Assessment of Tomato (Solanum lycopersicum) Landraces for Their Agronomic, Biochemical Characteristics and Resistance to Phytophthora infestans
Abstract
1. Introduction
2. Results
2.1. Morphological Traits
2.2. Biochemical Parameters
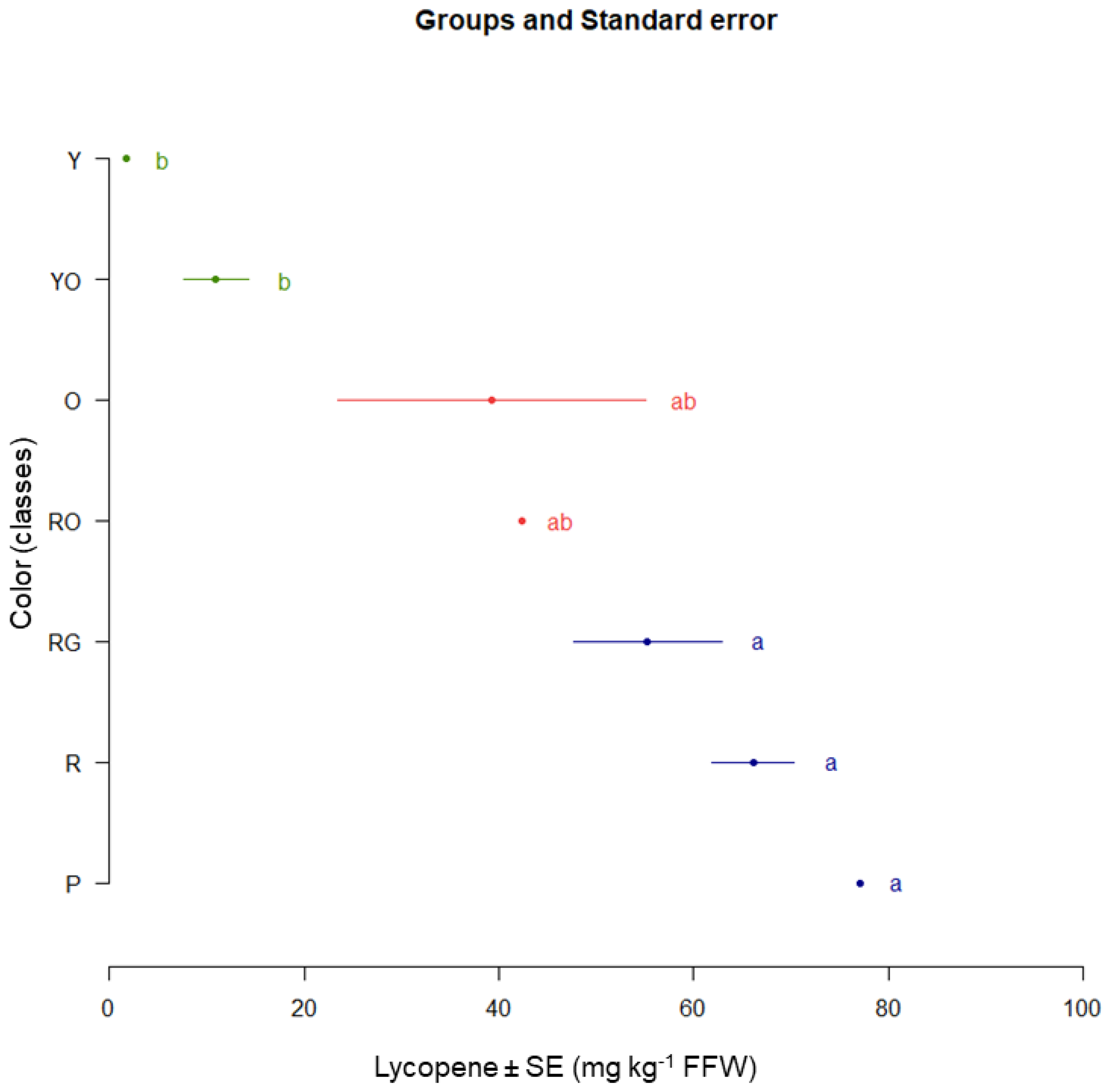
2.3. The Influence of Origin on Tomato Fruit Characteristics
2.4. The Influence of Lycopene on Tomato Fruit Characteristics
2.5. The Influence of Tomato Fruit Characteristics on Late Blight Disease
Assessment of Local Tomato Varieties to Late Blight Resistance
2.6. The Influence of Fruit Weight on Tomato Fruit Characteristics
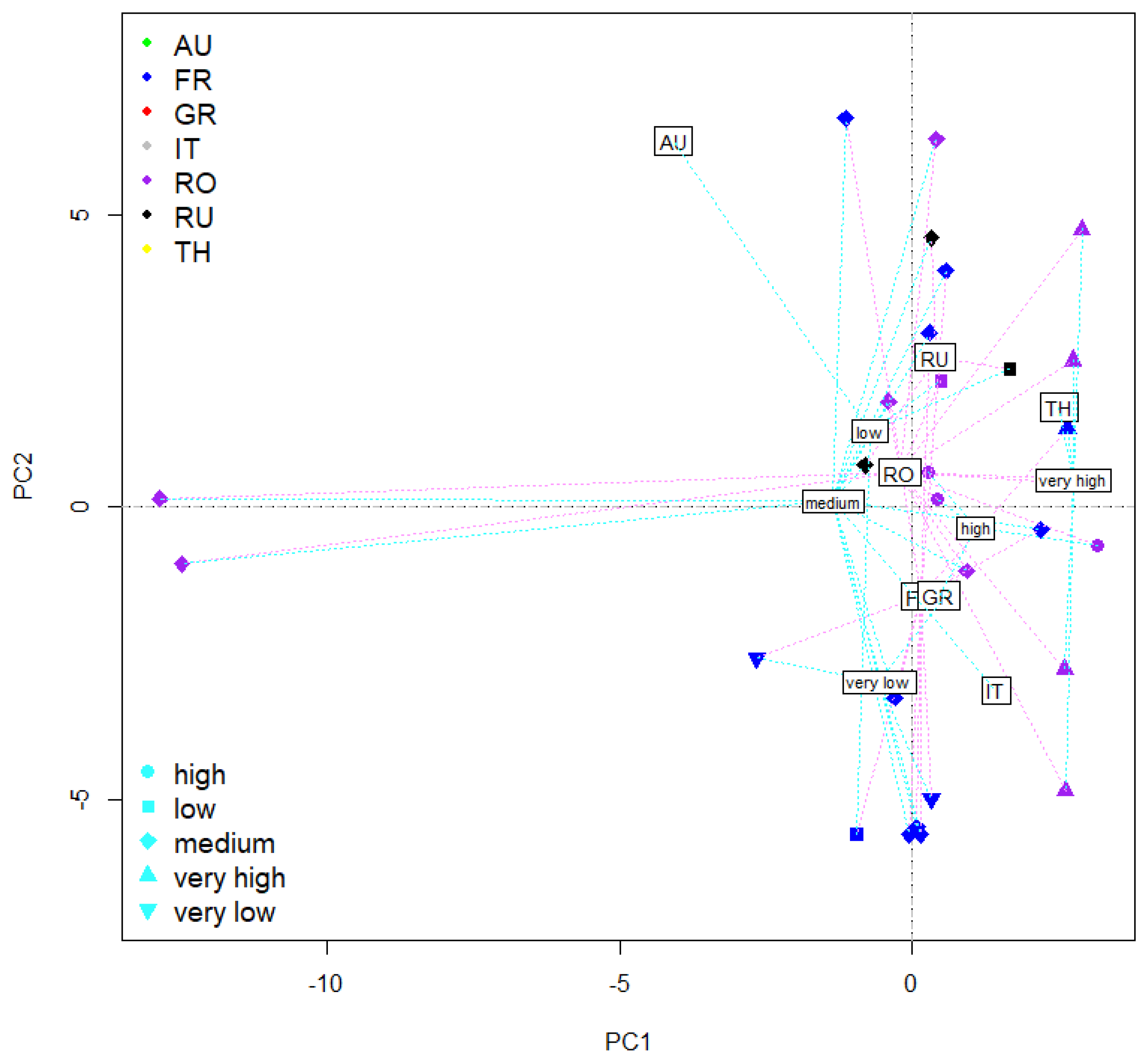
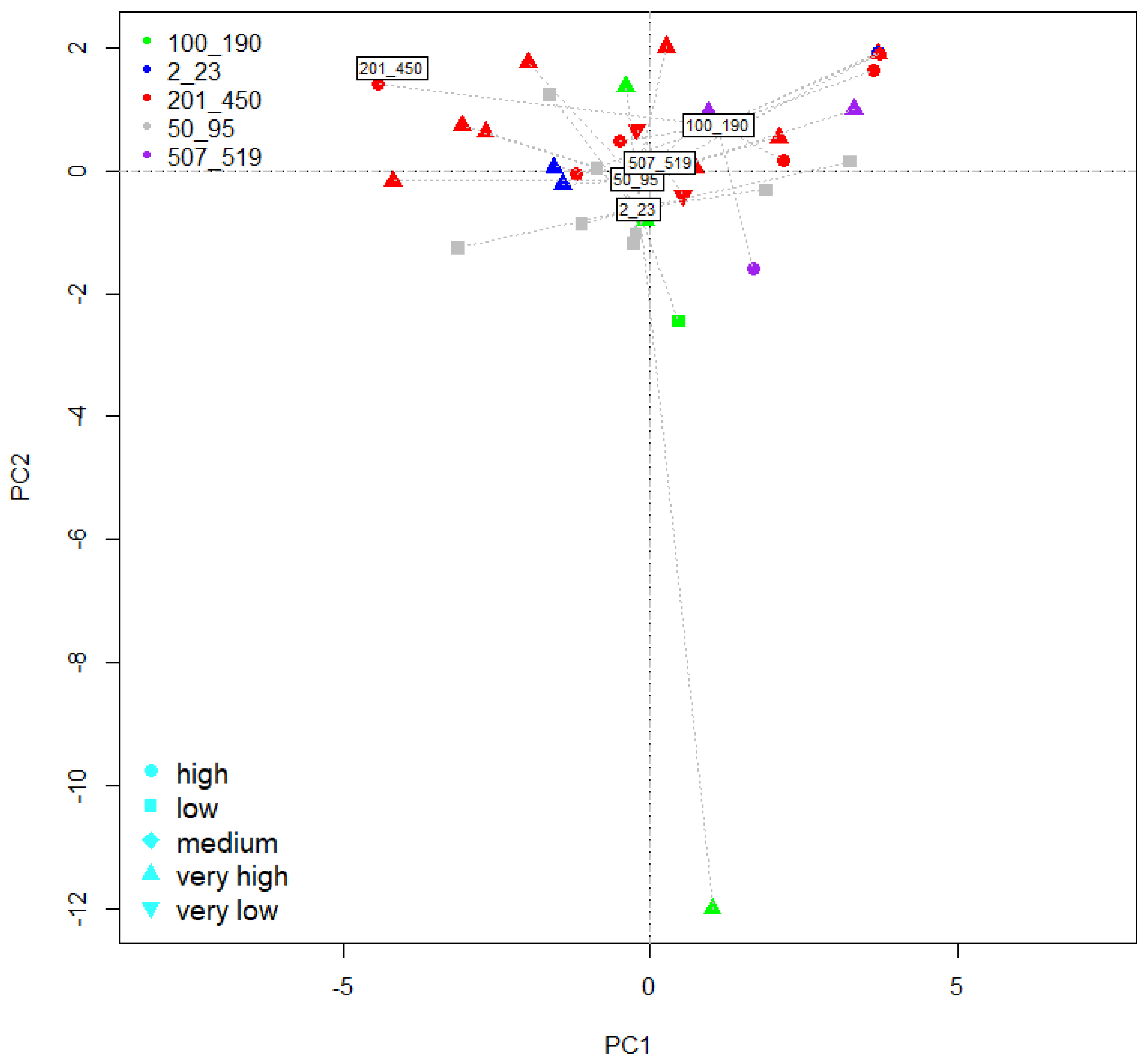
2.7. PCA Ordination of Tomato Fruit Characteristics as Influenced by Experimental Factors
3. Discussion
3.1. Characteristics of Tomato Landraces
3.2. Biochemical Characteristics of Tomato Landraces
4. Materials and Methods
4.1. Biologic Material and Experimental Conditions
4.2. Morphological, Agronomic Characterization and Assessment of Resistance to P. infestans of the Tomato Landraces
4.3. Determination of the Dry Matter Content and pH of Tomato Landraces
4.4. Ultrasound-Assisted Extraction (UAE) of Carotenoids
4.5. Quantitative and Qualitative Analysis of Carotenoids by HPLC/DAD
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hammer, K.; Diederichsen, A. Evolution, Status and Perspectives for Landraces in Europe. In European Cooperative Programme for Plant Genetic Resources; Biodiversity Technical Bulletin no. 15; Biodiversity International: Rome, Italy, 2009. [Google Scholar]
- Zaharia, H.; Kastler, G. European Union: Seeds and plants—Legislative progress? Citiz. Earth 2003, 6, 6. [Google Scholar]
- Maxim, A.; Străjeru, S.; Albu, C.; Sandor, M.; Mihalescu, L.; Pauliuc, S.E. Conservation of vegetable genetic diversity in Transylvania-Romania. Sci. Rep. 2020, 10, 18416. [Google Scholar] [CrossRef] [PubMed]
- Derpsch, R.; Roth, C.H.; Sidiras, N.; Köpke, U. Controle de Erosão no Paraná, Brasil: Sistema de Cobertura do Solo, Plantiodireto e Preparo Conservacionista do Solo; Sonderpublikation der GTZ, No. 245; TZ-Verlagsgesellschaft GmbH.: Rossdorf, Germany, 1991; p. 272. [Google Scholar]
- Negri, V. Landraces in central Italy: Where and why they are conserved and perspectives for their on farm conservation. Genet. Resour. Crop Evol. 2003, 50, 871–885. [Google Scholar] [CrossRef]
- Sanchez, E.; Sifres, A.; Casanas, F.; Nuez, F. The endangered future of organoleptically prestigious European landraces: Ganxet bean (Phaseolus vulgaris L.) as an example of a crop originating in the Americas. Genet. Resour. Crop Evol. 2008, 55, 45–52. [Google Scholar] [CrossRef]
- Bertoldo, J.G.; Coimbra, J.L.M.; Guidolin, A.F.; Braatz de Andrade, L.R.; Nodari, R.O. Agronomic potential of genebank landrace elite accessions for common bean genetic breeding. Sci. Agric. 2014, 71, 120–125. [Google Scholar] [CrossRef]
- Singh, S.; Waman, A.A.; Bohr, P.; Gautam, R.K.; Dam, R.S. Conservation and sustainable utilization of horticultural biodiversity in tropical Andaman and Nicobar Islands, India. Genet. Resour. Crop Evol. 2016, 63, 1431–1445. [Google Scholar] [CrossRef]
- Nyadanu, D.; Aboagye, L.M.; Akromah, R.; Dansi, A. Agro-biodiversity and challenges of on-farm conservation: The case of plant genetic resources of neglected and underutilized crop species in Ghana. Genet. Resour. Crop Evol. 2016, 63, 1397–1409. [Google Scholar] [CrossRef]
- Maxted, N.; Dulloo, M.E.; Ford-Lloyd, B.V. Enhancing Crop Genepool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; CAB eBooks: Wallingford, UK, 2016. [Google Scholar]
- Lammerts van Bueren, E.T.; Struik, P.C.; Jacobsen, E. Ecological aspects in organic farming and its consequences for an organic crop ideotype. Neth. J. Agric. Sci. 2002, 50, 1–26. [Google Scholar]
- Micheloni, C. On farm seed production: Integrity of organic farming system and biodiversity safeguard. In Proceedings of the First World Conference on Organic Seed, Rome, Italy, 5–7 July 2004; FAO: Rome, Italy, 2004. [Google Scholar]
- Chable, V. Conserving and Developing Crop Biodiversity—Biodiversity and Local Ecological Knowledge in France; Edition Cemagref, Cirad, Ifremer, Inr; Iddri, IFB: Paris, France, 2005. [Google Scholar]
- Conversa, G.; Lazzizera, C.; Bonasia, A.; Cifarelli, S.; Losavio, F.; Sonnante, G.; Elia, A. Exploring on-farm agro-biodiversity: A study case of vegetable landraces from Puglia region (Italy). Biodivers. Conserv. 2020, 29, 747–770. [Google Scholar] [CrossRef]
- Izahur, H.; Sher, A.K.; Sajid, A.A.F.; Naushad, A.; Sardar, A.; Shah, M.; Ijaz, H.; Kamran, A.; Haneef, R. Genetic Diversity among Tomato Accessions based on Agro-Morphological Traits (Kepelbagaian Genetikantara Penerimaan Tomato berdasarkan Ciri Agro Morfologi). Sains Malays. 2018, 47, 2637–2645. [Google Scholar]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Bauchet, G.; Causse, M. Genetic Diversity in Tomato (Solanum lycopersicum) and Its Wild Relatives. In Genetic Diversity in Plants; IN-TECH Education and Publishing: Rijeka, Croatia, 2012. [Google Scholar] [CrossRef]
- Ministerul Agriculturii și Dezvoltării Rurale din România. Catalogul Oficial al Soiurilor de Plante din România; Institutul de Stat Pentru Testarea și Înregistrarea Soiurilor, Ministerul Agriculturii și Dezvoltării Rurale din România: Bucharest, Romania, 2020. Available online: https://istis.ro/image/data/download/catalog-oficial/CATALOG%202020.pdf (accessed on 27 July 2022).
- Böhm, V.; Liets, G.; Olmedilla-Alonso, B.; Phelan, D.; Reboul, E.; Bánati, D.; Borel, P.; Corte-Real, J.; de Lera, A.R.; Desmarchelier, D.; et al. From carotenoid intake to carotenoid blood and tissue concentrations—Implications for dietary intake recommendations. Nutr. Rev. 2020, 79, 544–573. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease—Current state of knowledge. J. Funct. Foods 2017, 38 Pt A, 45–65. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Tudor-Radu, M.; Vîjan, L.E.; Tudor-Radu, C.M.; Tița, I.; Sima, R.; Mitrea, R. Assessment of Ascorbic Acid, Polyphenols, Flavonoids, Anthocyanins and Carotenoids Content in Tomato Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 477–483. [Google Scholar] [CrossRef]
- Kijlstra, A.; Tian, Y.; Kelly, E.R.; Berendschot Tos, T.J.M. Lutein: More than just a filter for blue light. Prog. Retin. Eye Res. 2012, 31, 303–315. [Google Scholar] [CrossRef]
- Fry, W.E.; Goodwin, S.B.; Dyer, A.T.; Matuszak, J.M.; Drenth, A.; Tooley, P.W.; Sujkowski, L.S.; Koh, Y.J.; Cohen, B.A.; Spielman, L.J.; et al. Historical and recentmigrationsof Phytophthora infestans: Chronology, pathways, andimplications. Plant Dis. 1993, 77, 653–661. [Google Scholar] [CrossRef]
- Hooker, W.J. (Ed.) Compendium of Potato Diseases; American Phytopathological Society: St. Paul, MN, USA, 1981; p. 141. [Google Scholar]
- Rylski, I.; Aloni, B.; Karni, L.; Zaidman, Z. Flowering, Fruit Set, Fruit Development and Fruit Quality under Different Environmental Conditions in Tomato and Pepper Crops. Acta Hortic. 1994, 366, 45–56. [Google Scholar] [CrossRef]
- Rana, N.; Kumar, M.; Walia, A.; Sharma, S. Tomato fruit quality under protected environment and open field conditions. Int. J. Bio-Resour. Stress Manag. 2014, 5, 422–426. [Google Scholar] [CrossRef]
- Adams, P.; Ho, L.C. Uptake and distribution of nutrients in relation to tomato fruit quality. Acta Hortic. 1995, 412, 374–387. [Google Scholar] [CrossRef]
- Sima, R.M.; Măniuţiu, D.N.; Cenariu, D.; Lazăr, V.; Sima, N.F. The impact of culture system and fertilization type on yield and fruit quality of greenhouse tomatoes. Adv. Agric. Bot.-Int. J. Bioflux Soc. 2010, 2, 49–54. [Google Scholar]
- Dumas, Y.; Dadomo, M.; Di Luca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidantcontent of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Hou, X.; Cui, J.; Liu, W.; Jiang, N.; Zhou, X.; Qi, H.; Meng, J.; Luan, Y. LncRNA39026 Enhances Tomato Resistance to Phytophthora infestans by Decoying miR168a and Inducing PR Gene Expression. Phytopathology 2020, 110, 873–880. [Google Scholar] [CrossRef]
- Leesutthiphonchai, W.; Andrea, L.V.; Audrey, M.V.A.-F.; Howard, S.J. How Does Phytophthora infestans Evade Control Efforts? Modern Insight Into the Late Blight Disease. Phytopatology 2018, 108, 916–924. [Google Scholar] [CrossRef]
- Meena, O.P.; Bahadur, V. Breeding potential of indeterminate tomato (Solanum lycopersicum L.) Accessions using D2 Analysis. SABRAO J. Breed. Genet. 2015, 47, 49–59. [Google Scholar]
- Maciel, G.M.; Fernandes, M.A.R.; Melo, O.D.; Oliveira, C.S. Agronomic potential of mini tomato hybrids with determinate and indeterminate growth habit. Hortic. Bras. 2016, 34, 144–148. [Google Scholar] [CrossRef]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a tomato landraces collection for fruit-related traits by the aid of a high-throughput genomic platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef]
- Rocha, M.C.; Gonçalves, L.S.A.; Rodrigues, R.; Alves da Silva, P.R.; Ferreira de Carmo, M.G.; Carlos de Souza Abboud, A. Uso do algoritmo de Gower nadeterminação da divergênciagenética entre acessos de tomateiro do grupocereja. Maringá 2010, 32, 423–431. [Google Scholar] [CrossRef][Green Version]
- Fernandes, M.O.; Bianchi, P.A.; Almeida da Silva, L.R.; Vianna, L.S.; Santos, E.A.; Moulin, M.M. Morpho-agronomic characterization and analysis of genetic divergence among accessions of tomatoes (Solanum lycopersicum L.). Ciência Rural. 2018, 48, e20180433. [Google Scholar] [CrossRef]
- Vargas, T.O.; Alves, E.; Carlos de Souza Abboud, A.; Leal, M.; Carmo, M. Diversidade genética em acessos de tomateiro heirloom. Hortic. Bras. 2015, 33, 174–180. [Google Scholar] [CrossRef]
- Agudelo, A.G.A.; Aguirre, N.C.; Ororco, F.J. Caracterización morfológica del tomatetipo cereza (Solanum lycopersicum L.). agron 2001, 19, 44–53. [Google Scholar]
- Terzopoulus, P.J.; Bebeli, P.J. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liberatore, K.L.; MacAlister, C.A.; Huang, Z.; Chu, Y.H.; Jiang, K.; Brroks, C.; Ogawa-Ohnishi, M.; Xiong, G.; Pauly, M.; et al. A cascade of arabinosyltransferases controls shoot meristem size in tomato. Nat. Genet. 2015, 47, 784–792. [Google Scholar] [CrossRef]
- Scarano, A.; Olivieri, F.; Gerardi, C.; Liso, M.; Chiesa, M.; Chieppa, M.; Frusciante, L.; Barone, A.; Santino, A.; Rigano, M.M. Selection of tomato landraces with high fruit yield and nutritional quality under elevated temperatures. J. Sci. Food Agric. 2020, 100, 2791–2799. [Google Scholar] [CrossRef]
- Tembe, K.O.; Chemining’wa, G.; Ambuko, J.; Owino, W. Evaluation of African tomato landraces (Solanum lycopersicum) based on morphological and horticultural traits. Agric. Nat. Resour. 2018, 52, 536–542. [Google Scholar] [CrossRef]
- Chávez-Servia, J.L.; Vera-Guzmán, A.M.; Linares Menéndez, L.R.; Carrillo-Rodríguez, J.C.; Aquino Bolaños, E.N. Agro morphological traits and mineral content in tomato accessions from El Salvador, Central America. Agron. J. 2018, 8, 32. [Google Scholar]
- Ortiz, R.; Izquierdo, J. Yield stability differences among tomato genotypes grown in Latin America and the Caribbean. Hortic. Sci. 1994, 29, 1175–1177. [Google Scholar] [CrossRef]
- Akhtar, K.P.; Saleem, M.Y.; Asghar, M.; Ali, S.; Sarwar, N.; Elahi, M.T. Resistance of Solanum species to Phytophthora infestans evaluated in the detached leaf assays and whole plant assays. Pak. J. Bot. 2012, 44, 1141–1146. [Google Scholar]
- Foolad, M.R.; Sullenberger, M.T.; Ashrafi, H. Detached leaflet evaluation of tomato germplasm for late blight resistance and its correspondence with field and greenhouse screenings. Plant Dis. 2015, 99, 718–722. [Google Scholar] [CrossRef]
- Irzhansky, I.; Cohen, Y. Inheritance of resistance against Phytophthora infestans in Lycopersicon pimpinellifolium L3707. Euphytica 2006, 149, 309–316. [Google Scholar] [CrossRef]
- Boziné-Pullai, K.; Csambalik, L.; Drexler, D.; Reiter, D.; Tóth, F.; Tóthné Bogdányi, F.; Ladányi, M. Tomato Landraces Are Competitive with Commercial Varieties in Terms of Tolerance to Plant Pathogens—A Case Study of Hungarian Gene Bank Accessions on Organic Farms. Diversity 2021, 13, 195. [Google Scholar] [CrossRef]
- Majid, R.F.; Heather, L.M.; Hamid, A. Genetics, Genomics and Breeding of Late Blight and Early Blight Resistance in Tomato. Crit. Rev. Plant Sci. 2008, 27, 75–107. [Google Scholar] [CrossRef]
- Lenucci, M.S.; Caccioppola, A.; Durante, M.; Serrone, L.; De Caroli, M.; Piro, G.; Dalessandro, G. Carotenoid content during tomato (Solanum lycopersicum L.) fruit ripening in traditional and high-pigment cultivars. Ital. J. Food Sci. 2009, 21, 461–472. [Google Scholar]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and flavonoid profile and antioxidant activity in “Pomodorino Vesuviano” tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical composition and antioxidant activity of high-lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, H.Y.; Chang, C.Y.; Liu, Y.C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; d’Acierno, A.; Nazzaro, F.; Riccardi, R.; Spigno, P. Qualitative Aspects of Some Traditional Landraces of the Tomato “Piennolo” (Solanum lycopersicum L.) of the Campania Region, Southern Italy. Antioxidants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.E.; Stushnoff, C.; Sampson, D.A. Relationship of fruit color and light exposure to lycopene content and antioxidant properties of tomato. Can. J. Plant Sci. 2003, 83, 913–919. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and Beyond Color: Comparative Overview of Functional Quality of Tomato and Watermelon Fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.J.; Scott, K.J. Development and evaluation of an HPLC method for the analysis of carotenoids in foods and measurement of the carotenoid content of vegetables and fruits commonly consumed in the UK. Food Chem. 1995, 54, 101–111. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and beta-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.M. Lutein: A Valuable Ingredient of Fruit and Vegetables, Critical Reviews in Food Science and Nutrition. Crit. Rev. Food Sci. Nutr. 2005, 45, 671–696. [Google Scholar]
- Tee, E.-S.; Lim, C.-L. Carotenoid composition and content of Malaysian vegetables and fruits by the AOAC and HPLC methods. Food Chem. 1991, 41, 309–339. [Google Scholar] [CrossRef]
- Grande, H.R.; Bencsath, F.A.; Dickey, R.W. Isolation of analogues of okadaic acid from cultures of Prorocentrum lima. Bull. Soc. Pathol. Exot. 1990, 85 Pt 2, 478–480. [Google Scholar]
- Müller, H. Determination of the carotenoid content in selected vegetables and fruit by HPLC and photodiode array detection. Z. Lebensm. Unters. Forsch. 1997, 204, 88–94. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in Carotenoids and Antioxidant Vitamins in Tomato as a Function of Varietal and Technological Factors. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Aruna, G.; Mamatha, B.S.; Baskaran, V. Lutein content of selected Indian vegetables and vegetable oils determined by HPLC. J. Food Comp. Anal. 2009, 22, 632–636. [Google Scholar] [CrossRef]
- Montesano, D.; Gennari, O.; Seccia, S.; Albrizio, S. A Simple and Selective Analytical Procedure for the Extraction and Quantification of Lutein from Tomato By-Products by HPLC–DAD. Food Anal. Methods 2012, 5, 710–715. [Google Scholar] [CrossRef]
- IPGRI. Descriptors of Tomato (Lycopersicon spp.). International Plant Genetic Resources Institute: Rome, Italy, 1996; Available online: https://www.bioversityinternational.org/e-library/publications/detail/descriptors-for-tomato-lycopersicon-spp/ (accessed on 27 July 2022).
- Masago, H.; Yoshikawa, H.; Fukada, M.; Nakanishi, H. Selective Inhibition of Pythium spp. on a Medium for Direct Isolation of Phythophthora spp. from Soils and Plants. Phytopathology 1977, 67, 425–428. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Teleky, B.E.; Eleni, P.; Boukouvalas, C.; Krokida, M.; Kapsalis, N.; Rus, A.V.; Socol, C.T.; Vodnar, D.C. Evaluation of the Bioactive Compounds Found in Tomato Seed Oil and Tomato Peels Influenced by Industrial Heat Treatments. Foods 2021, 10, 110. [Google Scholar] [CrossRef]
- Vâtcă, S.; Vidican, R.; Gâdea, Ș.; Horvat, M.; Vâtcă, A.; Stoian, V.A.; Stoian, V. Blackcurrant variety specific growth and yield formation as a response to foliar fertilizers. Agronomy 2020, 10, 2014. [Google Scholar] [CrossRef]
- Puia, C.; Vidican, R.; Szabó, G.; Stoian, V. Potential of biofertilisers to improve performance of local genotype tomatoes. Ital. J. Agron. 2017, 12, 12. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Wagner, H. Vegan: Community Ecology Package 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 31 July 2020).
- Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular mycorrhizas traits and yield of winter wheat profiled by mineral fertilization. Agronomy 2020, 10, 846. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, R package version 1.9.12; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- Vâtcă, S.; Gâdea, Ș.; Vâtcă, A.; Chînța, D.; Stoian, V. Black currant response to foliar fertilizers–modeling of varietal growth dynamics. J. Plant Nutr. 2020, 43, 2144–2151. [Google Scholar] [CrossRef]

| Tomato Parameters | |||||
|---|---|---|---|---|---|
| Class * | Lycopene (mg kg−1 FW **) | Lutein (mg kg−1 FW **) | β-Carotene (mg kg−1 FW **) | Total Carotene (mg kg−1 FW **) | pH |
| AU | 126.40 ± 3.78 a | 2.02 ± 0.25 b | 7.03 ± 0.25 de | 135.45 ± 3.26 a | 4.29 ± 0.02 a |
| FR | 41.21 ± 6.83 cd | 2.20 ± 0.26 b | 5.49 ± 0.81 e | 48.90 ± 7.38 c | 4.07 ± 0.04 ab |
| GR | 8.21 ± 5.81 d | 3.76 ± 0.27 ab | 65.50 ± 0.71 a | 77.46 ± 5.22 bc | 4.11 ± 0.03 ab |
| IT | 22.48 ± 4.13 cd | 2.68 ± 0.23 ab | 6.76 ± 0.60 de | 31.93 ± 6.07 c | 4.28 ± 0.02 a |
| RO | 59.94 ± 4.26 bc | 3.94 ± 0.27 a | 12.25 ± 0.65 bc | 76.13 ± 4.50 bc | 4.16 ± 0.02 a |
| RU | 82.33 ± 6.21 b | 4.80 ± 1.00 a | 9.70 ± 0.22 cd | 96.83 ± 6.10 ab | 4.23 ± 0.02 a |
| TH | 70.06 ± 5.26 bc | 3.65 ± 0.29 ab | 15.65 ± 0.24 b | 89.36 ± 4.90 abc | 3.92 ± 0.03 b |
| F test | 6.18 | 4.83 | 92.85 | 5.79 | 2.51 |
| p.val | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | 0.03 |
| Tomato Parameters | |||||
|---|---|---|---|---|---|
| Class * | FFW ** (g) | Lutein (mg kg−1 FW **) | β-Carotene (mg kg−1 FW **) | Total Carotene (mg kg−1 FW **) | pH |
| 1 | 110.75 ± 4.27 a | 0.35 ± 0.18 b | 0.33 ± 0.11 c | 1.48 ± 0.31 e | 4.12 ± 0.06 ab |
| 2 | 68.00 ± 12.01 a | 4.03 ± 0.81 a | 25.22 ± 1.07 a | 34.00 ± 10.89 d | 4.07 ± 0.02 b |
| 3 | 131.00 ± 31.71 a | 2.88 ± 0.29 a | 11.64 ± 0.99 b | 47.23 ± 2.56 c | 4.16 ± 0.04 ab |
| 4 | 91.33 ± 18.28 a *** | 3.93 ± 0.25 a | 10.07 ± 0.64 b | 83.85 ± 1.99 b | 4.10 ± 0.03 b |
| 5 | 113.60 ± 19.69 a | 3.75 ± 0.52 a | 11.59 ± 1.08 b | 131.79 ± 2.35 a | 4.24 ± 0.04 a |
| F test | 0.01 | 24.99 | 0.72 | 571.65 | 1.30 |
| p.val | 0.94 | p < 0.001 | 13.83 | p < 0.001 | 3.62 |
| Tomato Parameters | ||||||
|---|---|---|---|---|---|---|
| Class * | FFW ** (g) | Lycopene (mg kg−1 FW **) | Lutein (mg kg−1 FW **) | β-Carotene (mg kg−1 FW **) | Total Carotene (mg kg−1 FW **) | pH |
| very high | 14.88 ± 1.27 c | 57.56 ± 6.20 a | 4.17 ± 0.36 a | 12.57 ± 0.87 b | 74.29 ± 6.67 a | 4.12 ± 0.03 bc |
| high | 69.25 ± 11.72 bc | 41.43 ± 6.33 ab | 4.63 ± 0.38 a | 26.18 ± 7.05 a | 72.25 ± 1.14 a | 4.04 ± 0.03 c |
| medium | 147.63 ± 20.48 a | 58.13 ± 6.01 a | 2.71 ± 0.22 b | 7.26 ± 0.65 c | 68.11 ± 0.65 a | 4.09 ± 0.03 bc |
| low | 127.00 ± 20.88 ab | 70.56 ± 13.53 a | 3.34 ± 0.98 ab | 7.55 ± 1.45 bc | 81.45 ± 14.87 a | 4.34 ± 0.02 a |
| very low | 122.33 ± 16.99 ab | 23.50 ± 5.49 b | 1.87 ± 0.25 b | 9.16 ± 1.98 bc | 34.53 ± 6.23 b | 4.22 ± 0.06 ab |
| F test | 7.33 | 2.73 | 5.60 | 10.79 | 2.71 | 5.90 |
| p.val | p < 0.001 | 0.03 | p < 0.001 | p < 0.001 | 0.08 | p < 0.001 |
| Tomato Parameters | |||||
|---|---|---|---|---|---|
| Class * | Lycopene (mg kg−1 FW **) | Lutein (mg kg−1 FW **) | β-Carotene (mg kg−1 FW **) | Total Carotene (mg kg−1 FW **) | pH |
| 1 | 55.63 ± 5.60 b | 4.34 ± 0.33 a | 13.49 ± 0.93 a | 73.45 ± 5.93 b | 4.10 ± 0.03 a |
| 2 | 57.61 ± 5.51 b | 3.37 ± 0.32 b | 11.64 ± 2.26 ab | 72.61 ± 5.72 b | 4.13 ± 0.03 a |
| 3 | 39.06 ± 9.00 b | 2.25 ± 0.31 c | 6.91 ± 1.20 b | 48.23 ± 9.79 c | 4.13 ± 0.03 a |
| 4 | 126.40 ± 4.97 a | 2.02 ± 0.31 c | 7.03 ± 0.91 ab | 135.45 ± 3.98 a | 4.29 ± 0.03 a |
| 5 | 52.67 ± 3.27 b | 2.36 ± 0.04 bc | 10.42 ± 0.95 ab | 65.45 ± 2.73 bc | 4.20 ± 0.03 a |
| F test | 4.26 | 15.26 | 2.97 | 4.35 | 1.82 |
| p.val | 0.990.003 | p < 0.001 | 0.09 | 0.002 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maxim, A.; Albu, V.C.; Vodnar, D.C.; Mihăiescu, T.; Mang, Ș.M.; Camele, I.; Trotta, V.; Bonomo, M.G.; Mihalescu, L.; Sandor, M.; et al. Assessment of Tomato (Solanum lycopersicum) Landraces for Their Agronomic, Biochemical Characteristics and Resistance to Phytophthora infestans. Agronomy 2023, 13, 21. https://doi.org/10.3390/agronomy13010021
Maxim A, Albu VC, Vodnar DC, Mihăiescu T, Mang ȘM, Camele I, Trotta V, Bonomo MG, Mihalescu L, Sandor M, et al. Assessment of Tomato (Solanum lycopersicum) Landraces for Their Agronomic, Biochemical Characteristics and Resistance to Phytophthora infestans. Agronomy. 2023; 13(1):21. https://doi.org/10.3390/agronomy13010021
Chicago/Turabian StyleMaxim, Aurel, Vasile Cristian Albu, Dan Cristian Vodnar, Tania Mihăiescu, Ștefania Mirela Mang, Ippolito Camele, Vincenzo Trotta, Maria Grazia Bonomo, Lucia Mihalescu, Mignon Sandor, and et al. 2023. "Assessment of Tomato (Solanum lycopersicum) Landraces for Their Agronomic, Biochemical Characteristics and Resistance to Phytophthora infestans" Agronomy 13, no. 1: 21. https://doi.org/10.3390/agronomy13010021
APA StyleMaxim, A., Albu, V. C., Vodnar, D. C., Mihăiescu, T., Mang, Ș. M., Camele, I., Trotta, V., Bonomo, M. G., Mihalescu, L., Sandor, M., Ranga, F., & Borsai, O. (2023). Assessment of Tomato (Solanum lycopersicum) Landraces for Their Agronomic, Biochemical Characteristics and Resistance to Phytophthora infestans. Agronomy, 13(1), 21. https://doi.org/10.3390/agronomy13010021











