Variable Selection on Reflectance NIR Spectra for the Prediction of TSS in Intact Berries of Thompson Seedless Grapes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daniels, A.J.; Poblete-Echeverría, C.; Opara, U.L.; Nieuwoudt, H.H. Measuring Internal Maturity Parameters Contactless on Intact Table Grape Bunches Using NIR Spectroscopy. Front. Plant Sci. 2019, 10, 1517. [Google Scholar] [CrossRef]
- Vrochidou, E.; Tziridis, K.; Nikolaou, A.; Kalampokas, T.; Papakostas, G.A.; Pachidis, T.P.; Mamalis, S.; Koundouras, S.; Kaburlasos, V.G. An Autonomous Grape-Harvester Robot: Integrated System Architecture. Electronics 2021, 10, 1056. [Google Scholar] [CrossRef]
- Power, A.; Truong, V.K.; Chapman, J.; Cozzolino, D. From the Laboratory to The Vineyard—Evolution of The Measurement of Grape Composition using NIR Spectroscopy towards High-Throughput Analysis. High-Throughput 2019, 8, 21. [Google Scholar] [CrossRef]
- Das, A.J.; Wahi, A.; Kothari, I.; Raskar, R. Ultra-portable, wireless smartphone spectrometer for rapid, non-destructive testing of fruit ripeness. Sci. Rep. 2016, 6, 32504. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.; Zeb, A.; Qureshi, W.S.; Arslan, M.; Malik, A.U.; Alasmary, W.; Alanazi, E. Towards fruit maturity estimation using NIR spectroscopy. Infrared Phys. Technol. 2020, 111, 103479. [Google Scholar] [CrossRef]
- Hernández-Hierro, J.M.; Nogales-Bueno, J.; Rodríguez-Pulido, F.J.; Heredia, F.J. Feasibility Study on the Use of Near-Infrared Hyperspectral Imaging for the Screening of Anthocyanins in Intact Grapes during Ripening. J. Agric. Food Chem. 2013, 61, 9804–9809. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of Near-Infrared (NIR) Spectroscopy and Hyperspectral Imaging for Quality and Safety Assessment of Fruits: An Overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Lu, R.; van Beers, R.; Saeys, W.; Li, C.; Cen, H. Measurement of optical properties of fruits and vegetables: A review. Postharvest Biol. Technol. 2020, 159, 111003. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.Y.; Jing, L.I.; Zhang, H.; Ye, L.I.; Farooq, S.; Bacha, S.A.; Jie, W.A. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, J.; Cai, J.; Zhao, J. Rapid measurement of total acid content (TAC) in vinegar using near infrared spectroscopy based on efficient variables selection algorithm and nonlinear regression tools. Food Chem. 2012, 135, 590–595. [Google Scholar] [CrossRef]
- Costa, R.C.; Uchida, V.H.; Miguel, T.B.V.; Duarte, M.M.L.; Lima, K.M.G. Quantification of quality parameters in castanhola fruits by NIRS for the development of prediction models using PLS and variable selection algorithms on a laboratory scale. Anal. Methods 2017, 9, 352–357. [Google Scholar] [CrossRef]
- Friedel, M.; Patz, C.-D.; Dietrich, H. Comparison of different measurement techniques and variable selection methods for FT-MIR in wine analysis. Food Chem. 2013, 141, 4200–4207. [Google Scholar] [CrossRef] [PubMed]
- Foca, G.; Ferrari, C.; Ulrici, A.; Ielo, M.C.; Minelli, G.; Fiego, D.P.L. Iodine Value and Fatty Acids Determination on Pig Fat Samples by FT-NIR Spectroscopy: Benefits of Variable Selection in the Perspective of Industrial Applications. Food Anal. Methods 2016, 9, 2791–2806. [Google Scholar] [CrossRef]
- Liu, F.; He, Y. Application of successive projections algorithm for variable selection to determine organic acids of plum vinegar. Food Chem. 2009, 115, 1430–1436. [Google Scholar] [CrossRef]
- Sun, X.; Dong, X. Improved partial least squares regression for rapid determination of reducing sugar of potato flours by near infrared spectroscopy and variable selection method. J. Food Meas. Charact. 2015, 9, 95–103. [Google Scholar] [CrossRef]
- Wu, D.; Chen, X.; Cao, F.; Sun, D.-W.; He, Y.; Jiang, Y. Comparison of Infrared Spectroscopy and Nuclear Magnetic Resonance Techniques in Tandem with Multivariable Selection for Rapid Determination of ω-3 Polyunsaturated Fatty Acids in Fish Oil. Food Bioprocess Technol. 2014, 7, 1555–1569. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Cozzolino, D. Infrared Spectroscopy as a Versatile Analytical Tool for the Quantitative Determination of Antioxidants in Agricultural Products, Foods and Plants. Antioxidants 2015, 4, 482–497. [Google Scholar] [CrossRef]
- Cubero, S.; Aleixos, N.; Moltó, E.; Gómez-Sanchis, J.; Blasco, J. Advances in Machine Vision Applications for Automatic Inspection and Quality Evaluation of Fruits and Vegetables. Food Bioprocess Technol. 2011, 4, 487–504. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Vrochidou, E.; Bazinas, C.; Manios, M.; Papakostas, G.A.; Pachidis, T.P.; Kaburlasos, V.G. Machine Vision for Ripeness Estimation in Viticulture Automation. Horticulturae 2021, 7, 282. [Google Scholar] [CrossRef]
- Mishra, P.; Roger, J.M.; Rutledge, D.N.; Woltering, E. SPORT pre-processing can improve near-infrared quality prediction models for fresh fruits and agro-materials. Postharvest Biol. Technol. 2020, 168, 111271. [Google Scholar] [CrossRef]
- Cao, D.-S.; Xu, Q.-S.; Liang, Y.-Z.; Chen, X.; Li, H.-D. Prediction of aqueous solubility of druglike organic compounds using partial least squares, back-propagation network and support vector machine. J. Chemom. 2010, 24, 584–595. [Google Scholar] [CrossRef]
- Shezi, S.; Magwaza, L.S.; Tesfay, S.Z.; Mditshwa, A. Simple and Multiple Linear Regression Models for Predicting Maturity of ‘Mendez#1′ and ‘Hass’ Avocado Fruit Harvested from inside and outside Tree Canopy Positions. Int. J. Fruit Sci. 2020, 20, S1969–S1983. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kadioglu, A.; Bertoft, E.; Acar, C.; Turna, I. Effect of fruit maturation on sugar and organic acid composition in two blueberries (Vaccinium arctostaphylos and V. myrtillus) native to Turkey. N. Z. J. Crop Hortic. Sci. 2001, 29, 137–141. [Google Scholar] [CrossRef]
- Zhao, F.; Du, G.; Huang, Y. Exploring the use of Near-infrared spectroscopy as a tool to predict quality attributes in prickly pear (Rosa roxburghii Tratt) with chemometrics variable strategy. J. Food Compos. Anal. 2022, 105, 104225. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. Near-Infrared Technology in the Agricultural and Food Industries; American Association of ceral chemist: St. Paul, MN, USA, 2001. [Google Scholar]
- Nieuwoudt, H.H.; Prior, B.A.; Pretorius, I.S.; Manley, M.; Bauer, F.F. Principal Component Analysis Applied to Fourier Transform Infrared Spectroscopy for the Design of Calibration Sets for Glycerol Prediction Models in Wine and for the Detection and Classification of Outlier Samples. J. Agric. Food Chem. 2004, 52, 3726–3735. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-Wavelength Near-Infrared Spectra of Sucrose, Glucose, and Fructose with Respect to Sugar Concentration and Temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef]
- Courand, A.; Metz, M.; Héran, D.; Feilhes, C.; Prezman, F.; Serrano, E.; Bendoula, R.; Ryckewaert, M. Evaluation of a robust regression method (RoBoost-PLSR) to predict biochemical variables for agronomic applications: Case study of grape berry maturity monitoring. Chemom. Intell. Lab. Syst. 2022, 221, 104485. [Google Scholar] [CrossRef]
- Martins, R.C.; Barroso, T.G.; Jorge, P.; Cunha, M.; Santos, F. Unscrambling spectral interference and matrix effects in Vitis vinifera Vis-NIR spectroscopy: Towards analytical grade ‘in vivo’ sugars and acids quantification. Comput. Electron. Agric. 2022, 194, 106710. [Google Scholar] [CrossRef]
- Fernández-Novales, J.; Garde-Cerdán, T.; Tardáguila, J.; Gutiérrez-Gamboa, G.; Pérez-Álvarez, E.P.; Diago, M.P. Assessment of amino acids and total soluble solids in intact grape berries using contactless Vis and NIR spectroscopy during ripening. Talanta 2019, 199, 244–253. [Google Scholar] [CrossRef]
- Barnaba, F.E.; Bellincontro, A.; Mencarelli, F. Portable NIR-AOTF spectroscopy combined with winery FTIR spectroscopy for an easy, rapid, in-field monitoring of Sangiovese grape quality. J. Sci. Food Agric. 2014, 94, 1071–1077. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Peri, G.; Romaniello, R. Application of hyperspectral imaging for prediction of physico-chemical and sensory characteristics of table grapes. Comput. Electron. Agric. 2012, 87, 142–151. [Google Scholar] [CrossRef]
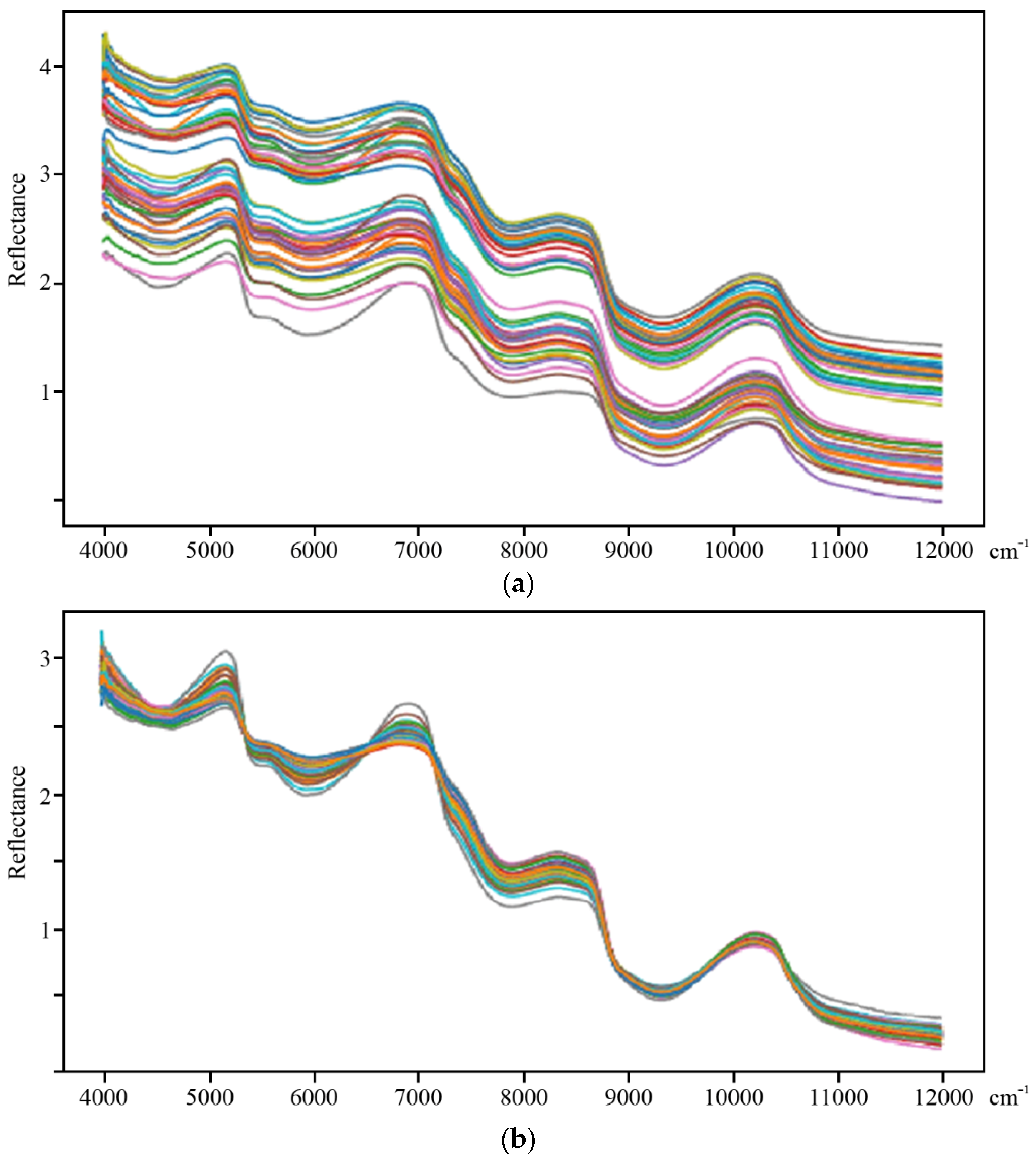
| Dataset 1 Harvest 1-2016 | Dataset 2 Harvest 2-2016 | Dataset 3 2016 | Dataset 4 2016 and 2017 |
|---|---|---|---|
| 52 spectra 1037 wavenumbers | 41 spectra 1037 wavenumbers | 93 spectra 1037 wavenumbers | 165 spectra 1037 wavenumbers |
| opt_Xc and absxcoefs | opt_Xc and absxcoefs | opt_Xc and absxcoefs | opt_Xc and absxcoefs |
| 52 spectra 103 wavenumbers | 41 spectra 181 wavenumbers | 93 spectra 334 wavenumbers | 165 spectra 134 wavenumbers |
| Parameter | Dataset 1 Harvest 1 2016 | Dataset 2 Harvest 2 2016 | Dataset 3 both Harvests 2016 | Dataset 4 both Harvests 2016 and 2017 |
|---|---|---|---|---|
| N | 52 | 41 | 93 | 165 |
| Mean | 17.51 | 18.69 | 18.03 | 17.54 |
| Median | 17.76 | 18.86 | 18.17 | 17.66 |
| Min | 13.44 | 14.07 | 13.44 | 10.18 |
| Max | 20.93 | 22.42 | 22.42 | 22.42 |
| Range | 7.49 | 8.35 | 8.89 | 12.24 |
| Standard deviation | 1.74 | 1.89 | 1.90 | 2.20 |
| Coefficient of variation | 0.10 | 0.10 | 0.10 | 0.12 |
| Dataset | MLR | PLS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entire Dataset | opt_Xc | absxcoefs | Entire Dataset | opt_Xc | absxcoefs | |||||||
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
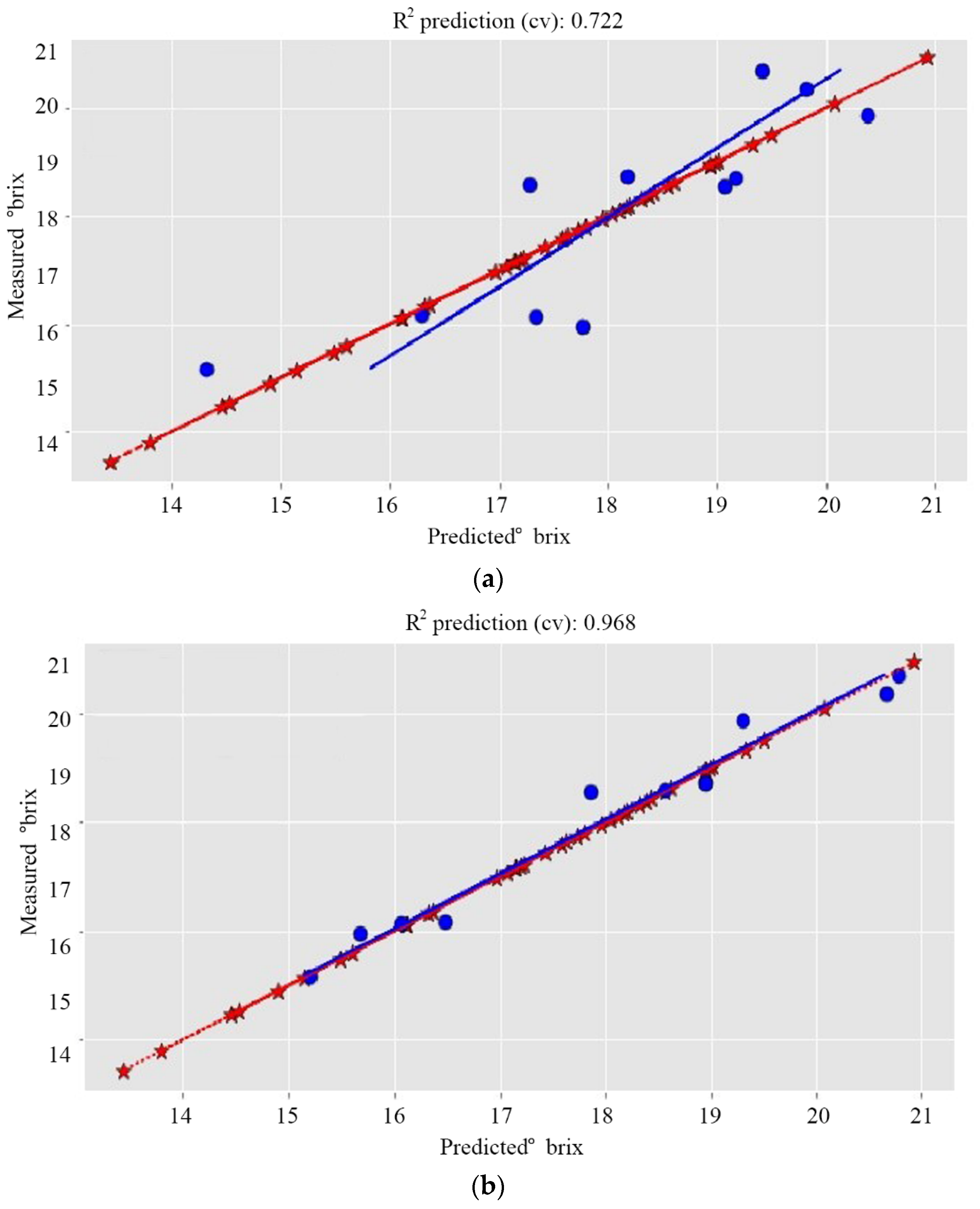
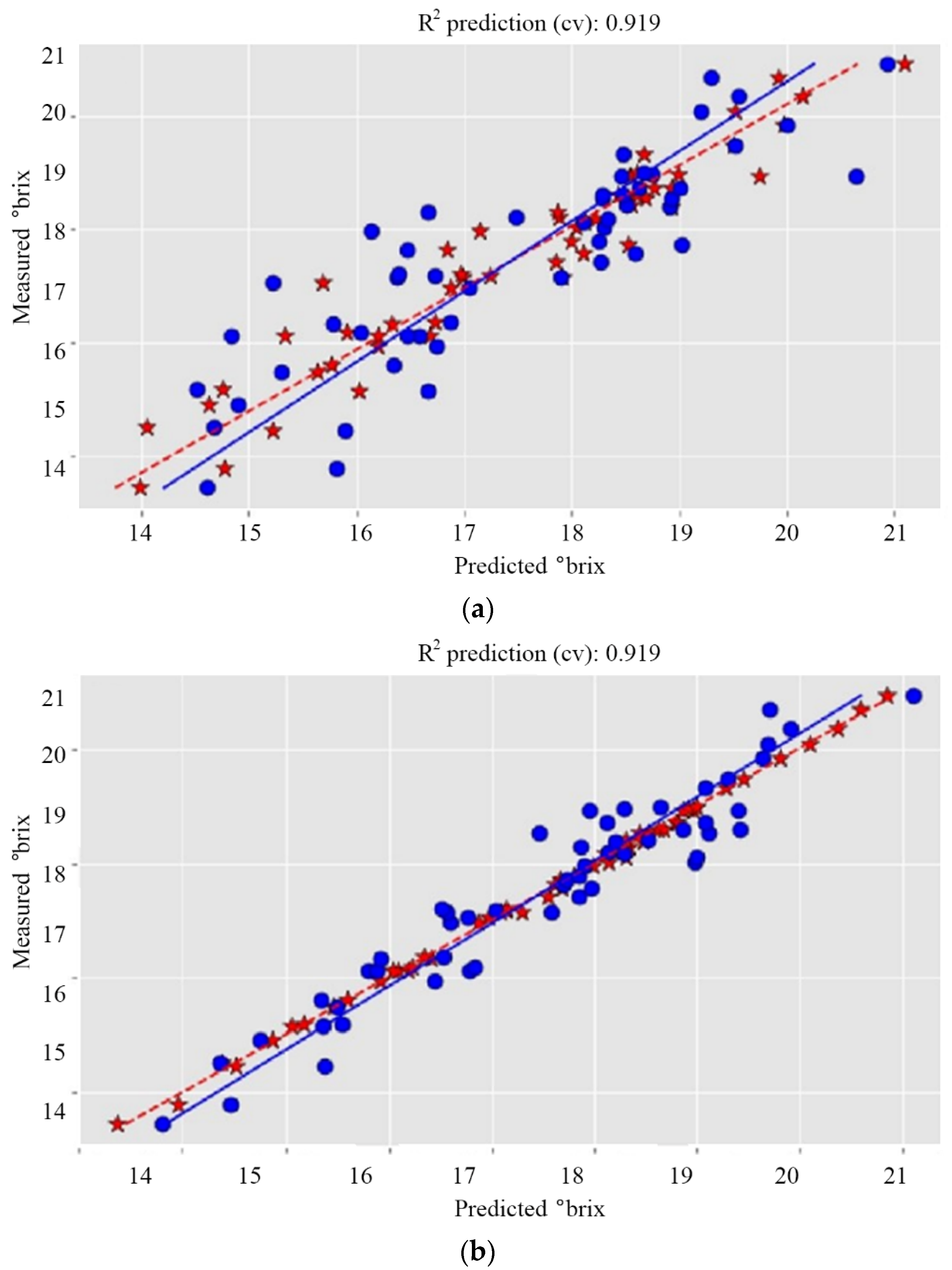
| Dataset 1 | 0.722 | 0.965 | 0.880 | 0.866 | 0.972 | 0.306 | 0.751 | 0.869 | 0.926 | 0.472 | 0.926 | 0.472 |
| Dataset 2 | −3.315 | 102.47 | 0.789 | 0.586 | 0.969 | 0.275 | 0.344 | 1.536 | 0.893 | 0.621 | 0.893 | 0.621 |
| Dataset 3 | 0.729 | 1.080 | 0.770 | 0.881 | 0.696 | 1.143 | 0.777 | 1.027 | 0.892 | 0.715 | 0.891 | 0.626 |
| Dataset 4 | 0.747 | 1.146 | −4.66 | 0.859 | −6.29 | 6.149 | 0.738 | 1.130 | 0.887 | 0.741 | 0.887 | 0.741 |
| Dataset | MLR | PLS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entire Dataset | opt_Xc | absxcoefs | Entire Dataset | opt_Xc | absxcoefs | |||||||
| R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | R2 | RMSE | |
| Dataset 1 | 0.722 | 0.965 | 0.911 | 0.544 | 0.968 | 0.324 | 0.751 | 0.869 | 0.919 | 0.495 | 0.919 | 0.495 |
| Dataset 2 | −3.315 | 102.47 | 0.503 | 1.099 | 0.569 | 1.023 | 0.189 | 1.710 | 0.871 | 0.683 | 0.871 | 0.683 |
| Dataset 3 | 0.543 | 1.092 | 0.570 | 1.158 | 0.687 | 0.988 | 0.740 | 0.972 | 0.899 | 0.605 | 0.899 | 0.605 |
| Dataset 4 | 0.747 | 1.070 | −5.693 | 5.893 | −5.693 | 5.893 | 0.727 | 1.154 | 0.884 | 0.751 | 0.884 | 0.751 |
| Dataset 1 Harvest 1 2016 | Dataset 2 Harvest 2 2016 | Dataset 3 both Harvests 2016 | Dataset 4 both Harvests 2016 and 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| wr | r | wr | r | wr | r | wr | r | |||
| Entire dataset | c | R2 | 1.000 | 0.942 | 1.000 | 0.592 | 1.000 | 0.958 | 1.000 | 0.724 |
| RMSE | 0.000 | 0.403 | 0.000 | 1.118 | 0.000 | 1.166 | 0.000 | 1.067 | ||
| p | R2 | 0.722 | 0.737 | −3.314 | 0.240 | 0.543 | 0.154 | 0.746 | 0.705 | |
| RMSE | 0.969 | 0.939 | 102.46 | 1.166 | 1.092 | 0.620 | 1.070 | 1.111 | ||
| opt_Xc | c | R2 | 1.000 | 0.983 | 1.000 | 0.363 | 1.000 | 0.980 | 0.999 | 0.418 |
| RMSE | 0.000 | 0.212 | 0.000 | 1.256 | 0.000 | 0.513 | 0.031 | 1.655 | ||
| p | R2 | 0.911 | 0.927 | 0.503 | 0.435 | 0.570 | 0.759 | −5.693 | 0.418 | |
| RMSE | 0.544 | 0.964 | 1.099 | 1.172 | 1.158 | 0.867 | 5.893 | 1.737 | ||
| absxcoefs | c | R2 | 1.000 | 0.991 | 1.000 | 0.252 | 1.000 | 0.974 | 0.999 | 0.563 |
| RMSE | 0.000 | 0.324 | 0.000 | 1.307 | 0.000 | 0.861 | 0.031 | 1.434 | ||
| p | R2 | 0.968 | 0.969 | 0.569 | 0.493 | 0.687 | 0.762 | −5.693 | 0.560 | |
| RMSE | 0.324 | 0.320 | 1.023 | 1.168 | 0.988 | 0.928 | 5.893 | 1.511 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chariskou, C.; Vrochidou, E.; Daniels, A.J.; Kaburlasos, V.G. Variable Selection on Reflectance NIR Spectra for the Prediction of TSS in Intact Berries of Thompson Seedless Grapes. Agronomy 2022, 12, 2113. https://doi.org/10.3390/agronomy12092113
Chariskou C, Vrochidou E, Daniels AJ, Kaburlasos VG. Variable Selection on Reflectance NIR Spectra for the Prediction of TSS in Intact Berries of Thompson Seedless Grapes. Agronomy. 2022; 12(9):2113. https://doi.org/10.3390/agronomy12092113
Chicago/Turabian StyleChariskou, Chrysanthi, Eleni Vrochidou, Andries J. Daniels, and Vassilis G. Kaburlasos. 2022. "Variable Selection on Reflectance NIR Spectra for the Prediction of TSS in Intact Berries of Thompson Seedless Grapes" Agronomy 12, no. 9: 2113. https://doi.org/10.3390/agronomy12092113
APA StyleChariskou, C., Vrochidou, E., Daniels, A. J., & Kaburlasos, V. G. (2022). Variable Selection on Reflectance NIR Spectra for the Prediction of TSS in Intact Berries of Thompson Seedless Grapes. Agronomy, 12(9), 2113. https://doi.org/10.3390/agronomy12092113










