain the positive effects of diverse crop rotations on maize and soybean performance [7]. Previous studies have demonstrated that the structure of the soil microbial community was significantly affected by different cropping systems [8,9]. It has been shown that soil bacterial community composition and function were significantly altered by corn insertions in a long-term continuous soybean cropping system [10]. A recent meta-analysis also revealed that crop type was a significant factor that influenced crop yield in the rotation system [11]. Meier et al., reported that rhizosphere microbiomes could respond to host species and nitrogen fertilization at the genus and subgenus levels in a maize-soybean rotation system [12]. Meanwhile, different cropping systems also affect microbial species and the relative abundances of soil-enriched microorganisms [13]. For instance, a previous study reported that continuous cropping of soybean increased the relative abundance of the beneficial fungi Mortierella sp. and Paecilomyces lilacinus, as well as potentially pathogenic fungi Fusarium oxysporum and Lectera longa [14]. Liu et al., showed that continuous soybean cultivation for 13 years and crop rotation with maize alternatively for five years increased the relative abundance of potentially beneficial bacteria Bradyrhizobium sp. and Gemmatimonas sp. and fungi Mortierella sp. and Paecilomyces sp. but decreased the relative abundance of the pathogenic fungi Fusarium sp. [3]. In contrast, other studies have reported no significant differences in microbial communities between crop rotation and continuous cropping systems. The fungal abundance did not differ between wheat/maize/soybean rotation and long-term continuous wheat cropping systems [15]. These inconsistencies between these studies were primarily due to the type of rotation systems, the variety of planting years, different research methods, and various soil types. These studies indicate that a complex mechanism is associated with the continuous soybean cropping system, which should be investigated under different conditions.
This study aimed to investigate the effects of two cropping systems, i.e., continuous wheat/soybean (W/S) and wheat/soybean-wheat/maize rotation system (W/S-W/M) on the abundance, composition, and species diversity of fungal and bacterial communities in the rhizosphere soil of soybean plants in the Huang-Huai-Hai region of China. We hypothesized that fungal and bacterial community composition and diversity would differ between the two cropping systems, which could explain the reason why continuous wheat/soybean cropping induces soybean yield reduction. This study also investigated the potential relationship among rhizosphere environmental changes, soil microbial communities, and soybean yield under different cropping systems. This is the first study to examine the effect of soybean continuous cropping on soybean rhizosphere microbes in a wheat/soybean double cropping system.
2. Materials and Methods
2.1. Experiment Design and Soil Sampling
The experiments were conducted from 2016 to 2020 at Jiaozhou City, Shandong Province (36.09° N, 110.00° E). The soil type in this region is fluvo-aquic. This region has a temperate continental monsoon climate, and belongs to a semi-humid and drought-prone area. Annual mean temperatures averaged 9~16 °C and precipitation averaged 475 mm per year, most rainfall is concentrated in June-August. Two cropping systems were established in October 2016 in a randomized block experimental design with three replications. The area of each plot was 962 m2 (148 m × 6.5 m). Two cropping systems were designed as follows: (1) continuous winter wheat/summer soybean cropping system (W/S) with two harvests in one year; (2) winter wheat/summer soybean-winter wheat/summer maize cropping system (W/S-W/M) with four harvests in two years. Winter wheat was sowed in early October and harvested in mid-June, and summer soybean was sowed in mid-June and harvested in late September. In this study, the cultivars of wheat, maize, and soybean were “Jimai 22”, “Zhengdan 958”, and “Qihuang 34”, respectively. Subsoiling tillage before winter wheat sowing and no-tillage before either summer maize or soybean sowing was conducted with wheat and maize straw returning. Nitrogen, phosphorus, and potassium (NPK) compound fertilizer (15-15-15) were applied before planting at 600 kg/hm2, 450 kg/hm2, and 150 kg/hm2 for wheat, maize, and soybean, respectively. An additional 225 kg/ hm2 of urea was top-dressed at the V12 stage in maize. In terms of irrigation, in addition to natural precipitation, we timely check the soil water content in the field to ensure the water supply and the normal growth of crops. Other aspects were managed according to normal crop management measures.
Soybean rhizosphere soil was collected at the maturity stage in 2019. A total of six rhizosphere soil samples (2 groups × 3 replicates) were collected, each soil sample consisted of five plants root that were randomly selected in each plot, then mixed rhizosphere soil thoroughly. Soybean plants were carefully uprooted using a shove, and afterwards, loosely attached soil was shaken off vigorously. Then, the rhizosphere soil adhering to plant roots was collected using a sterile brush and transferred to sterilized bags. We immediately transported these soil samples in iceboxes to the laboratory. For each sample, the soil was partially placed into sterilized bags and kept in a −80 °C freezer for microbiota analysis. The remaining soil samples were dried at room temperature to analyze the chemical properties.
2.2. Analysis of the Chemical Properties of Soil
Soil pH, soil organic matter (SOM), total nitrogen (TN), ammonium-nitrogen (NH4+-N), nitrate-nitrogen (NO3−-N), available potassium (AK), and available phosphorus (AP) were measured according to standard analytical methods as reported previously [16].
2.3. DNA Extraction and qPCR
DNA in the soil samples was extracted using the E.N.Z.A.TM Soil DNA Kit (Omega, St. Louis, MO, USA). A NanoDrop 2000 spectrophotometer (Thermo, Branchburg, NJ, USA) was used to determine the concentration and purity of isolated DNA. Furthermore, 1% agarose gel electrophoresis was used to check the DNA quality. PCR amplification and library preparation were conducted, as mentioned previously [17]. Bacterial 16S rRNA V4 region and the ITS1 region of the fungal ITS were amplified using the primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′): 806R (5′-GGACTACHVGGGTWTCTAAT-3′) and ITS1-F (5′-CTTGGTCATTTAGAGGAAGTAA-3′): ITS2-R (5′-GCTGCGTTCTTCATCGATGC-3′), respectively [18,19]. The assay was performed in a 30 μL PCR reaction mixture containing 10 μL DNA (approximately 10 ng), 1 μL forward/reverse primers (1 μM), 15 μL Phusion® High-Fidelity PCR Master Mix (NEB), and 3 μL ultra-pure water. The PCR reactions were carried out in a Bio-rad T100 Gradient PCR thermocycler and the thermocycler was set to 1 minute at 98 °C for initial denaturation, followed by 30 cycles of 98 °C for 10 s for denaturation, 30 s at 56 °C for 16S or 52 °C for ITS for annealing, and 30 s at 72 °C for extension followed by a final extension at 72 °C for 10 min. The PCR products were pooled and purified using a Universal DNA purification kit (TianGen, Beijing, China). NEB Next® Ultra DNA Library Prep Kit was used for library construction. The Illumina sequencing was performed using a NovaSeq 6000 sequencer (Illumina, San Diego, CA, USA).
2.4. High-Throughput Sequencing Data Analysis
Microbiome sequencing data were analyzed with the bioinformatics pipeline of Quantitative Insights into Microbial Ecology (QIIME2 version 2020.6) [20]. The NovaSeq PE250 platform was used to generate paired-end reads. The paired-end reads were merged using FLASH software (V1.2.11, http://ccb.jhu.edu/software/FLASH/ accessed on 20 May 2022) to get raw tags [21]. We used the fastp (V 0.20.0) software to control the quality of the raw tags and obtain high-quality tags with a minimum overlap of 10 bp and a maximum allowed 10% mismatches within the overlap region. Chimeric sequences were removed using the Vsearch tool to obtain effective tags [22]. DADA2 version 1.10.0 plugin for QIIME2 was used to cluster the tags into amplicon sequence variants (ASVs); i.e., to produce 100% Operational Taxonomic Units (OTUs) representing the entirety of unique sequences observed in the dataset [23]. The annotation and relative abundance of ASVs were performed using the classify-sklearn algorithm of QIIME2 trained with Silva 138.1 database for bacterial and UNITEv8.2 for fungi [20]. In the NCBI database, raw sequences were deposited under SRA accession: PRJNA890146 and PRJNA889694.
Fungal and bacterial alpha- and beta-diversity indexes were calculated using the rarefied ASVs. The alpha-diversity index including Shannon, Simpson, Chao1, and Pielou_e was analyzed using the R-package in QIIME2. To analyze the beta diversity between the two groups, Non-Metric Multi-Dimensional Scaling (NMDS) was carried out which was based on the Bray-Curtis distance using the “vegan” package in R. MetaStat analysis at the level of the genus was performed to determine significant differences between groups using the R package. The linear discriminant analysis (LDA) effect size (LEfSe) analyses were conducted to elucidate the biomarkers in each treatment using the Galaxy web application via the LEfSe algorithm [24]. In each treatment, LDA scores over 4.0 were considered important biomarkers.
2.5. Statistical Analysis
The soybean yield, soil chemical properties, relative abundance of the most abundant bacterial and fungal, and alpha-diversity indexes between the W/S-W/M and W/S groups were compared with a one-way analysis of variance (ANOVA), followed by Duncan’s test (p < 0.05). To identify genera that were potentially pathogenic and beneficial fungi, significant differences between the W/S-W/M and W/S were tested with MetaStat analysis (p < 0.05). The relationship between microbial parameters and soil characteristics was determined using Spearman’s correlation coefficient. SPSS version 20.0 software was used for all statistical analyses.
3. Results
3.1. Variation of Soybean Yield in Two Cropping Systems
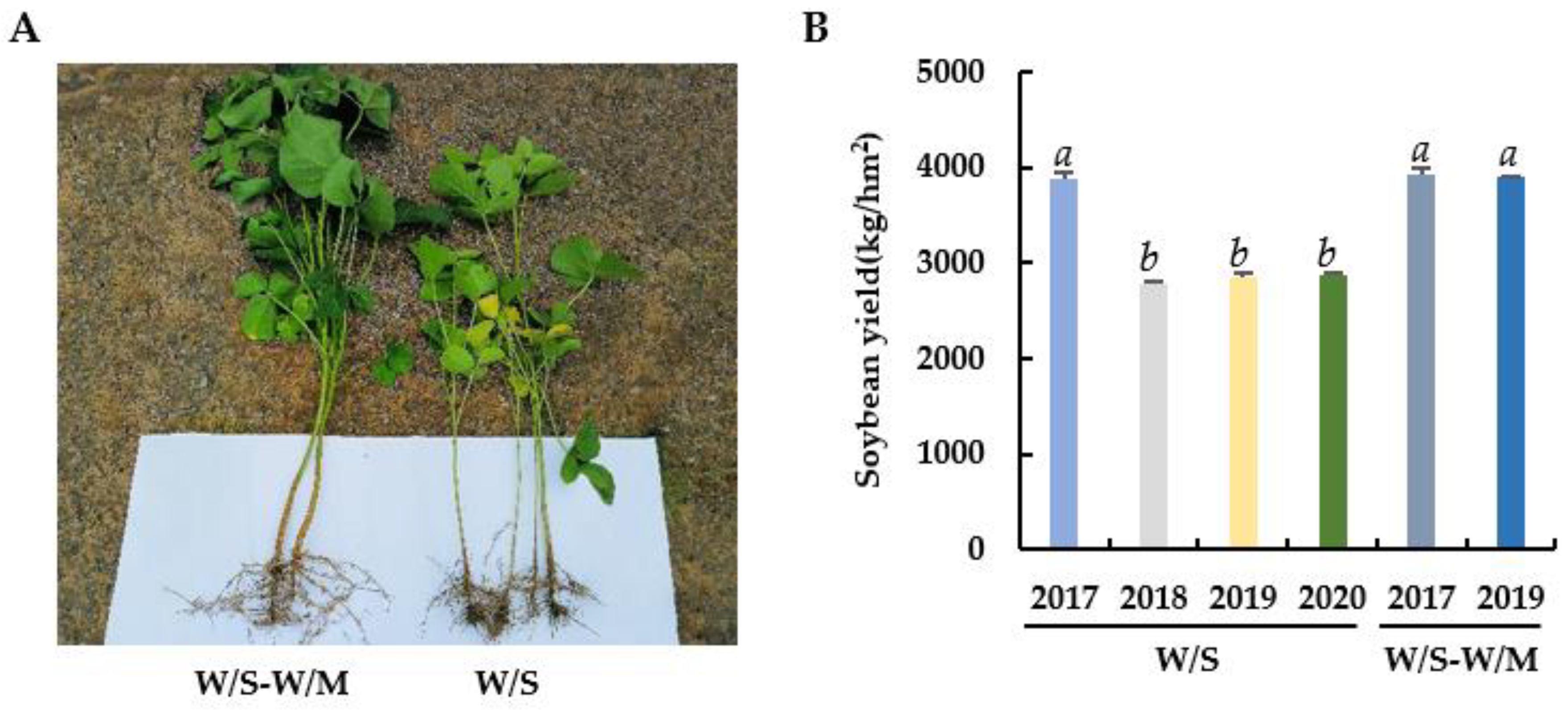
The growth of soybean root and shoot was notably stunted in the W/S group compared with the W/S-W/M group (Figure 1A). The highest soybean yield in the W/S group was observed in 2017, however, the yields declined significantly by 28.24% (p < 0.05) from 2018 onwards. No significant change was observed in the soybean yield from 2018 to 2020 in the W/S group. The soybean yield in the W/S-W/M group was 36.78% higher than the continuous W/S group in 2019. However, there was no significant change in soybean yield from 2017 to 2019 in the W/S-W/M group (Figure 1B).
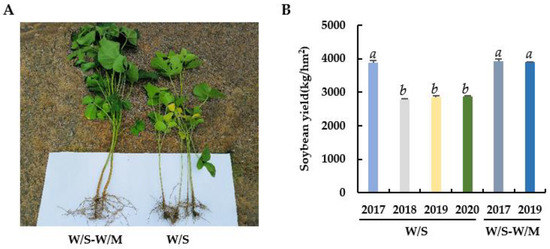
Figure 1.
Comparison of soybean plant morphology (A) and yield in each year (B) between two cropping systems. W/S-W/M, wheat/soybean-wheat/maize rotation system; W/S, continuous wheat/soybean cropping system. Different letters above columns signified statistically significant difference (p < 0.05).
3.2. Soil Chemical Properties
The effects of the two different cropping systems on the fundamental physicochemical characteristics of soil were summarized in Table 1. The soil pH in the W/S group was 5.86, which was around 0.42 pH units higher than in the W/S-W/M group. Compared with the W/S-W/M group, the levels of NH4+-N and NO3−-N significantly increased by 18.75% and 51.62% (p < 0.05) in the continuous W/S group. The content of AP increased significantly by 23.24% in the W/S-W/M group compared with the W/S group (p < 0.05), whereas other soil properties, such as SOM, TN, and AK did not change significantly between the W/S and W/S-W/M groups (p > 0.05).

Table 1.
Effects of different cropping systems on soil chemical properties.
3.3. Composition of Bacterial and Fungal Communities
A total of 481,803 bacterial 16S rRNA and 463,925 fungal ITS1 qualified reads were obtained in the six soil samples, which were clustered into 4623 and 603 ASVs, respectively. The bacterial ASVs identified in all the samples were divided into 41 phyla, 103 classes, 232 orders, 339 families, and 584 genera. The fungal ASVs identified in all the samples were divided into 6 phyla, 17 classes, 42 orders, 85 families, and 152 genera.
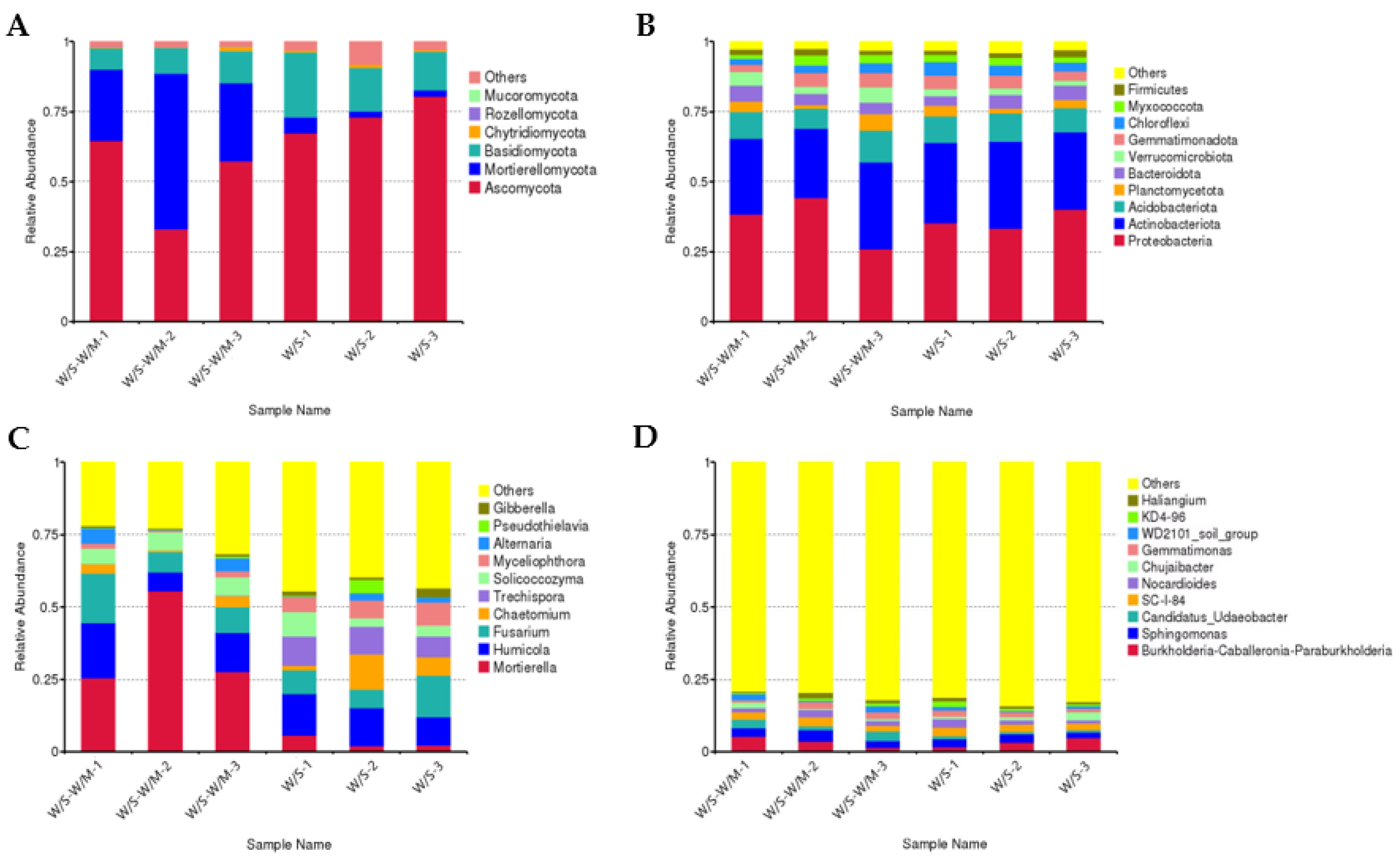
The fungal and bacterial community composition in the W/S-W/M and W/S groups at the phylum and genus levels were also compared (Figure 2A–D). Analysis of the fungal abundance at the phylum level showed enrichment of Ascomycota (51.78–73.64%), Mortierellomycota (3.37–36.23%), and Basidiomycota (9.43–17.54%) phyla in both the W/S-W/M and W/S groups. Chytridiomycota, Rozellomycota, and Mucoromycota were all minor phyla with less than 1% abundance on average (Figure 2A). A significant difference was observed in the percentage of the Mortierellomycota and Rozellomycota phylum, which showed relatively low abundance in the W/S group (Supplementary Table S1). Genus-level fungal abundance analysis showed that the most prevalent genus was Mortierella (33.37–36.23%), followed by Humicola (12.42–13.07%), Fusarium (9.58–10.97%), Chaetomium (2.61–6.70%), Trechispora (0.08–9.02%), Solicoccozyma (4.98–5.93%), Myceliophthora (1.47–6.35%), Alternaria (1.50–3.31%), Pseudothielavia (0.03–1.50%), and Gibberella (0.64–1.83%) (Figure 2C). The relative abundance of Mortierella and Humicola was higher in the W/S-W/M group compared with the W/S group, while the abundance of Trechispora and Myceliophthora was higher in the W/S group (Supplemental Table S1).
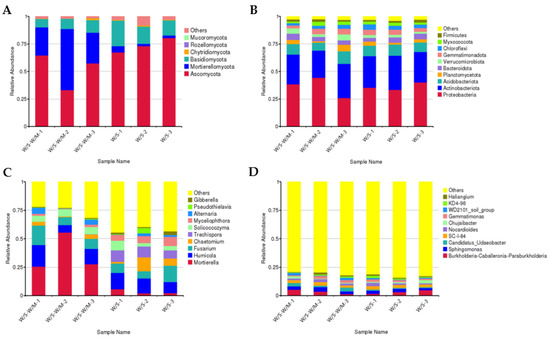
Figure 2.
The fungal and bacterial community composition in the rhizosphere soil. (A,B): The relative abundance of fungi (A) and bacteria (B) between the W/S-W/M and W/S at the phylum level. (C,D): The relative abundance of fungi (C) and bacteria (D) between the W/S-W/M and W/S at the genus level. W/S-W/M-1, W/S-W/M-2, and W/S-W/M-3 represent the three replications of W/S-W/S treatment. W/S -1, W/S-2, and W/S-3 represent the three replications of W/S treatment.
In W/S-W/M and W/S groups, the bacterial composition included Proteobacteria (36.24–36.27%), Actinobacteriota (27.59–29.18%), Acidobacteriota (9.37–9.38%), Planctomycetota (2.86–3.70%), Bacteroidota (4.43–4.50%), Verrucomicrobiota (2.17–4.32%), Gemmatimonadota (4.20–4.25%), Chloroflexi (2.74–3.88%), Myxococcota (2.40–2.70%), Firmicutes (1.90–1.92%), and some other phyla (Figure 2B). Genus-level bacterial abundance analysis showed that Burkholderia-Caballeronia-Paraburkholderia (3.22–3.49%), Sphingomonas (2.54–3.02%), Candidatus_Udaeobacter (0.96–2.60%), SC-I-84 (2.52–2.59%), Nocardioides (1.88–1.89%), Chujaibacter (1.01–1.49%), and Gemmatimonas (1.65–1.87%) were the dominant genus (Figure 2D). Nevertheless, there was no significant difference in the bacterial abundance for the top ten dominant members between the W/S and W/S-W/M groups either at the phylum or genus levels (Supplemental Table S2).
3.4. Response of Alpha and Beta Diversity to Different Cropping Systems
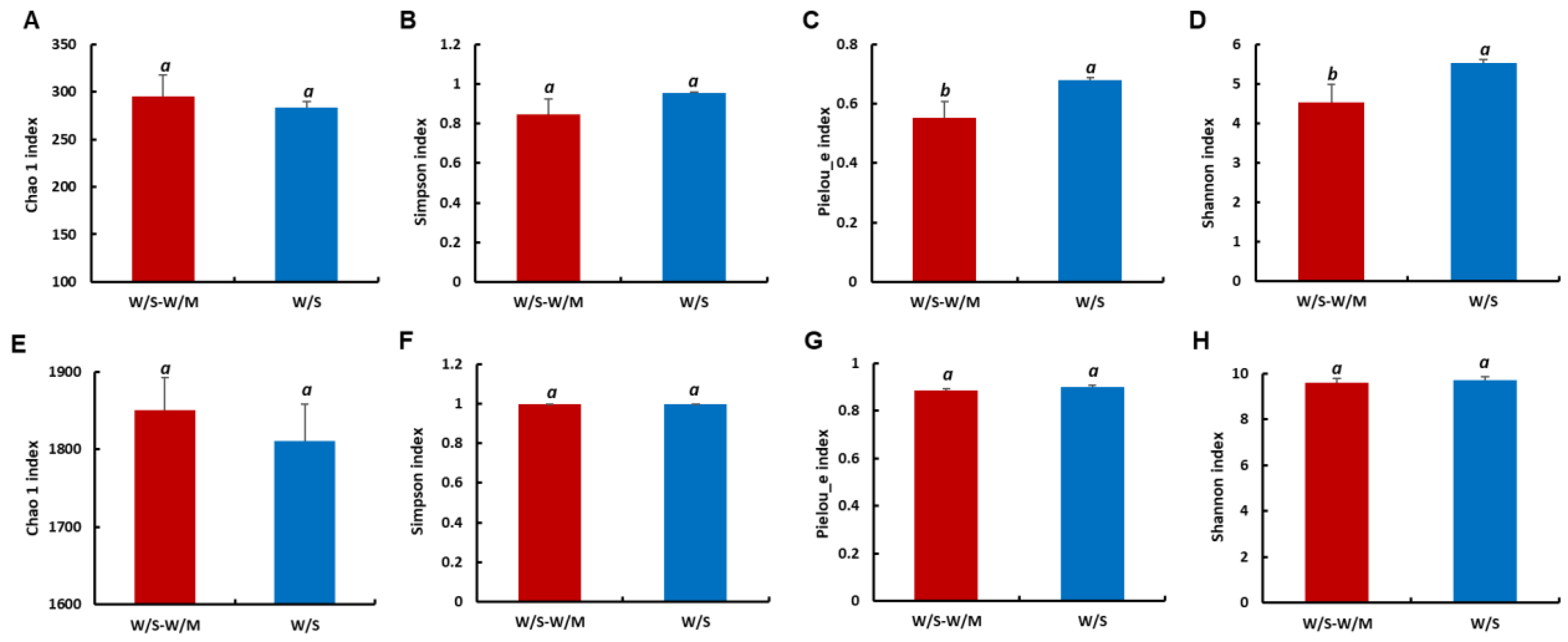
The alpha diversity was used to determine the richness, diversity, and evenness of the fungi and bacteria in the soil. For the fungal community, the Pielou_e and Shannon indexes of the W/S group showed significantly 22.08% and 23.01% higher values, respectively, indicating relatively higher fungal diversity and evenness compared with the W/S-W/M group (p < 0.05). Chao 1 and Simpson indexes showed no significant differences, representing indiscriminate richness between the W/S-W/M and W/S groups (Figure 3A–D). For the bacterial community, the difference in Chao1, Simpson, Pielou_e, and Shannon indexes between the W/S-W/M and W/S group was insignificant (p > 0.05, Figure 3E–H).
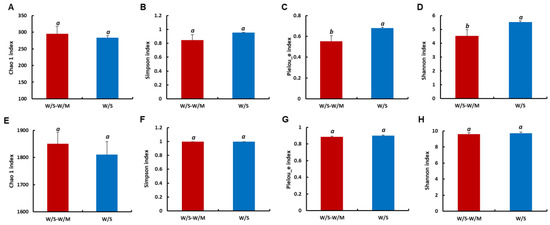
Figure 3.
Richness (Chao 1 and Simpson) and diversity (Pielou_e and Shannon) of the fungal (A–D) and bacterial (E–H) communities in the rhizosphere soils. Different letters above columns signified statistically significant difference (p < 0.05) according to Duncan’s test; the same letters are not significantly different between two cropping systems. W/S-W/M, wheat/soybean-wheat/maize rotation system; W/S, continuous wheat/soybean cropping system.
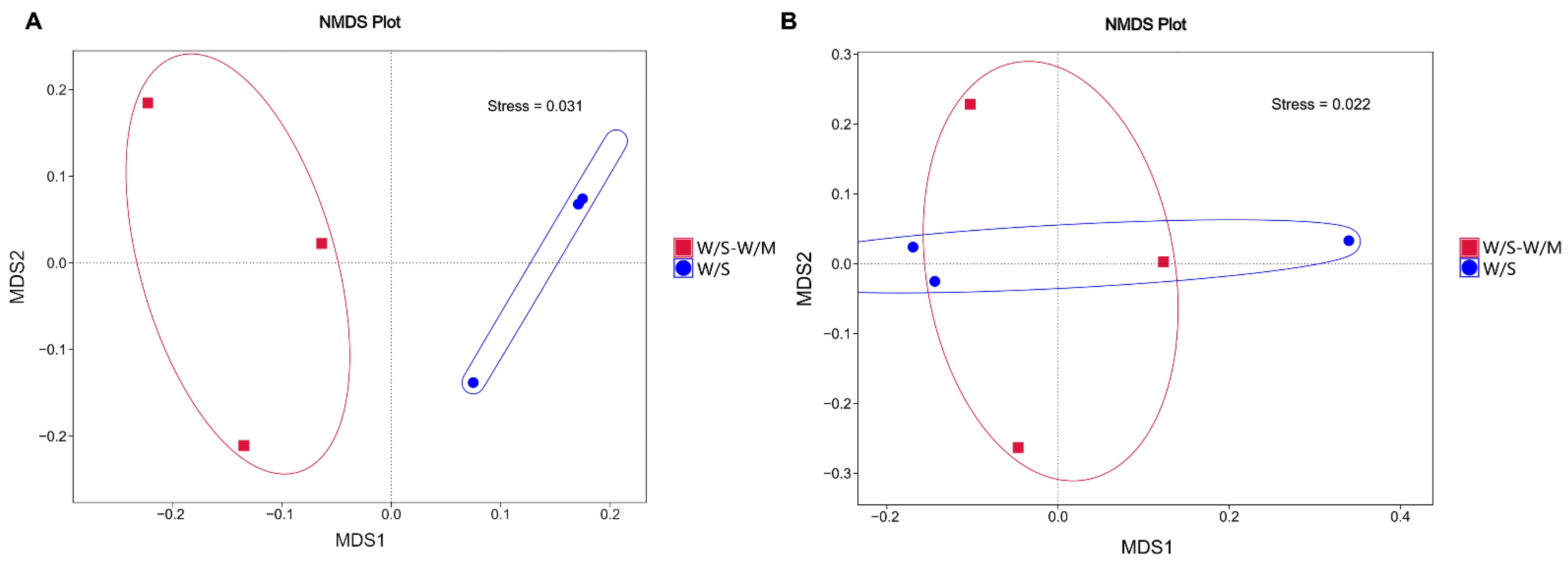
The beta diversity of the W/S-W/M and W/S groups was determined to unravel the fungal and bacterial community structure of the soil. Non-metric multidimensional scaling (NMDS) analysis was used to cluster the ordination space to reveal the soil fungal or bacterial community composition in the different cropping systems. The results of the beta diversity index analysis showed that the fungal community structure was completely different between the W/S and W/S-W/M groups (Figure 4A). However, the bacterial community structure was not entirely separated between the W/S and W/S-W/M groups. The distance between the W/S and W/S-W/M groups was not notable and overlapped in the NMDS results (Figure 4B).
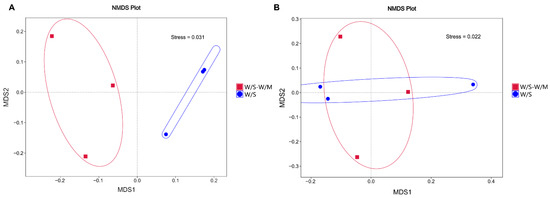
Figure 4.
Non-metric multidimensional scaling (NMDS) analysis of rhizosphere soil fungi (A) and bacteria (B) in different cropping systems. W/S-W/M, wheat/soybean-wheat/maize rotation system; W/S, continuous wheat/soybean cropping system.
3.5. Potentially Pathogenic and Beneficial Fungi Analysis
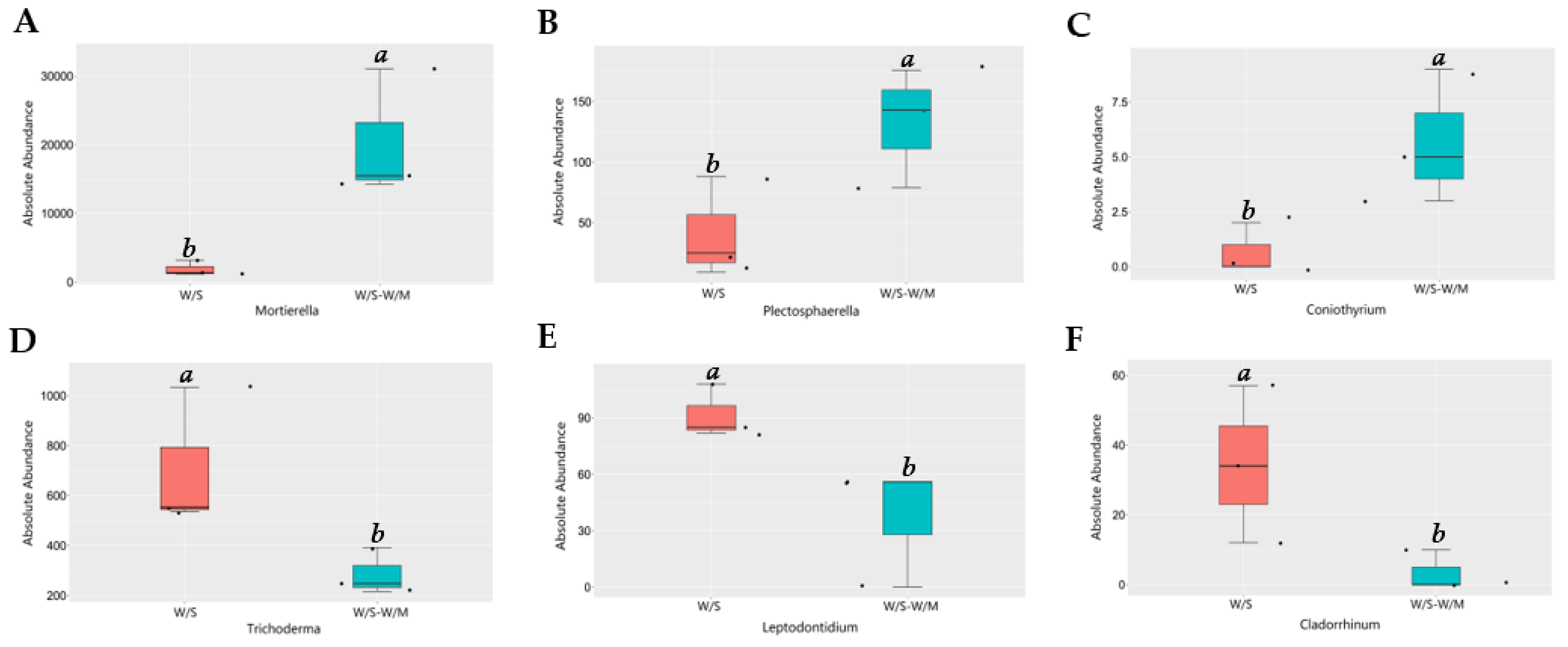
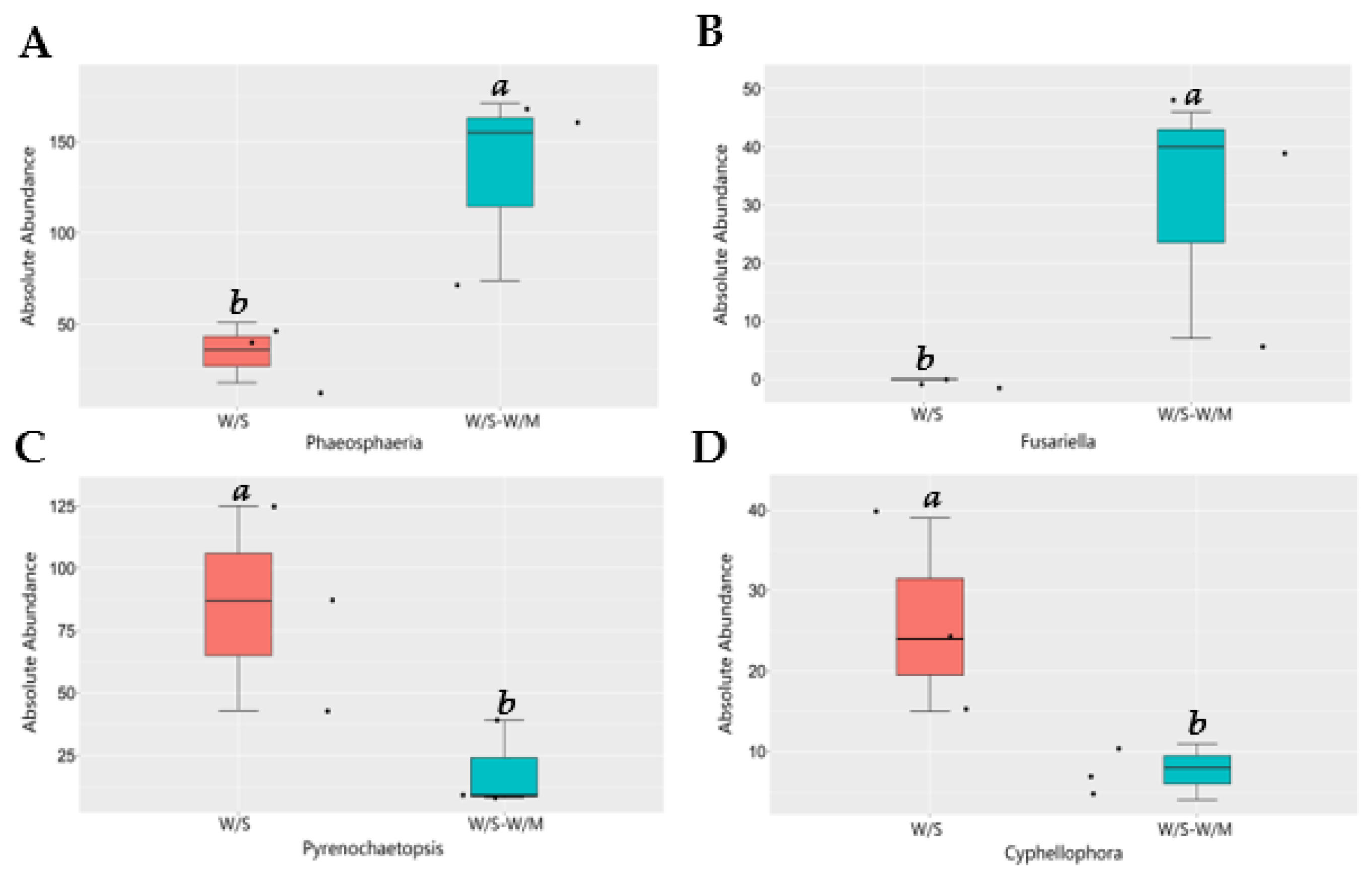
Since the two cropping systems differed in their fungal community composition, we performed a functional analysis of the potentially pathogenic and beneficial fungi. Based on MetaStat analysis at the genus level, the variance in fungal relative abundance between the W/S and W/S-W/M groups was illustrated. Compared with the W/S-W/M group, the W/S group exhibited a significant shift in the relative abundance of 21 genera, where the relative abundance of 13 genera increased and that of 8 genera decreased. The FunGuild analysis revealed that the most abundant guilds were saprotrophs, followed by pathotroph-saprotroph, pathotroph-saprotroph-symbiotroph, and pathotroph (Supplemental Figure S1). The most noteworthy findings of this study were the higher relative abundance of plant growth-promoting fungi Mortierella, nematophagous fungi Plectosphaerella, and biological control fungi Coniothyrium that were dramatically enhanced in the W/S-W/M group compared with the W/S group. However, the potentially beneficial fungi Trichoderma, Leptodontidium, and Metapochonia had a higher relative abundance in the W/S group than in the W/S-W/M group (Figure 5). In contrast, certain potential pathogens, such as Pyrenochaetopsis and Cyphellophora were significantly increased in the W/S group. However, a higher abundance of potentially pathogenic fungi Phaeosphaeria and Fusariella was also observed in the W/S-W/M group (Figure 6). In addition, we also observed that lignocellulose-degrading fungi Trechispora, Myceliophthora, Botryotrichum, Rhizophlyctis, and Coniochaeta were significantly enriched in the W/S group compared with the W/S-W/M group (Supplemental Figure S2).
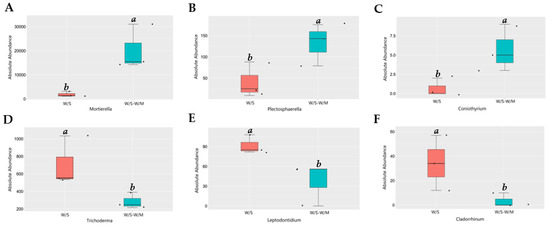
Figure 5.
Relative abundance of potentially beneficial fungi in two cropping systems. Different letters above columns signified statistically significant difference (p < 0.05) according to MetaStat analysis. The dots represent three replicates for each treatment. W/S, continuous wheat/soybean cropping system; W/S-W/M, wheat/soybean-wheat/maize rotation system. (A)-Mortierella; (B)-Plectosphaerella; (C)-Coniothyrium; (D)-Trichoderma; (E)-Leptodontidium; (F)-Cladorrhinum.
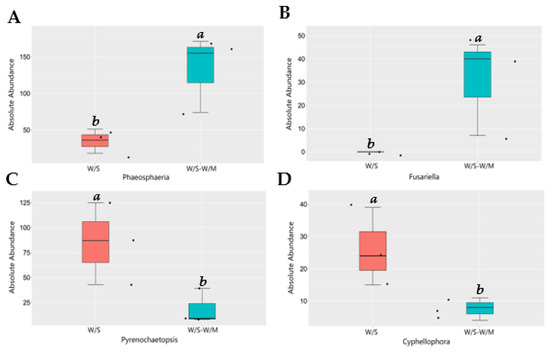
Figure 6.
Relative abundance of potentially pathogenic fungi in two cropping systems. Different letters above columns signified statistically significant difference (p < 0.05) according to MetaStat analysis. The dots represent three replicates for each treatment. W/S, continuous wheat/soybean cropping system; W/S-W/M, wheat/soybean-wheat/maize rotation system. (A)-Phaeosphaeria; (B)-Fusariella; (C)-Pyrenochaetopsis; (D)-Cyphellophora.
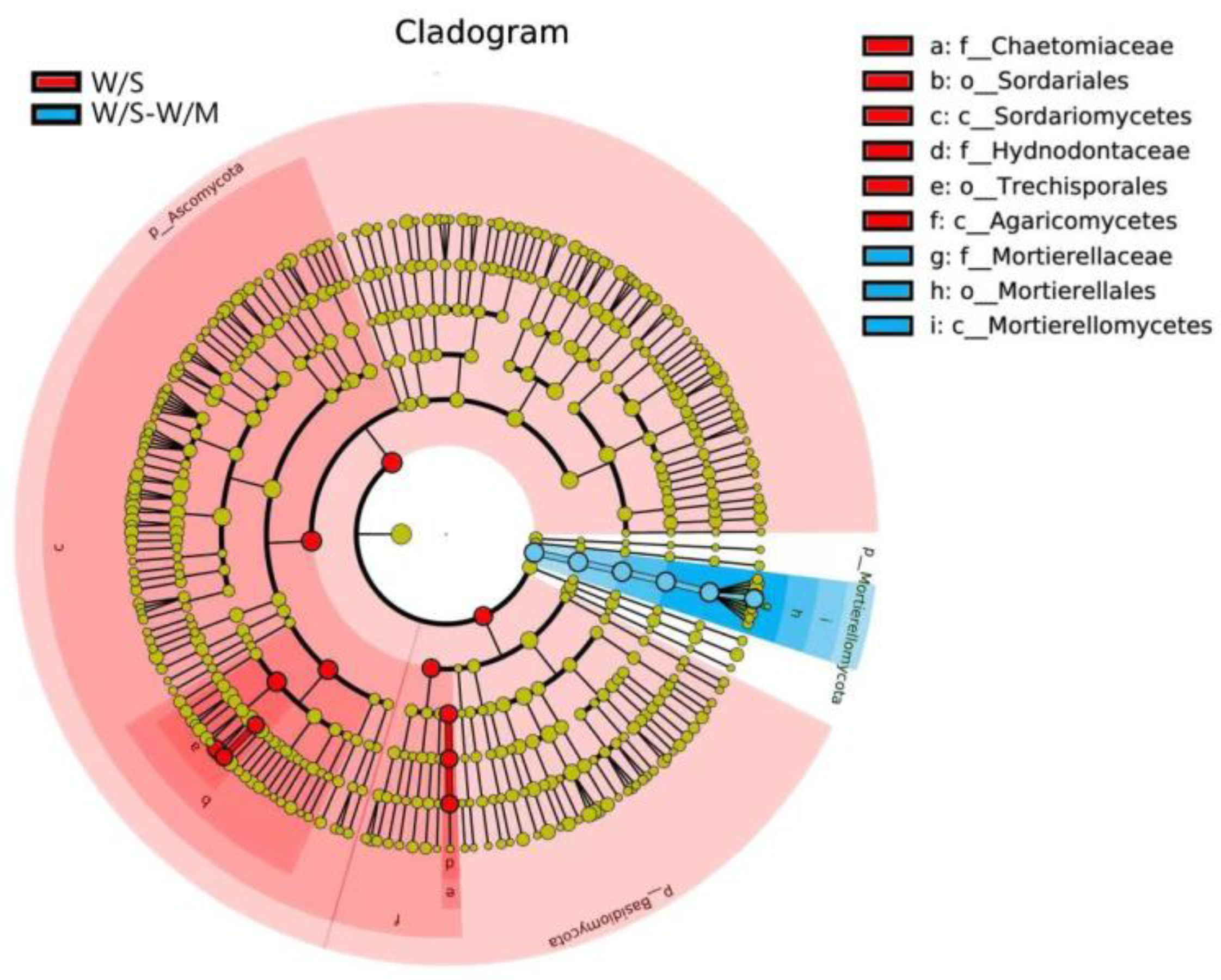
LEfSe analysis was carried out to investigate the primary taxa that were responsible for the differences in the fungal compositions between the two groups. The cladogram analysis revealed a total of 15 characteristics showed a significant difference in the abundance between the two cropping systems at an LDA threshold of 4.0, including 3 phylum, 3 classes, 3 orders, 3 families, 3 genera, and 3 species (Figure 7). The W/S group exhibited differential enrichment of genera Myceliophthora and Trechispora, and the LDA score of Trechispora was higher than that of Myceliophthora. However, the genus Mortierella was enriched in the W/S-W/M group (Supplemental Figure S3).
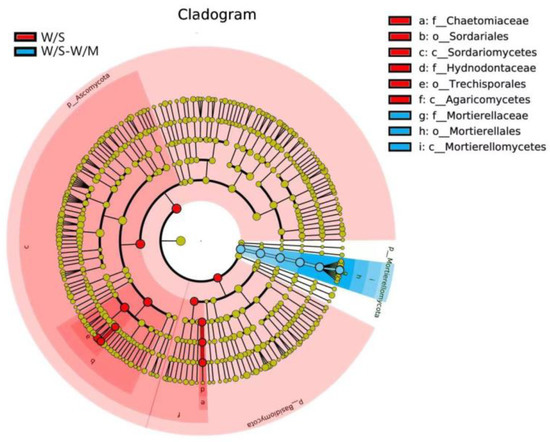
Figure 7.
Taxonomic cladogram produced from the LEfSe analysis. The phylum, class, order, family, genus, and species levels are listed in order from inside to outside of the cladogram, and the labels for levels of the family and genus are abbreviated using a single letter. Red and blue showed taxa enriched in W/S and W/S-W/M, respectively, while the yellow circles represented the taxa without significant differences among the two cropping systems.
3.6. Correlations between Soil Properties and Bacterial and Fungal Communities
The correlation between the soil properties and microbial communities was evaluated using Spearman’s correlation analysis. For fungal communities, the SOM contents were significantly (p < 0.05) and negatively correlated with the relative abundances of Gibberella. The NO3−-N content was significantly (p < 0.01) and positively correlated to Trechispora, and negatively (p < 0.05) correlated to Mortierella. The AP content was significantly (p < 0.05) and positively correlated to Mortierella, and negatively (p < 0.01) correlated to Trechispora, respectively. The soil pH was significantly (p < 0.05) and positively correlated to Trechispora and Myceliophthora, but negatively (p < 0.01) correlated to Mortierella (Table 2). For bacteria, the TN contents were significantly (p < 0.05) and positively correlated to the Chujaibacter genus. The NH4+-N contents were significantly (p < 0.05) and positively correlated to KD4.96, and negatively (p < 0.05) correlated to Burkholderia genera. The soil pH was significantly (p < 0.05) and negatively correlated to Candidatus_Udaeobacter genera (Table 3).

Table 2.
The Spearman’s correlations between soil properties and fungal abundance at the genus level.

Table 3.
The Spearman’s correlations between soil properties and bacterial abundance at the genus level.
4. Discussion
Continuous cropping practice hampers soybean production. Crop rotation is beneficial for soybean growth, but the effect of different cropping systems on soybean rhizosphere soil properties and microbes in continuous wheat/soybean double-cropping systems remains unknown. In the present study, the rhizosphere soil fungal and bacterial characteristics of different soybean cropping systems were investigated to assess the potential association between microbial community and continuous soybean cropping obstacles. The outcomes of this study showed that the continuous wheat-soybean cropping system decreased soybean yield and soil available P levels. The fungal community diversity and evenness were also influenced by the different cropping systems while the bacterial community diversity, richness, and evenness remained unaltered. The relative abundance of certain fungal taxa was influenced by the cropping system, which could be involved in changing soil fertility and crop yield.
4.1. Soil Chemical Properties and Soybean Yield
The rotation of crops is a traditional and effective method for improving soil quality, replanting for disease alleviation, and increasing crop yield [25]. In this study, wheat/soybean-wheat/maize rotation systems contained higher soil available phosphorus (AP) than continuous wheat/soybean cropping systems. Previous studies showed that soybean-corn rotation treatments had significantly higher AP contents of soil than continuously cropped soybean [26], which indicated that crop rotation could increase soil phosphorus content. However, NH4+-N and NO3−-N levels and soil pH were significantly higher in the continuous W/S cropping system than in the W/S-W/M crop rotation system in contrast to a previous study, which reported that the soil pH and available nitrogen were significantly higher in wheat-soybean/wheat-maize crop rotation systems [3]. The difference may be due to the difference in the planting years, soil type, and different cropping systems. In addition, the findings of no significant differences in the SOM and TN content between the W/S and W/S-W/M groups are in line with the previous reports, which showed that the overall carbon and nitrogen concentration was less susceptible to various cropping systems [27].
In this study, compared with continuous wheat/soybean cropping, the wheat/soybean-wheat/maize crop rotation system showed a greater effect on promoting soybean growth. This might be attributed to an improved soil environment, including soil nutrition, microbial activity, and growth-promoting substances. Perez-Montano et al., found that some beneficial microorganisms may increase the plant’s growth either by using their metabolism (solving phosphates, producing hormones, and fixing nitrogen) or by directly influencing the metabolism of the plant (increasing water and minerals uptake) [28]. Benitez et al., also found that rhizosphere microbial communities played an important role in explaining the benefits of diverse crop rotations for maize and soybeans [7]. Continuous cropping of soybean could enrich root exudates and organic compounds in the rhizosphere, which cause the growth of pathogens [29]. These studies could well explain our results that the soybean yield in the W/S-W/M group was significantly higher than that in the W/S group.
4.2. Microbial Abundance and Community Composition
Previous studies have demonstrated differences in the rhizosphere microbial populations between soybean continuous and rotation cropping systems [30,31]. In this study, the dominant bacterial phyla were the Proteobacteria, Actinobacteriota, and Acidobacteriota in both W/S and W/S-W/M systems. This result was consistent with previous studies, which reported that the same bacterial phyla were most prevalent in rotation fields of soybeans and corn [32,33]. At the genus level, Burkholderia-Caballeronia-Paraburkholderia, Sphingomonas, and Candidatus_Udaeobacter were the dominant genus. However, an insignificant difference was observed between the bacterial communities in the W/S and W/S-W/M groups at the phylum level.
A distinct difference was observed between the two cropping systems in the composition of the fungal community at the phylum and genus levels. At the phylum level, Ascomycota, Mortierellomycota, and Basidiomycota emerged as the dominant fungal phyla in the W/S and W/S-W/M groups. These findings were in line with other investigations which reported that in various crop rotation systems, these three fungi phyla were dominant, suggesting their ubiquitous nature and critical role in ecosystems [34,35]. A significant variation was observed in the relative abundance of fungal phyla Mortierellomycota and Rozellomycota, which were found to be less abundant in the W/S group. At the genus level, the outcomes of the current study demonstrated that the relative abundance of Mortierella and Humicola was higher in the W/S-W/M group, while the abundance of Trechispora and Myceliophthora was higher in the W/S group. Mortierella is known to be a large genus of plant growth-promoting fungi in agricultural soil. It significantly increases crop production by positively influencing the activity of beneficial microorganisms [36]. Trechispora and Myceliophthora are known to be a large genus of saprotrophs that play a significant role in wood degradation and may be related to underdeveloped roots [37,38]. However, previous studies reported that continuous soybean cropping enhanced the relative abundance of various species, including Alternaria, Clonostachys, Fusarium, Humicola, and Mortierella [9,29,39]. Variations in the composition of microbial communities are caused by a variety of factors, such as the physicochemical characteristics of the soil, allelopathic autotoxicity accumulation, and root exudates.
4.3. Microbial Alpha Diversity
The effect of two cropping systems on both fungal and bacterial alpha diversity was examined for soybean rhizosphere soil. The current study reported a higher soil fungal diversity and evenness in the continuous W/S cropping system. This was in line with the previous investigation which showed that the soil fungal diversity increased in the continuous soybean cropping over a long time [9]. However, the previous study had reported contrasting findings where it was reported that the continuous cropping of soybean reduced the fungal community diversity in both the bulk and rhizosphere soil compared with the soybean rotation cropping system [14]. Higher fungal diversity in the rhizosphere soil of the W/S group could be attributed to the ongoing pressure from continuous soybean croppings, such as root exudates in the rhizosphere soil and altered soil chemical properties. In addition, the current study also reported that the cropping system did not significantly alter the diversity, richness, and evenness of the bacterial community. It was reported that a canola-soybean cropping system similarly affected bacterial community diversity and richness with the soybean-soybean system [40]. However, another finding showed that corn-soybean crop rotation treatment differs from continuous soybean cropping in bacterial diversity and community composition [33,41]. These inconsistencies between these studies might be due to inconsistent cropping systems and soil environment as all these factors could affect the microbial community.
4.4. Potentially Pathogenic and Beneficial Fungi
Identification of core microbial species is crucial for unraveling microbial consortium ecology [42]. According to this study, the relative abundance of the plant growth-promoting fungi Mortierella, nematophagous fungi Plectosphaerella and biological control fungi Coniothyrium were dramatically enhanced in the W/S-W/M group compared with the W/S group. Mortierella is a growth-promoting soil-dwelling saprobe with diverse characteristics [43]. According to a previous study, it promoted the decomposition of plant litter and degraded aromatic hydrocarbons, and helped plants and mycorrhizal fungus acquire phosphorus [44,45]. A previous study had reported that Mortierella was substantially more prevalent in the soybean soil of CR5 and CC13 [3]. Both LEfSe and MetaStat analyses have shown that the abundance of Mortierella was remarkably higher in the rhizosphere soil of the W/S-W/M group compared with the W/S group, which might be associated with the increased soil fertility index and the improved plant health and yield production. Long-term continuous cropping could result in an increased population of soybean cyst nematode. In this study, the abundance of Plectosphaerella was significantly higher in the rhizosphere soil of the W/S-W/M group than in the W/S group. Plectosphaerella cucumerina, a nematophagous fungus, has shown a 60% reduction in potato cyst nematodes population size in field trials when used as a biological control agent [46]. Consequently, Plectosphaerella might be responsible for the development of a suppressive soil for soybean cyst nematodes in the W/S-W/M group. Certain species of fungi Coniothyrium, such as Coniothyrium minitans are promising fungal biological control for plant pathogen Sclerotinia sclerotiorum, which is the main cause of rot disease [47,48]. The higher abundance of Coniothyrium in the W/S-W/M group might play a crucial role in the biocontrol of plant disease. Therefore, the inoculation of beneficial microorganisms into continuous cropping soil might be an effective strategy for overcoming the continuous cropping obstacles. We also discovered that certain biocontrol microbes, such as Trichoderma, Leptodontidium, and Metapochonia were increased in the W/S group. These microbes are environmentally friendly that can antagonize various plant pathogens. The genus Trichoderma comprises a large number of rhizocompetent filamentous fungal strains found in a large variety of ecosystems which can antagonize a variety of pathogens [49]. Leptodontidium sp. is a genus of dark septate endophytes which could colonize healthy roots and decrease the growth of the root phytopathogens [50]. A biological control agent based on Metapochonia rubescens was used against nematodes that parasitize plants [51]. All these potentially beneficial fungi may be sensitive to soybean continuous cropping and play an important role in antagonizing soil-borne pathogens.
In this study, compared with the W/S-W/M group, certain plant pathogens, such as Pyrenochaetopsis and Cyphellophora were significantly increased in the W/S group. The genus Pyrenochaetopsis consists of several saprophytic and endophytic fungal species that are involved in plant pathogenesis [52]. Previous studies had demonstrated that Pyrenochaetopsis was enriched in the soybean roots after continuous cropping [53]. Certain Cyphellophora species are also known to be potential pathogens [54]. The outcomes of this study were consistent with a recent study, which reported that more than ten years of continuous alfalfa cropping led to a significantly higher rate of Cyphellophora compared with cropping for less than ten years [55]. However, the relative abundances of certain potentially pathogenic fungi, such as Phaeosphaeria and Fusariella increased in the W/S-W/M group compared with the W/S group. Phaeosphaeria species are reported to cause disease in barley, wheat, and a wide range of wild grasses [56]. Previous studies had focused on the isolation and identification of new species of Fusariella, and there were few reports on their pathogenicity [57]. Therefore, despite an increase in the abundance of certain pathogens in the rotation system, these pathogens may not harm soybean plants.
In addition, we also observed that certain lignocellulose-degrading fungi, such as Trechispora, Myceliophthora, Botryotrichum, and Coniochaeta that were significantly enriched in the W/S group which could enhance soil organic matter conversion by converting plant residues. A previous study had demonstrated that as continuous cropping was extended, an improvement in soil nutrient availability and content was observed. It could be largely attributed to the increased abundance of beneficial microbial taxa that were involved in soil material cycling and lignin degradation in long-term continuous cropping systems [3]. Although the content of soil organic matter did not change in the two cropping systems, it might increase with the increasing planting years in the W/S group.
5. Conclusions
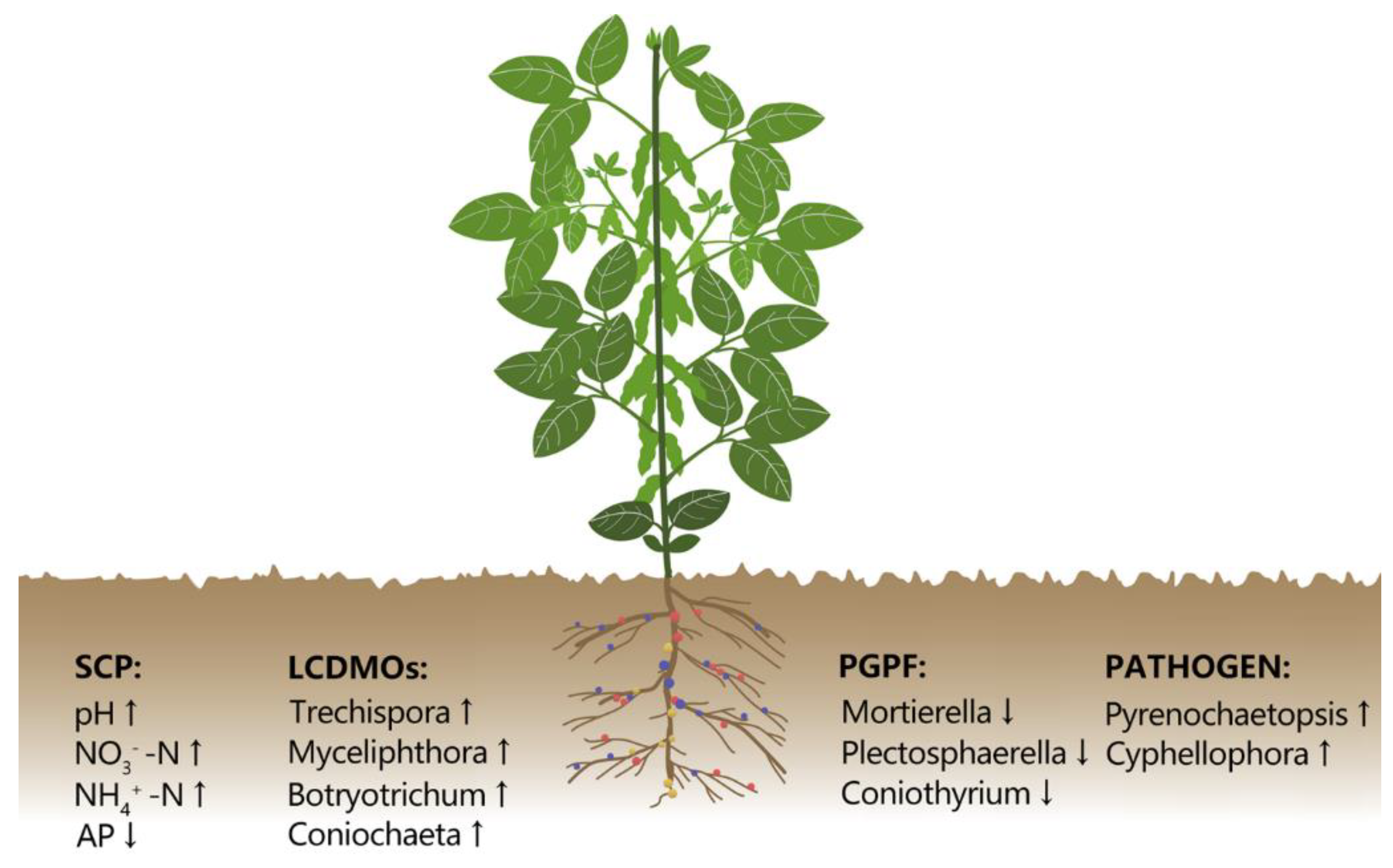
In this study, we observed that in the continuous wheat/soybean cropping system, plant growth retardation and yield reduction were closely related to fungi around the rhizosphere (Figure 8). Wheat-soybean/wheat-maize rotation system improved soybean yield by approximately 36.8% and soil available P content by 23.24% in contrast to the continuous wheat/soybean cropping system. The W/S-W/M rotation system also changed the fungal community structure and decreased the fungal diversity. In addition, several keystone fungal genera, such as Mortierella, Myceliophthora, and Trechispora emerged as important biomarkers that could be involved in changing soil fertility, soil-borne disease, and crop yield. Therefore, these results shed light on further understanding of the mechanisms associated with soybean continuous cropping obstacles in the wheat/soybean double cropping system. Further investigations on the effects of long-term continuous W/S cropping on the dynamic change of rhizosphere soil microbial communities at different development stages of the soybean, as well as the responses of the rhizoplane and endophytic microbial communities, would be interesting.
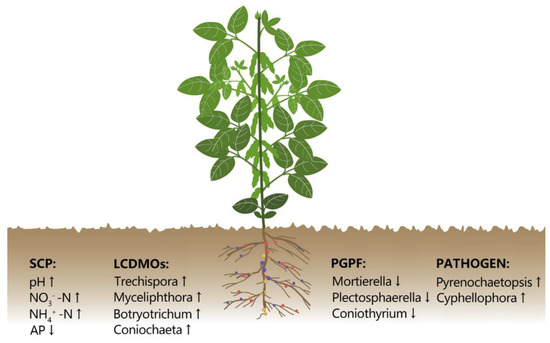
Figure 8.
A conceptual paradigm explaining how long-term continuous wheat/soybean cropping influences rhizosphere soil chemical properties and microbial community. SCP: soil chemical properties; LCDMOs: lignocellulose degrading microorganisms; PGPF: plant growth-promoting fungi; PATHOGEN: plant pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13010028/s1, Figure S1: The FunGuild analysis of fungal community in the rhizosphere soils.; Figure S2: Relative abundance of lignocellulose-degrading fungi in two cropping systems. W/S, continuous wheat/soybean cropping system; W/S-W/M, wheat/soybean-wheat/maize rotation system; Figure S3: Histogram of the linear discriminant analysis (LDA) scores. Taxa enriched in W/S are shown in red and W/S-W/M in blue; Table S1: Relative abundances of the fungal phyla and genera in the rhizosphere soils under continuous wheat/soybean cropping system (W/S) and wheat/soybean-wheat/maize rotation system (W/S-W/M); Table S2: Relative abundances of the bacterial phyla and genera in the rhizosphere soils under continuous wheat/soybean cropping system (W/S) and wheat/soybean-wheat/maize rotation system (W/S-W/M).
Author Contributions
Conceptualization, Q.S. and W.J.; methodology, Q.S.; software, P.Z.; validation, Z.Z. and X.S.; formal analysis, X.L.; investigation, Q.S., P.Z., Z.Z., X.L., X.S. and W.J.; data curation, Q.S.; writing—original draft preparation, Q.S.; writing—review and editing, Q.S. and W.J.; visualization, Q.S. and W.J.; supervision, P.Z.; project administration, Z.Z.; funding acquisition, W.J. and Q.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shandong Modern Agricultural Industrial Technology System Construction Fund, grant number SAIT-02-06, Youth Program of the Natural Science Foundation of Shandong, grant number ZR2020QC108, National Key Research and Development Program, grant number 2016YFD0300803, and Qingdao Agricultural University High-level Talents Research Foundation.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Haiping Ni from the State Key Laboratory of Microbial Technology of Shandong University for help and guidance in the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Yu, T.; Shi, Q.; Han, D.; Yu, K.; Wang, L.; Wang, S.; Xiang, H.; Wen, R.; Nian, H.; et al. Rhizosphere soil bacterial communities of continuous cropping-tolerant and sensitive soybean genotypes respond differently to long-term continuous cropping in Mollisols. Front. Microbiol. 2021, 12, 729047. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Yu, Z.; Yao, Q.; Li, Y.; Liang, A.; Zhang, W.; Mi, G.; Jin, J.; Liu, X.; et al. Long-term continuous cropping of soybean is comparable to crop rotation in mediating microbial abundance, diversity and community composition. Soil Tillage Res. 2020, 197, 104503. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Penuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Sadiqi, S.; Hamza, M.; Ali, F.; Alam, S.; Shakeela, Q.; Ahmed, S.; Ayaz, A.; Ali, S.; Saqib, S.; Ullah, F.; et al. Molecular characterization of bacterial isolates from soil samples and evaluation of their antibacterial potential against MDRS. Molecules 2022, 27, 6281. [Google Scholar] [CrossRef] [PubMed]
- Benitez, M.S.; Ewing, P.M.; Osborne, S.L.; Lehman, R.M. Rhizosphere microbial communities explain positive effects of diverse crop rotations on maize and soybean performance. Soil Boil Biochem. 2021, 159, 108309. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Shi, Z.; Wang, S.; Wang, Z.; Liu, Y.; Wen, X.; Mo, F.; Han, J.; Liao, Y. Crop development has more influence on shaping rhizobacteria of wheat than tillage practice and crop rotation pattern in an arid agroecosystem. Appl. Soil Ecol. 2021, 165, 104016. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. Response of soil fungal community structure to long-term continuous soybean cropping. Front. Microbiol. 2018, 9, 3316. [Google Scholar] [CrossRef]
- Rao, D.; Meng, F.; Yan, X.; Zhang, M.; Yao, X.; Kim, K.S.; Zhao, J.; Qiu, Q.; Xie, F.; Zhang, W. Changes in soil microbial activity, bacterial community composition and function in a long-term continuous soybean cropping system after corn insertion and fertilization. Front. Microbiol. 2021, 12, 638326. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Does crop rotation yield more in China? A meta-analysis. Field Crops Res. 2020, 245, 107659. [Google Scholar] [CrossRef]
- Meier, M.A.; Lopez-Guerrero, M.G.; Guo, M.; Schmer, M.R.; Herr, J.R.; Schnable, J.C.; Alfano, J.R.; Yang, J. Rhizosphere microbiomes in a historical maize-soybean rotation system respond to host species and nitrogen fertilization at the genus and subgenus levels. Appl. Environ. Microbiol. 2021, 87, e0313220. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C.; Samac, D.A.; Gutknecht, J.L.; Sadowsky, M.J.; Rosen, C.J.; Schlatter, D.; Kinkel, L.L. Impacts of cover crops and nitrogen fertilization on agricultural soil fungal and bacterial communities. Plant Soil. 2021, 466, 139–150. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Q.; Li, Y.; Zhang, W.; Mi, G.; Chen, X.; Yu, Z.; Wang, G. Continuous cropping of soybean alters the bulk and rhizospheric soil fungal communities in a Mollisol of Northeast PR China. Land Degrad. Dev. 2019, 30, 1725–1738. [Google Scholar] [CrossRef]
- Liu, H.; Pan, F.; Han, X.; Song, F.; Zhang, Z.; Yan, J.; Xu, Y. A comprehensive analysis of the response of the fungal community structure to long-term continuous cropping in three typical upland crops. J. Integr. Agric. 2020, 19, 866–880. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Xu, X.; Hu, J.; Sun, X.; Xiao, Y.; Zhang, Y. Trichoderma improves the growth of Leymus chinensis. Biol. Fert. Soils 2018, 54, 685–696. [Google Scholar] [CrossRef]
- Abid, S.; Farid, A.; Abid, R.; Rehman, M.U.; Alsanie, W.F.; Alhomrani, M.; Alamri, A.S.; Asdaq, S.M.B.; Hefft, D.I.; Saqib, S.; et al. Identification, biochemical characterization, and safety attributes of locally isolated Lactobacillus fermentum from Bubalus bubalis (buffalo) milk as a probiotic. Microorganisms 2022, 10, 954. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-Medellin, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Cicek, H.; Entz, M.H.; Martens, J.R.T.; Bullock, P.R. Productivity and nitrogen benefits of late-season legume cover crops in organic wheat production. Can. J. Plant Sci. 2014, 94, 771–783. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, F. Community assembly correlates with alfalfa production by mediating rhizosphere soil microbial community composition in different planting years and regimes. Plant Soil. 2022, 479, 355–370. [Google Scholar] [CrossRef]
- Zou, J.; Yao, Q.; Liu, J.; Li, Y.; Song, F.; Liu, X.; Wang, G. Changes of diazotrophic communities in response to cropping systems in a Mollisol of Northeast China. PeerJ. 2020, 8, e9550. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef]
- Perez-Montano, F.; Alias-Villegas, C.; Bellogin, R.A.; del Cerro, P.; Espuny, M.R.; Jimenez-Guerrero, I.; Lopez-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef]
- Bai, L.; Cui, J.; Jie, W.; Cai, B. Analysis of the community compositions of rhizosphere fungi in soybeans continuous cropping fields. Microbiol. Res. 2015, 180, 49–56. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Kong, W.; Wu, Y.; Wang, J. Effect of monoculture soybean on soil microbial community in the Northeast China. Plant Soil 2009, 330, 423–433. [Google Scholar] [CrossRef]
- Tang, H.; Xiao, C.; Ma, J.; Yu, M.; Li, Y.; Wang, G.; Zhang, L. Prokaryotic diversity in continuous cropping and rotational cropping soybean soil. FEMS Microbiol. Lett. 2009, 298, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.A.; Bolton, M.L.; Cox, M.S.; Suen, G.; Conley, S.P.; Ané, J.-M. Crop rotation, but not cover crops, influenced soil bacterial community composition in a corn-soybean system in southern Wisconsin. Appl. Soil Ecol. 2020, 154, 103603. [Google Scholar] [CrossRef]
- Lyu, J.; Jin, L.; Jin, N.; Xie, J.; Xiao, X.; Hu, L.; Tang, Z.; Wu, Y.; Niu, L.; Yu, J. Effects of different vegetable rotations on fungal community structure in continuous tomato cropping matrix in greenhouse. Front. Microbiol. 2020, 11, 829. [Google Scholar] [CrossRef] [PubMed]
- Bolaji, A.J.; Wan, J.C.; Manchur, C.L.; Lawley, Y.; de Kievit, T.R.; Fernando, W.G.D.; Belmonte, M.F. Microbial community dynamics of soybean (Glycine max) is affected by cropping sequence. Front. Microbiol. 2021, 12, 632280. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Karnaouri, A.; Topakas, E.; Antonopoulou, I.; Christakopoulos, P. Genomic insights into the fungal lignocellulolytic system of Myceliophthora thermophila. Front. Microbiol. 2014, 5, 281. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.L. The Phylogenetic relationship revealed three new wood-inhabiting fungal species from genus Trechispora. Front. Microbiol. 2021, 12, 650195. [Google Scholar] [CrossRef]
- Wu, L.; Chen, J.; Wu, H.; Wang, J.; Wu, Y.; Lin, S.; Khan, M.U.; Zhang, Z.; Lin, W. Effects of consecutive monoculture of Pseudostellaria heterophylla on soil fungal community as determined by pyrosequencing. Sci. Rep. 2016, 6, 26601. [Google Scholar] [CrossRef]
- Lay, C.Y.; Bell, T.H.; Hamel, C.; Harker, K.N.; Mohr, R.; Greer, C.W.; Yergeau, E.; St-Arnaud, M. Canola root-associated microbiomes in the Canadian Prairies. Front. Microbiol. 2018, 9, 1188. [Google Scholar] [CrossRef]
- Ashworth, A.J.; DeBruyn, J.M.; Allen, F.L.; Radosevich, M.; Owens, P.R. Microbial community structure is affected by cropping sequences and poultry litter under long-term no-tillage. Soil Bio. Biochem. 2017, 114, 210–219. [Google Scholar] [CrossRef]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: The hunt for a core microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, L.; Redmile-Gordon, M.; Zhang, J.B.; Zhang, C.Z.; Ning, Q.; Li, W. Mortierella elongata’s roles in organic agriculture and crop growth promotion in a mineral soil. Land Degrad. Dev. 2018, 29, 1642–1651. [Google Scholar] [CrossRef]
- Ellegaard-Jensen, L.; Aamand, J.; Kragelund, B.B.; Johnsen, A.H.; Rosendahl, S. Strains of the soil fungus Mortierella show different degradation potentials for the phenylurea herbicide diuron. Biodegradation 2013, 24, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Tamayo-Velez, A.; Osorio, N.W. Co-inoculation with an arbuscular mycorrhizal fungus and a phosphate-solubilizing fungus promotes the plant growth and phosphate uptake of avocado plantlets in a nursery. Botany 2017, 95, 539–545. [Google Scholar] [CrossRef]
- Atkins, S.D.; Clark, I.M.; Sosnowska, D.; Hirsch, P.R.; Kerry, B.R. Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, real-time PCR, selective media, and baiting. Appl. Environ. Microbiol. 2003, 69, 4788–4793. [Google Scholar] [CrossRef]
- de Vrije, T.; Antoine, N.; Buitelaar, R.M.; Bruckner, S.; Dissevelt, M.; Durand, A.; Gerlagh, M.; Jones, E.E.; Luth, P.; Oostra, J.; et al. The fungal biocontrol agent Coniothyrium minitans: Production by solid-state fermentation, application and marketing. Appl. Microbiol. Biotechnol. 2001, 56, 58–68. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, H.; Luo, C.; Du, L.; Cheng, J.; Xie, J.; Jiang, D.; Fu, Y. CmAim24 is essential for mitochondrial morphology, conidiogenesis, and mycoparasitism in Coniothyrium minitans. Appl. Environ. Microbiol. 2020, 86, e02291-19. [Google Scholar] [CrossRef]
- Brotman, Y.; Kapuganti, J.G.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, R390–R391. [Google Scholar] [CrossRef]
- Berthelot, C.; Leyval, C.; Chalot, M.; Blaudez, D. Interactions between dark septate endophytes, ectomycorrhizal fungi and root pathogens in vitro. FEMS Microbiol. Lett. 2019, 366, fnz158. [Google Scholar] [CrossRef]
- Moosavi, M.R.; Zare, R. Fungi as biological control agents of plant-parasitic nematodes, in plant defence. Biol. Control. 2012, 67–107. [Google Scholar]
- da Silva, R.R.; da Rosa, N.G.; de Oliveira, L.C.G.; Juliano, M.A.; Juliano, L.; Rosa, J.C.; Cabral, H. Biochemical properties and catalytic specificity of a novel neutral serine peptidase secreted by fungus Pyrenochaetopsis sp. Appl. Biochem. Biotechnol. 2019, 187, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Neupane, A.; Bulbul, I.; Wang, Z.; Lehman, R.M.; Nafziger, E.; Marzano, S.L. Long term crop rotation effect on subsequent soybean yield explained by soil and root-associated microbiomes and soil health indicators. Sci. Rep. 2021, 11, 9200. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, X.; Wang, Q.; Ongena, M.; Wei, D.; Ding, J.; Guan, D.; Cao, F.; Zhao, B.; Li, J. Responses of fungal community composition to long-term chemical and organic fertilization strategies in Chinese Mollisols. Microbiologyopen 2018, 7, e00597. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, Y.; Liu, X.; Liu, J.; Huang, X.; Yang, W.; Yang, Z.; Lan, L.; Zhou, J.; Wang, G. Dynamics of soil properties and fungal community structure in continuous-cropped alfalfa fields in Northeast China. PeerJ 2019, 7, e7127. [Google Scholar] [CrossRef]
- Ghaderi, F.; Habibi, A.; Sharifnabi, B. Phylogenetic analysis of Phaeosphaeria species using mating type genes and distribution of mating types in Iran. Plant Pathol. J. 2022, 38, 78–89. [Google Scholar] [CrossRef]
- Lin, C.-G.; Chen, Y.; McKenzie, E.H.C.; Bhat, D.J.; Ariyawansa, H.A.; Hyde, K.D.; Wang, Y. The genus Fusariella. Mycol. Prog. 2016, 15, 1313–1326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).