Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth and Biological Activity of Selected Arable Soils under Model Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungus Strain
2.2. Production and Chemical Composition of Feather Hydrolysate
2.3. Determination of Keratin Hydrolysate Phytotoxicity
2.3.1. Soils Used for Phytotoxicity Tests of Feather Hydrolysates
2.3.2. Plants Used for Phytotoxicity Tests of Feather Hydrolysates
2.3.3. Phytotoxicity Determination
2.4. Determination of the Effect of Keratin Hydrolysates on Biological Activity of Soils and Plant Growth
2.4.1. Soils Used in the Study
2.4.2. Experimental Variants, Test Plant, and Experimental Conditions
- Variant I (control 1): soil, water;
- Variant II (control 2): soil, water, oilseed rape;
- Variant III: soil, keratin hydrolysate;
- Variant IV: soil, keratin hydrolysate, oilseed rape.
2.4.3. Abundance of Soil Microorganisms
2.4.4. Respiratory and Soil Enzyme Activity
2.4.5. DNA Concentration Measurements
2.4.6. Oilseed Rape Biomass Determination
2.4.7. Determination of Soil Chemical Parameters
2.5. Statistical Analysis
3. Results
3.1. Phytotoxicity
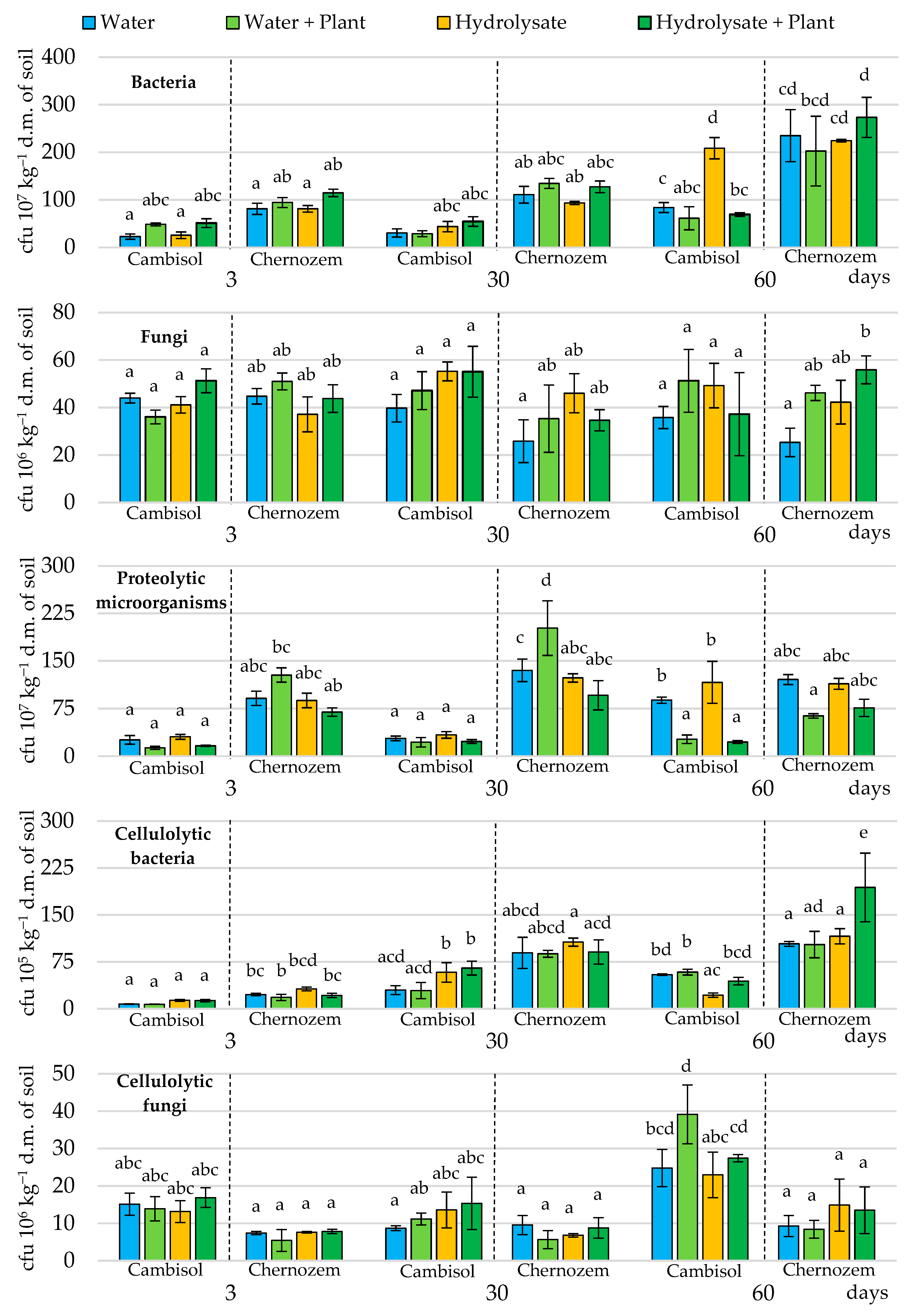
3.2. Abundance of Soil Microorganisms
3.3. Respiratory and Enzymatic Activity
3.4. Total DNA Pool
3.5. Plant Biomass
3.6. Chemical Parameters
3.6.1. pH
3.6.2. NPK and TOC
3.7. Statistical Analysis
3.7.1. Correlations
3.7.2. PCA
4. Discussion
4.1. Phytotoxicity
4.2. Abundance of Soil Microorganisms in Variants Treated and Non-Treated with Feather Hydrolysate
4.3. Enzymatic Activity of Soils Fertilized and Not Fertilized with Feather Hydrolysate
4.4. Oilseed Rape Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korniłłowicz–Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Sala, L.; Kalil, S.J. Microbial enzymes for bioconversion of poultry waste into added–value products. Food Res. Int. 2015, 73, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Możejko, M.; Bohacz, J. Optimization of conditions for feather waste biodegradation by geophilic Trichophyton ajelloi fungal strains towards further agricultural use. Int. J. Environ. Res. Public Health 2022, 19, 10858. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World J. Microb. Biotechnol. 2017, 33, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohacz, J.; Korniłłowicz–Kowalska, T. Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process Biochem. 2019, 80, 119–128. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz–Kowalska, T.; Kitowski, I.; Ciesielska, A. Degradation of chicken feathers by Aphanoascus keratinophilus and Chrysosporium tropicum strains from pellets of predatory birds and its practical aspect. Int. Biodeter. Biodegr. 2020, 151, 104968. [Google Scholar] [CrossRef]
- Bohacz, J.; Możejko, M.; Kitowski, I. Arthroderma tuberculatum and Arthroderma multifidum isolated from soils in rook (Corvus frugilegus) colonies as producers of keratinolytic enzymes and mineral forms of N and S. Int. J. Environ. Res. Public Health 2020, 17, 9162. [Google Scholar] [CrossRef]
- Bohacz, J. Microbial strategies and biochemical activity during lignocellulosic waste composting in relation to the occurring biothermal phases. J. Environ. Manag. 2018, 206, 1052–1062. [Google Scholar] [CrossRef]
- Thakur, V.; Sharma, E.; Guleria, A.; Sangar, S.; Singh, K. Modification and management of lignocellulosic waste as an ecofriendly biosorbent for the application of heavy metal ions sorption. Mater. Today Proc. 2020, 32, 608–619. [Google Scholar] [CrossRef]
- Pylak, M.; Oszust, K.; Frąc, M. Review report on the role of bioproducts, biopreparations, biostimulants and microbial inoculants in organic production of fruit. Rev. Environ. Sci. Bio/Technol. 2020, 18, 597–616. [Google Scholar] [CrossRef]
- Dasgupta, D.; Kumar, K.; Miglani, R.; Mishra, R.; Panda, A.K.; Bisht, S.S. Microbial biofertilizers: Recent trends and future outlook. In Recent Advancement in Microbial Biotechnology, Agricultural and Industrial Approach, 1st ed.; Manda, S.D., Passari, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 1–26. [Google Scholar] [CrossRef]
- De Silva, R.R. Enzymatic synthesis of protein hydrolysates from animal proteins: Exploring microbial peptidases. Front. Microbiol. 2011, 9, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, C.; Zamboni, A.; Varanini, Z.; Pandolfini, T. Growth stimulatory effects and genome–wide transcriptional changes produced by protein hydrolysates in maize seedlings. Front. Plant Sci. 2017, 8, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berechet, M.D.; Simion, D.; Stanca, M.; Alexe, C.A.; Chelaru, C.; Râpă, M. Keratin hydrolysates extracted from sheep wool with potential use as organic fertilizer. Leather Footwear J. 2020, 20, 267–276. [Google Scholar] [CrossRef]
- Gaidau, C.; Stanca, M.; Niculescu, M.D.; Alexe, C.A.; Becheritu, M.; Horoias, R.; Cioineag, C.; Râpă, M.; Stanculescu, I.R. Wool keratin hydrolysates for bioactive additives preparation. Materials 2021, 14, 4696. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Das Mohapatra, P.K.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate Paenibacillus woosongensis TKB2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Korniłłowicz–Kowalska, T.; Bohacz, J. The influence of keratin–bark and keratin–bark–straw composts and development of bacteria and fungi in two soils under different plant cultivation systems. Adv. Agric. Sci. Probl. Issues 2005, 506, 245–259. (In Polish) [Google Scholar]
- Meena, V.S.; Maurya, B.R.; Verma, R.; Meena, R.S.; Jatav, G.K.; Meena, S.K.; Meena, R.; Meena, S.K. Soil microbial population and selected enzyme activities as influenced by concentrate manure and inorganic fertilizer in Alluvium soil of Varanasi. Bioscan 2013, 8, 931–935. [Google Scholar]
- Szwed, A.; Bohacz, J. Enzymatic activity and certain chemical properties of grey–brown podzolic soil (Haplic Luvisol) amended with compost of tobacco wastes. Arch. Environ. Prot. 2014, 40, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.A.; Nunes, L.A.P.L.; Melo, W.J.; Araújo, A.S.F. Tannery sludge compost amendment rates on soil microbial biomass of two different soils. Eur. J. Soil Biol. 2011, 47, 146–151. [Google Scholar] [CrossRef]
- Šimon, T.; Czakó, A. Influence of long–term application of organic and inorganic fertilizers on soil properties. Plant Soil Environ. 2014, 60, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Groth, D.A.; Sokólski, M.; Jankowski, K.J. A multi–criteria evaluation of the effectiveness of nitrogen and sulfur fertilization in different cultivars of winter rapeseed—Productivity, economic and energy balance. Energies 2020, 13, 4654. [Google Scholar] [CrossRef]
- Stepaniuk, M.; Głowacka, A. Yield of winter oilseed rape (Brassica napus L. var. napus) in a short–term monoculture and the macronutrient accumulation in relation to the dose and method of sulphur application. Agronomy 2022, 12, 68. [Google Scholar] [CrossRef]
- Marazzi, C.; Städler, E. Influence of plant sulphur nutrition on oviposition and larval performance of the diamondback moth. Entomol. Exp. Appl. 2004, 111, 225–232. [Google Scholar] [CrossRef]
- Jakubus, M. Sulfur in the Environment, 1st ed.; Agricultural Academy in Poznan Publishing House: Poznan, Poland, 2006; pp. 1–48. (In Polish) [Google Scholar]
- Kozłowska–Strawska, J.; Badora, A. Selected problems of sulfur management in crops. Pol. J. Nat. Sci. 2013, 28, 309–316. [Google Scholar]
- Podleśna, A. The effect of sulfur fertilization on concentration and uptake of nutrients by winter oilseed rape. Oilseeds 2005, 25, 627–636. (In Polish) [Google Scholar]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef]
- World Reference Base of Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Recourses Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Bohacz, J.; Możejko, M.; Korniłłowicz–Kowalska, T.; Siebielec, G. Impact of ecological factors on the occurrence and spatial–taxonomic structure of keratinophilic fungi and their co–occurrence in arable soils. Agriculture 2022, 12, 194. [Google Scholar] [CrossRef]
- Masciandaro, G.; Ceccanti, B.; Garcia, C. Soil agro-ecological management: Fertirrigation and vermicompost treatments. Bioresour. Technol. 1997, 59, 199–206. [Google Scholar] [CrossRef]
- Czop, M.; Żorawik, K.; Grochowska, S.; Kulkińska, L.; Januszewska, W. Tests of phytotoxicity of mining wastes on selected group of plants. Arch. Waste Manag. Environ. Prot. 2016, 18, 33–44. (In Polish) [Google Scholar]
- Carvalho Neves, L.; de Souza, J.B.; de Souza Vidal, C.M.; Herbert, L.T.; de Souza, K.V.; Martins, K.G.; Young, B.J. Phytotoxicity indexes and removal of color, COD, phenols and ISA from pulp and paper mill wastewater post–treated by UV/H2O2 and photo–fenton. Ecotox. Environ. Saf. 2020, 202, 110939. [Google Scholar] [CrossRef]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological evaluation of compost maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Martin, J.P. Use of acid, rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Rühling, Å.; Tyler, G. Heavy metal pollution and decomposition of spruce needle litter. Oikos 1973, 24, 402–416. [Google Scholar] [CrossRef]
- Thalmann, A. Zur methodik der bestimmung der dehydrogenaseactivität im boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsch Forsch 1968, 21, 249–258. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.A.H. Short–term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Olson, N.D.; Morrow, J.B. DNA extract characterization process for microbial detection methods development and validation. BMC Res. Notes 2012, 5, 668. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, M.; Andreas, L.; Lagerkvist, A. Effect of accelerated carbonation and zero valent iron on metal leaching from bottom ash. Waste Manag. 2016, 51, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Wang, L.; Jia, J.; Fu, X.; Le, Y.; Chen, X.; Sun, Y. Response of soil microbial community in Jiuduansha wetland to different successional stages and its implications for soil microbial respiration and carbon turnover. Soil Biol. Biochem. 2011, 43, 638–646. [Google Scholar] [CrossRef]
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar] [CrossRef]
- Mocek–Płóciniak, A. Utilisation of enzymatic activity for the evaluation of the impact of anthropogenic changes caused by heavy metals in soil environment. Sci. Nat. Technol. 2010, 4, 1–10. (In Polish) [Google Scholar]
- Joniec, J. Enzymatic activity as an indicator of regeneration processes in degraded soil reclaimed with various types of waste. Int. J. Environ. Sci. Technol. 2018, 15, 2241–2252. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Jain, A.; Rawat, N.; Nair, M.; Gumashta, R. Feather hydrolysate from Streptomyces sampsonii GS 1322: A potential low cost soil amendment. J. Biosci. Bioeng. 2016, 121, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, D.A.; Obideyi, O.A.; Evinemi, O.T.; Adetunji, O.A. Phytotoxicity assessment of compost-type biofertilizer using co-composting and post composting fortification methods. Asian J. Agric. Food Sci. 2020, 8, 44–48. [Google Scholar] [CrossRef]
- Rys, M.; Saja–Garbarz, D.; Skoczowski, A. Phytotoxic effects of selected herbal extracts on the germination, growth and metabolism of mustard and oilseed rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Nustorova, M.; Braikova, D.; Gousterova, A.; Vasileva–Tonkova, E.; Nedkov, P. Chemical, microbiological and plant analysis of soil fertilized with alkaline hydrolysate of sheep’s wool waste. World J. Microb. Biotechnol. 2006, 22, 383–390. [Google Scholar] [CrossRef]
- Bhavsar, P.S.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Rovero, G.; Giansetti, M.; Tonin, C. Superheated water hydrolysis of waste wool in a semi–industrial reactor to obtain nitrogen fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 6722–6731. [Google Scholar] [CrossRef]
- Gousterova, A.; Nustorova, M.; Paskaleva, D.; Naydenov, M.; Neshev, G.; Vasileva–Tonkova, E. Assessment of feather hydrolysate from thermophilic actinomycetes for soil amendment and biological control application. Int. J. Environ. Res. 2011, 5, 1065–1070. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Futa, B.; Bik–Mołodzińska, M.; Szewczuk, C.; Sugier, D. The impact of fertilizing agents on the enzymatic activity of soils. J. Res. Appl. Agric. Eng. 2013, 58, 15–19. (In Polish) [Google Scholar]
- Wrońska, I.; Onyszko, M.; Cybulska, K.; Telesiński, A.; Mahdi–Oraibi, S. The content of live microbial biomass and its number in horticultural soil enriched with biological preparation. Proc. ECOpole 2015, 9, 795–801. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, X. Changes in proteolytic bacteria in paddy soils in response to organic management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2017, 67, 583–589. [Google Scholar] [CrossRef]
- Różyło, K.; Bohacz, J. Microbial and enzyme analysis of soil after the agricultural utilization of biogas digestate and mineral mining waste. Int. J. Environ. Sci. Technol. 2020, 17, 1051–1062. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.M. Ecology of Soil Fungi, 1st ed.; Chapman and Hall: London, UK, 1973; pp. 1–193. [Google Scholar]
- Shreiner, R.P.; Koide, R.T. Antifungal compounds from the roots of mycotrophic and non–mycotrophic plant species. New Phytol. 1993, 123, 99–105. [Google Scholar] [CrossRef]
- Yasumoto, S.; Suzuki, K.; Matsuzaki, M.; Hiradate, S.; Oose, K.; Hirokane, H.; Okada, K. Effects of plant residue, root exudate and juvenile plants of rapeseed (Brassica napus L.) on the germination, growth, yield, and quality of subsequent crops in successive and rotational cropping systems. Plant Prod. Sci. 2011, 14, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Smyk, B.; Barabasz, W.; Różycki, E. The effect of the use of mineral nitrogen fertilizers (N and NPK) on the occurrence of nitrosamines and mycotoxins in mountain soils and lowland grassland ecosystems. Adv. Agric. Sci. Probl. Issues 1989, 380, 1–9. (In Polish) [Google Scholar]
- Borymski, S. Characteristics of Biodiversity of Microorganism Assemblies Inhabiting the Metallophytes Rhizosphere in Soils Contaminated with Heavy Metals. Ph.D. Thesis, University of Silesia in Katowice, Katowice, Poland, 2019. (In Polish). [Google Scholar]
- Ahemad, M.; Zaidi, A.; Khan, S.; Oves, M. Biological importance of phosphorus and phosphate solubilizing microbes—An overview. In Phosphate Solubilizing Microbes for Crop Improvement, 1st ed.; Khan, M.S., Zaidi, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; Volume 1, pp. 1–14. [Google Scholar]
- Kurek, E.; Jaroszuk, J. Siderophores and their role in the soil environment. Adv. Microb. 1993, 32, 71–81. (In Polish) [Google Scholar]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant–driven selection of microbes. Plant Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z.; Szymańska, E. Dehydrogenase activity of soil microorganisms and the total DNA level in soil of different use. J. Agric. Sci. Technol. B 2013, 3, 613–622. [Google Scholar]
- Zhu, C.; Ma, Y.; Wu, H.; Sun, T.; La Pierre, K.J.; Sun, Z.; Yu, Q. Divergent effects of nitrogen addition on soil respiration in a semiarid grassland. Sci. Rep. 2016, 6, 33541. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. In Dehydrogenases, 1st ed.; Canuto, R.A., Ed.; IntechOpen: London, UK, 2012; Volume 8, pp. 183–210. [Google Scholar] [CrossRef] [Green Version]
- Greenfield, L.M.; Puissant, J.; Jones, D.L. Synthesis of methods used to assess soil protease activity. Soil Biol. Biochem. 2021, 158, 108277. [Google Scholar] [CrossRef]
- Vranova, V.; Rejsek, K.; Formanek, P. Proteolytic activity in soil: A review. Appl. Soil Ecol. 2013, 70, 23–32. [Google Scholar] [CrossRef]
- Caballero, P.; Macías–Benítez, S.; Revilla, E.; Tejada, M.; Parrado, J.; Castaño, A. Effect of subtilisin, a protease from Bacillus sp., on soil biochemical parameters and microbial biodiversity. Eur. J. Soil Biol. 2020, 101, 103244. [Google Scholar] [CrossRef]
- Bielińska, E.J. Methods of determination of phosphatase activity. Acta Agrophys. Dissert. Monog. 2005, 3, 63–74. (In Polish) [Google Scholar]
- Klaczyński, E. Phosphorus in the environment, its importance and possibilities of recovery from sewage sludge. ABC Technol. 2015, 6, 35–41. (In Polish) [Google Scholar]
- Sun, Y.; Goll, D.S.; Ciais, P.; Peng, S.; Margalef, O.; Asensio, D.; Sardans, J.; Peñuelas, J. Spatial pattern and environmental drivers of acid phosphatase activity in Europe. Front. Big Data 2020, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Prakash, V.; Kundu, S.; Kumar, N.; Mina, B.L. Soil enzymatic activity as affected by long term application of farm yard manure and mineral fertilizer under a rainfed soybean–wheat system in N–W Himalaya. Eur. J. Soil. Biol. 2008, 44, 309–315. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Harasim, E.; Feledyn–Szewczyk, B.; Antonkiewicz, J. Enzymatic activity of loess soil in organic and conventional farming systems. Agriculture 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Jiang, N.; Condron, L.M.; Dunfield, K.E.; Chen, Z.; Wang, J.; Chen, L. Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long–term nitrogen and manure inputs. Geoderma 2019, 349, 36–44. [Google Scholar] [CrossRef]
- Chang, E.H.; Chung, R.S.; Tsai, Y.H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2007, 53, 132–140. [Google Scholar] [CrossRef]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste–derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]



| Soil | Plant | [%] | R:S | |||
|---|---|---|---|---|---|---|
| GC | IR | IS | GI | |||
| Feather hydrolysate | ||||||
| Cambisol I | Brassica napus L. var. napus | 100.00 | 48.66 | 42.11 | 51.34 | 3.15 |
| Lepidium sativum L. | 100.00 | 41.82 | 21.76 | 58.18 | 1.47 | |
| Chernozem | Brassica napus var. napus | 100.00 | 17.78 | 21.94 | 82.22 | 2.71 |
| Lepidium sativum L. | 100.00 | −7.63 | −27.81 | 107.63 | 1.71 | |
| Feather hydrolysate dilluted 1:2 | ||||||
| Cambisol I | Brassica napus L. var. napus | 100.00 | −1.63 | −59.47 | 101.63 | 2.26 |
| Lepidium sativum L. | 100.00 | 17.76 | 17.13 | 82.24 | 1.97 | |
| Cambisol II | Brassica napus L. var. napus | 100.00 | 10.92 | −2.19 | 89.08 | 2.71 |
| Lepidium sativum L. | 100.00 | −2.02 | −26.25 | 102.02 | 1.38 | |
| Chernozem | Brassica napus L. var. napus | 100.00 | −26.55 | −70.22 | 126.55 | 1.91 |
| Lepidium sativum L. | 100.00 | −13.95 | −13.90 | 113.95 | 2.03 | |
| Soil | Experimental Variants | Days of Measurement | |||
|---|---|---|---|---|---|
| 3 A | 14 B | 21 C | 30 D | ||
| Cambisol A | Water A | 0.88 ± 0.03 a | 2.30 ± 0.02 c | 4.81 ± 0.15 e | 6.61 ± 0.17 b |
| Hydrolysate B | 1.13 ± 0.02 a | 4.12 ± 0.16 d | 7.00 ± 0.18 b | 10.00 ± 0.32 f | |
| Chernozem B | Water A | 1.42 ± 0.07 a | 4.35 ± 0.18 c | 8.14 ± 0.22 e | 12.65 ± 0.42 b |
| Hydrolysate B | 2.12 ± 0.03 a | 6.59 ± 0.27 d | 12.94 ± 0.20 b | 19.87 ± 0.80 f | |
| Soil | Days | 3 | 30 | 60 | Mean |
|---|---|---|---|---|---|
| pH [−log10[H+]] | |||||
| Cambisol | Water | 3.71 ± 0.01 | 3.67 ± 0.01 | 3.61 ± 0.01 | 3.66 ± 0.01 aA |
| Water + Plant | 3.72 ± 0.01 | 3.68 ± 0.01 | 3.57 ± 0.01 | 3.66 ± 0.01 aA | |
| Hydrolysate | 3.76 ± 0.01 | 3.81 ± 0.06 | 3.42 ± 0.01 | 3.67 ± 0.02 aA | |
| Hydrolysate + Plant | 3.75 ± 0.01 | 3.78 ± 0.04 | 3.40 ± 0.01 | 3.64 ± 0.02 aA | |
| Water | 6.20 ± 0.02 | 6.21 ± 0.08 | 6.17 ± 0.04 | 6.19 ± 0.05 bC | |
| Chernozem | Water + Plant | 6.08 ± 0.10 | 6.21 ± 0.12 | 6.06 ± 0.07 | 6.11 ± 0.09 bC |
| Hydrolysate | 6.15 ± 0.05 | 5.87 ± 0.07 | 5.16 ± 0.03 | 5.72 ± 0.05 aB | |
| Hydrolysate + Plant | 6.31 ± 0.03 | 5.56 ± 0.26 | 5.11 ± 0.20 | 5.66 ± 0.17 aB | |
| Soil | Days of Analyses | Sample | TOC (g kg−1) | N (g kg−1) | P (mg kg−1) | K (mg kg−1) |
|---|---|---|---|---|---|---|
| Cambisol | 3 | Water | 5.40 | 0.20 | 32.00 | 348.00 |
| Water + Plant | 6.20 | 0.60 | 31.20 | 485.00 | ||
| Hydrolysate | 6.05 | 0.40 | 43.00 | 447.00 | ||
| Hydrolysate + Plant | 5.95 | 0.40 | 40.60 | 397.00 | ||
| 60 | Water | 6.20 | 0.60 | 29.10 | 483.00 | |
| Water + Plant | 5.87 | 0.50 | 44.90 | 352.00 | ||
| Hydrolysate | 5.90 | 0.60 | 89.10 | 370.00 | ||
| Hydrolysate + Plant | 5.73 | 0.30 | 106.80 | 678.00 | ||
| Chernozem | 3 | Water | 13.57 | 0.00 | 17.80 | 1310.00 |
| Water + Plant | 13.75 | 1.50 | 25.40 | 2980.00 | ||
| Hydrolysate | 13.07 | 1.20 | 33.90 | 2520.00 | ||
| Hydrolysate + Plant | 12.97 | 0.40 | 27.30 | 2620.00 | ||
| 60 | Water | 14.41 | 1.10 | 37.70 | 2700.00 | |
| Water + Plant | 16.41 | 0.40 | 37.60 | 2080.00 | ||
| Hydrolysate | 14.51 | 0.40 | 99.80 | 2260.00 | ||
| Hydrolysate + Plant | 13.49 | 0.50 | 104.60 | 3040.00 |
| Soil | Experimental Variant | Analyzed Parameters | TOC | N | P | K |
|---|---|---|---|---|---|---|
| Cambisol | Water | Soil bacteria | 0.598 * | 0.666 * | 0.025 | 0.504 |
| Soil fungi | −0.339 | –0.245 | 0.600 * | −0.594 * | ||
| Proteolytic microorganisms | 0.336 | 0.325 | −0.371 | 0.399 | ||
| Cellulolytic bacteria | 0.316 | 0.436 | 0.486 | −0.034 | ||
| Cellulolytic fungi | 0.047 | 0.213 | 0.742 ** | −0.296 | ||
| Soil respiration | −0.145 | −0.010 | 0.344 | −0.265 | ||
| Dehydrogenase | −0.265 | −0.393 | −0.443 | 0.051 | ||
| Protease | 0.122 | 0.306 | 0.728 ** | −0.181 | ||
| Acid phosphatase | −0.163 | −0.153 | −0.426 | 0.190 | ||
| Alkaline phosphatase | 0.487 | 0.426 | −0.038 | 0.337 | ||
| pH | −0.180 | −0.315 | −0.620 * | 0.205 | ||
| dsDNA | −0.043 | 0.111 | 0.941 *** | −0.516 | ||
| Hydrolysate | Soil bacteria | −0.159 | 0.826 *** | 0.527 | −0.337 | |
| Soil fungi | 0.184 | 0.227 | −0.243 | −0.370 | ||
| Proteolytic microorganisms | 0.087 | 0.855 *** | 0.356 | −0.439 | ||
| Cellulolytic bacteria | −0.720 ** | −0.434 | 0.856 *** | 0.868 *** | ||
| Cellulolytic fungi | −0.421 | −0.063 | 0.807 ** | 0.503 | ||
| Soil respiration | −0.185 | 0.703 * | 0.574 | −0.153 | ||
| Dehydrogenase | 0.547 | −0.309 | −0.944 *** | −0.347 | ||
| Protease | 0.400 | −0.436 | −0.720 ** | −0.187 | ||
| Acid phosphatase | 0.148 | 0.385 | –0.258 | –0.501 | ||
| Alkaline phosphatase | −0.639 * | 0.133 | 0.511 | 0.197 | ||
| pH | 0.627 * | −0.183 | −0.980 *** | −0.449 | ||
| dsDNA | −0.700 * | 0.034 | 0.989 *** | 0.585 * | ||
| Chernozem | Water | Soil bacteria | 0.426 | 0.117 | 0.772 ** | 0.246 |
| Soil fungi | 0.025 | −0.086 | −0.462 | −0.143 | ||
| Proteolytic microorganisms | −0.652 * | 0.745 ** | −0.156 | 0.624 * | ||
| Cellulolytic bacteria | 0.632 * | −0.032 | 0.869 *** | 0.134 | ||
| Cellulolytic fungi | 0.141 | −0.143 | 0.267 | −0.170 | ||
| Soil respiration | −0.128 | 0.438 | 0.431 | 0.481 | ||
| Dehydrogenase | −0.537 | 0.053 | −0.835 *** | −0.112 | ||
| Protease | −0.129 | 0.893 *** | 0.153 | 0.884 *** | ||
| Acid phosphatase | −0.613 * | 0.316 | −0.259 | 0.248 | ||
| Alkaline phosphatase | −0.004 | 0.683 * | 0.625 * | 0.736 ** | ||
| pH | −0.502 | −0.237 | −0.340 | −0.371 | ||
| dsDNA | 0.837 *** | 0.071 | 0.946 *** | 0.273 | ||
| Hydrolysate | Soil bacteria | 0.535 | −0.586 * | 0.927 *** | 0.281 | |
| Soil fungi | −0.203 | –0.387 | 0.430 | 0.600 * | ||
| Proteolytic microorganisms | 0.555 | −0.023 | 0.381 | −0.577 * | ||
| Cellulolytic bacteria | 0.449 | −0.353 | 0.885 *** | 0.487 | ||
| Cellulolytic fungi | 0.325 | −0.311 | 0.577 * | −0.049 | ||
| Soil respiration | 0.566 | −0.750 ** | 0.600 * | −0.316 | ||
| Dehydrogenase | −0.664 * | 0.368 | −0.986 *** | −0.102 | ||
| Protease | −0.171 | 0.263 | 0.121 | 0.519 | ||
| Acid phosphatase | −0.523 | −0.020 | −0.012 | 0.916 *** | ||
| Alkaline phosphatase | −0.749 ** | 0.328 | −0.441 | 0.695 * | ||
| pH | −0.654 * | 0.414 | −0.988 *** | −0.143 | ||
| dsDNA | 0.551 | −0.370 | 0.981 *** | 0.273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Możejko, M.; Bohacz, J. Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth and Biological Activity of Selected Arable Soils under Model Conditions. Agronomy 2023, 13, 187. https://doi.org/10.3390/agronomy13010187
Możejko M, Bohacz J. Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth and Biological Activity of Selected Arable Soils under Model Conditions. Agronomy. 2023; 13(1):187. https://doi.org/10.3390/agronomy13010187
Chicago/Turabian StyleMożejko, Michał, and Justyna Bohacz. 2023. "Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth and Biological Activity of Selected Arable Soils under Model Conditions" Agronomy 13, no. 1: 187. https://doi.org/10.3390/agronomy13010187
APA StyleMożejko, M., & Bohacz, J. (2023). Effect of Keratin Hydrolysates Obtained from Feather Decomposition by Trichophyton ajelloi on Plant Germination, Growth and Biological Activity of Selected Arable Soils under Model Conditions. Agronomy, 13(1), 187. https://doi.org/10.3390/agronomy13010187







