Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Sensitivity to Efflux Pump Substrates
2.3. Statistical Analysis
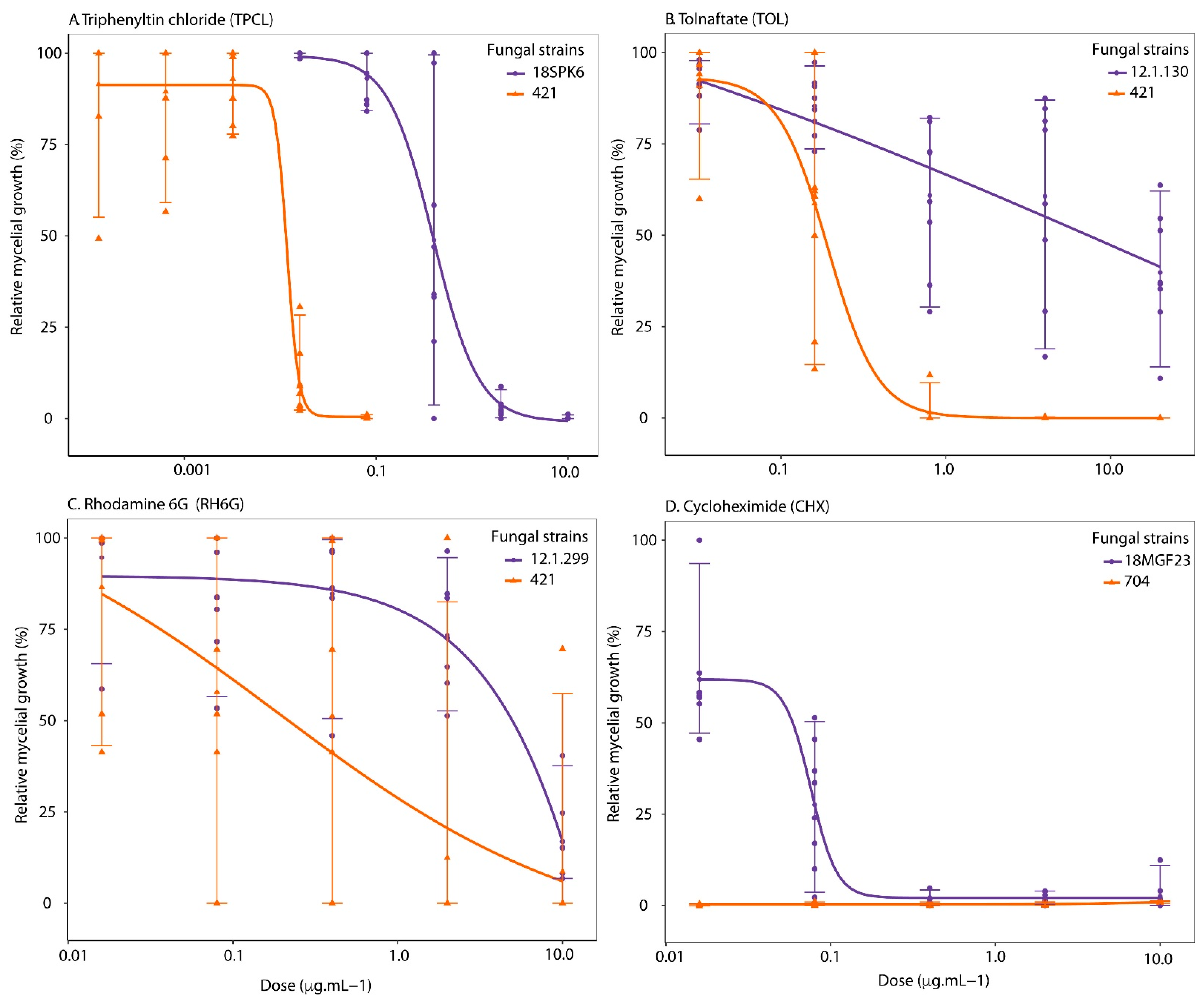
3. Results
3.1. Sensitivity to Efflux Pumps Substrates
3.2. Correlation Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castroagudín, V.L.; Moreira, S.I.; Pereira, D.A.S.; Moreira, S.S.; Brunner, P.C.; Maciel, J.L.N.; Crous, P.W.; McDonald, B.A.; Alves, E.; Ceresini, P.C. Pyricularia graminis-tritici, a new Pyricularia species causing wheat blast. Pers. Mol. Phylogeny Evol. Fungi 2016, 37, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.C.; Castroagudin, V.L.; Rodrigues, F.A.; Rios, J.A.; Eduardo Aucique-Perez, C.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat Blast: Past, Present, and Future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef] [PubMed]
- Ceresini, P.C.; Castroagudin, V.L.; Rodrigues, F.A.; Rios, J.A.; Aucique-Perez, C.E.; Moreira, S.I.; Croll, D.; Alves, E.; de Carvalho, G.; Maciel, J.L.N.; et al. Wheat blast: From its origins in South America to its emergence as a global threat. Mol. Plant Pathol. 2019, 20, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.F.; Prestes, A.M.; Maciel, J.L.N.; Scheeren, P.L. Resistência parcial à brusone de genótipos de trigo comum e sintético nos estádios de planta jovem e de planta adulta. Trop. Plant Pathol. 2010, 35, 24–31. [Google Scholar] [CrossRef]
- Pagani, A.P.S.; Dianese, A.C.; Café-Filho, A.C. Management of wheat blast with synthetic fungicides, partial resistance and silicate and phosphite minerals. Phytoparasitica 2014, 42, 609–617. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Ceresini, P.C.; Oliveira, S.C.; Reges, J.T.; Maciel, J.L.; Bonato, A.L.; Dorigan, A.F.; McDonald, B.A. Resistance to QoI fungicides Is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 2015, 105, 284–294. [Google Scholar] [CrossRef]
- Dorigan, A.F.; Carvalho, G.d.; Poloni, N.M.; Negrisoli, M.M.; Maciel, J.L.N.; Ceresini, P.C. Resistance to triazole fungicides in Pyricularia species is associated with invasive plants from wheat fields in Brazil. Acta Sci. Agron. 2019, 41. [Google Scholar] [CrossRef]
- Oliveira, S.C.; Castroagudín, V.L.; Maciel, J.L.N.; Pereira, D.A.S.; Ceresini, P.C. Resistência cruzada aos fungicidas IQo azoxistrobina e piraclostrobina no patógeno da brusone do trigo Pyricularia oryzae no Brasil. Summa Phytopathol. 2015, 41, 298–304. [Google Scholar] [CrossRef]
- Poloni, N.M.; Carvalho, G.; Nunes Campos Vicentini, S.; Francis Dorigan, A.; Nunes Maciel, J.L.; McDonald, B.A.; Intra Moreira, S.; Hawkins, N.; Fraaije, B.A.; Kelly, D.E.; et al. Widespread distribution of resistance to triazole fungicides in Brazilian populations of the wheat blast pathogen. Plant Pathol. 2021, 70, 436–448. [Google Scholar] [CrossRef]
- Vicentini, S.N.C.; Casado, P.S.; de Carvalho, G.; Moreira, S.I.; Dorigan, A.F.; Silva, T.C.; Silva, A.G.; Custódio, A.A.P.; Gomes, A.C.S.; Nunes Maciel, J.L.; et al. Monitoring of Brazilian wheat blast field populations reveals resistance to QoI, DMI, and SDHI fungicides. Plant Pathol. 2022, 71, 304–321. [Google Scholar] [CrossRef]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.; Fraaije, B.; et al. Proposal for a unified nomenclature for target-site mutations associated with resistance to fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Leroch, M.; Mosbach, A.; Walker, A.-S.; Fillinger, S.; Mernke, D.; Schoonbeek, H.-J.; Pradier, J.-M.; Leroux, P.; De Waard, M.A.; et al. Fungicide-Driven Evolution and Molecular Basis of Multidrug Resistance in Field Populations of the Grey Mould Fungus Botrytis cinerea. PLoS Pathog. 2009, 5, e1000696. [Google Scholar] [CrossRef] [PubMed]
- Omrane, S.; Sghyer, H.; Audeon, C.; Lanen, C.; Duplaix, C.; Walker, A.S.; Fillinger, S. Fungicide efflux and the MgMFS1 transporter contribute to the multidrug resistance phenotype in Zymoseptoria tritici field isolates. Environ. Microbiol. 2015, 17, 2805–2823. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Meng, F.-Z.; Zhang, M.-M.; Yin, L.-F.; Yin, W.-X.; Lin, Y.; Hsiang, T.; Peng, Y.-L.; Wang, Z.-H.; Luo, C.-X. A Putative Zn(2)Cys(6) Transcription Factor Is Associated With Isoprothiolane Resistance in Magnaporthe oryzae. Front. Microbiol. 2018, 9, 2608. [Google Scholar] [CrossRef]
- Perlin, M.H.; Andrews, J.; Toh, S.S. Essential letters in the fungal alphabet: ABC and MFS transporters and their roles in survival and pathogenicity. Adv. Genet. 2014, 85, 201–253. [Google Scholar]
- Paulsen, I.T.; Sliwinski, M.K.; Saier, M.H., Jr. Microbial genome analyses: Global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 1998, 277, 573–592. [Google Scholar] [CrossRef]
- Roohparvar, R. Drug Transporters of the Fungal Wheat Pathogen Mycosphaerella Graminicola; Wageningen University: Wageningen, The Netherlands, 2007. [Google Scholar]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Driessen, A.J.M.; Rosen, B.P.; Konings, W.N. Diversity of transport mechanisms: Common structural principles. Trends Biochem. Sci. 2000, 25, 397–401. [Google Scholar] [CrossRef]
- Yan, N. Structural Biology of the Major Facilitator Superfamily Transporters. Annu. Rev. Biophys. 2015, 44, 257–283. [Google Scholar] [CrossRef]
- Esquivel, B.D.; White, T.C. Accumulation of Azole Drugs in the Fungal Plant Pathogen Magnaporthe oryzae Is the Result of Facilitated Diffusion Influx. Front. Microbiol. 2017, 8, 1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.B.; Suresh, A.; Deng, Y.Z.; Naqvi, N.I. A multidrug resistance transporter in Magnaporthe is required for host penetration and for survival during oxidative stress. Plant Cell 2006, 18, 3686–3705. [Google Scholar] [CrossRef] [PubMed]
- Omrane, S.; Audéon, C.; Ignace, A.; Duplaix, C.; Aouini, L.; Kema, G.; Walker, A.S.; Fillinger, S. Plasticity of the MFS1 Promoter Leads to Multidrug Resistance in the Wheat Pathogen Zymoseptoria tritici. mSphere 2017, 2, e00393-17. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhai, P.; Wang, T.; Bromley, M.J.; Zhang, Y.; Lu, L. The C(2)H(2) Transcription Factor SltA Contributes to Azole Resistance by Coregulating the Expression of the Drug Target Erg11A and the Drug Efflux Pump Mdr1 in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2021, 65, e01839-20. [Google Scholar] [CrossRef]
- Schubert, S.; Popp, C.; Rogers, P.D.; Morschhäuser, J. Functional dissection of a Candida albicans zinc cluster transcription factor, the multidrug resistance regulator Mrr1. Eukaryot. Cell 2011, 10, 1110–1121. [Google Scholar] [CrossRef]
- Nakajima, M.; Suzuki, J.; Hosaka, T.; Hibi, T.; Akutsu, K. Functional Analysis of an ATP-Binding Cassette Transporter Gene in Botrytis cinerea by Gene Disruption. J. Gen. Plant Pathol. 2001, 67, 212–214. [Google Scholar] [CrossRef]
- Zwiers, L.H.; Stergiopoulos, I.; Gielkens, M.M.; Goodall, S.D.; De Waard, M.A. ABC transporters of the wheat pathogen Mycosphaerella graminicola function as protectants against biotic and xenobiotic toxic compounds. Mol. Genet. Genom. MGG 2003, 269, 499–507. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Yamamoto, K.; Hamamoto, H.; Nakaune, R.; Hibi, T. A novel ABC transporter gene ABC2 involved in multidrug susceptibility but not pathogenicity in rice blast fungus, Magnaporthe grisea. Pestic. Biochem. Physiol. 2005, 81, 13–23. [Google Scholar] [CrossRef]
- Chang, M.; Sionov, E.; Khanal Lamichhane, A.; Kwon-Chung, K.J.; Chang, Y.C. Roles of Three Cryptococcus neoformans and Cryptococcus gattii Efflux Pump-Coding Genes in Response to Drug Treatment. Antimicrob. Agents Chemother. 2018, 62, e01751-17. [Google Scholar] [CrossRef]
- Leroux, P.; Walker, A.S. Multiple mechanisms account for resistance to sterol 14alpha-demethylation inhibitors in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2010, 67, 44–59. [Google Scholar] [CrossRef]
- Yamashita, M.; Fraaije, B. Non-target site SDHI resistance is present as standing genetic variation in field populations of Zymoseptoria tritici. Pest Manag. Sci. 2018, 74, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic Diversity and Azole Fungicide Sensitivity in Pseudocercospora musae Field Populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed]
- Arango Isaza, R.E.; Castañeda Sánchez, D.A.; Rodríguez Beltrán, E.; Peláez Montoya, J.E.; Vásquez David, L.E.; Díaz Brito, T.J. Utilización de un ensayo de diluición en microplatos para medir la actividad antifúngica de sustancias contra Mycosphaerella fijiensis. Rev. Fac. Nac. Agron. 2006, 59, 3425–3433. [Google Scholar]
- Vargas, M.H. ED50plus v1.0; Instituto Nacional de Enfermedades Respiratorias: Mexico City, Mexico, 2000. [Google Scholar]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lawana, V.; Korrapati, M.C.; Mehendale, H.M. Cycloheximide. In Encyclopedia of Toxicology; Academic Press: London, UK, 2014; pp. 1103–1105. [Google Scholar]
- Hu, M.; Chen, S. Non-Target Site Mechanisms of Fungicide Resistance in Crop Pathogens: A Review. Microorganisms 2021, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Lamping, E.; Monk, B.C.; Niimi, K.; Holmes, A.R.; Tsao, S.; Tanabe, K.; Niimi, M.; Uehara, Y.; Cannon, R.D. Characterization of three classes of membrane proteins involved in fungal azole resistance by functional hyperexpression in Saccharomyces cerevisiae. Eukaryot. Cell 2007, 6, 1150–1165. [Google Scholar] [CrossRef]
- Hollingworth, R. Inhibitors and Uncouplers of Mitochondrial Oxidative Phosphorylation; Academic Press: San Diego, CA, USA, 2001; pp. 1169–1261. [Google Scholar]
- Man, D.; Podolak, M.; Engel, G.; Boniewska, E. Effect of Chlorotriphenyl Derivatives of Sn and Pb upon Biophysical Properties of Membranes. J. Biomed. Biotechnol. 2009, 2009, 969480. [Google Scholar] [CrossRef]
- Gear, A.R. Rhodamine 6G. A potent inhibitor of mitochondrial oxidative phosphorylation. J. Biol. Chem. 1974, 249, 3628–3637. [Google Scholar] [CrossRef]
- Maesaki, S.; Marichal, P.; Vanden Bossche, H.; Sanglard, D.; Kohno, S. Rhodamine 6G efflux for the detection of CDR1-overexpressing azole-resistant Candida albicans strains. J. Antimicrob. Chemother. 1999, 44, 27–31. [Google Scholar] [CrossRef]
- Posteraro, B.; Sanguinetti, M.; Fiori, B.; La Sorda, M.; Spanu, T.; Sanglard, D.; Fadda, G. Caspofungin activity against clinical isolates of azole cross-resistant Candida glabrata overexpressing efflux pump genes. J. Antimicrob. Chemother. 2006, 58, 458–461. [Google Scholar] [CrossRef]
- Georgopapadakou, N.H.; Bertasso, A. Effects of squalene epoxidase inhibitors on Candida albicans. Antimicrob. Agents Chemother. 1992, 36, 1779–1781. [Google Scholar] [CrossRef]
- Cannon, R.D.; Lamping, E.; Holmes, A.R.; Niimi, K.; Baret, P.V.; Keniya, M.V.; Tanabe, K.; Niimi, M.; Goffeau, A.; Monk, B.C. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 2009, 22, 291–321. [Google Scholar] [CrossRef] [PubMed]
- de Waard, M.A.; Andrade, A.C.; Hayashi, K.; Schoonbeek, H.J.; Stergiopoulos, I.; Zwiers, L.H. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 2006, 62, 195–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakaune, R.; Hamamoto, H.; Imada, J.; Akutsu, K.; Hibi, T. A novel ABC transporter gene, PMR5, is involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Mol. Genet. Genom. MGG 2002, 267, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Pasrija, R.; Banerjee, D.; Prasad, R. Structure and function analysis of CaMdr1p, a major facilitator superfamily antifungal efflux transporter protein of Candida albicans: Identification of amino acid residues critical for drug/H+ transport. Eukaryot. Cell 2007, 6, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.; Salat, M.; Frey, R.; Mosbach, A.; Luksch, T.; Balmer, D.; Hansen, R.; Widdison, S.; Logan, G.; Dietrich, R.A.; et al. A dispensable paralog of succinate dehydrogenase subunit C mediates standing resistance towards a subclass of SDHI fungicides in Zymoseptoria tritici. PLoS Pathog. 2019, 15, e1007780. [Google Scholar] [CrossRef]
- King, K.; Kirikyali, N.; West, J.; Fraaije, B. Rapid LAMP Assays to Detect MgCYP 51 and/or MgMFS 1 Overexpressing Strains of Zymoseptoria tritici in Leaf Samples. In The Modern Fungicides and Antifungal Compounds; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2017; pp. 67–72. [Google Scholar]
- West, J.; Atkins, S.; Emberlin, J.; Fitt, B. PCR to predict risk of airborne disease. Trends Microbiol. 2008, 16, 380–387. [Google Scholar] [CrossRef]
- West, J.; Kimber, R. Innovations in air sampling to detect plant pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef]
- Prates, R.A.; Kato, I.T.; Ribeiro, M.S.; Tegos, G.P.; Hamblin, M.R. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J. Antimicrob. Chemother. 2011, 66, 1525–1532. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, V.K.; Pathania, R. Efflux pump inhibitors for bacterial pathogens: From bench to bedside. Indian J. Med. Res. 2019, 149, 129–145. [Google Scholar]
- Tegos, G.P.; Masago, K.; Aziz, F.; Higginbotham, A.; Stermitz, F.R.; Hamblin, M.R. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2008, 52, 3202–3209. [Google Scholar] [CrossRef]
- Wong, K.; Ma, J.; Rothnie, A.; Biggin, P.C.; Kerr, I.D. Towards understanding promiscuity in multidrug efflux pumps. Trends Biochem. Sci. 2014, 39, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Fultang, N.; Illendula, A.; Lin, J.; Pandey, M.K.; Klase, Z.; Peethambaran, B. ROR1 regulates chemoresistance in Breast Cancer via modulation of drug efflux pump ABCB1. Sci. Rep. 2020, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- Green, A.T.; Moniruzzaman, M.; Cooper, C.J.; Walker, J.K.; Smith, J.C.; Parks, J.M.; Zgurskaya, H.I. Discovery of multidrug efflux pump inhibitors with a novel chemical scaffold. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129546. [Google Scholar] [CrossRef] [PubMed]
- Vega-Chacón, Y.; Albuquerque, M.; Pavarina, A.; Goldman, G.; Mima, E. Verapamil inhibits efflux pumps in Candida albicans, exhibits synergism with fluconazole, and increases survival of Galleria mellonella. Virulence 2021, 12, 231–243. [Google Scholar] [CrossRef]
| Source of Variation | df | Triphenyltin Chloride | Tolnaftate | Rhodamine 6G | Cycloheximide | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean Square | F Values | Mean Square | F Values | Mean Square | F Values | Mean Square | F Values | ||
| Strain (S) | 20 | 0.0802 | 53.923 ** | 5,043 | 43.241 ** | 2.946 | 6.325 ** | 0.5575 | 20.851 ** |
| Block | 3 | 0.0021 | 1.431 NS | 0.0965 | 0.828 NS | 0.1237 | 0.265 NS | 0.0881 | 3.295 NS |
| Residuals | 122 | 0.0015 | 0,1166 | 0.4659 | 0.0267 | ||||
| a Species and Isolates | b Previously Determined Fungicide Resistance Phenotype | f,g,h,i Mean EC50 (μg·mL−1), Standard Deviation and RF | j MDR Phenotype | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c QoI Azoxystrobin | d DMI Tebuconazole | e SDHI Fluxapyroxad | Triphenyltin Chloride (TPCL) | RF TPCL | Tolnaftate (TOL) | RF TOL | Rhodamine 6G (RH6G) | RF RH6G | Cycloheximide (CHX) | RF CHX | ||||||
| Pyricularia oryzae Triticum lineage—PoTl | ||||||||||||||||
| 12.1.015 | S | R | RS | 0.030 (0.01) | d | 3.5 | 0.25 (0.1) | d | 0.73 | 1.01 (0.9) | b | 29 | 0.10 (0.08) | d | 0.83 | MDRP1 |
| 12.1.045i | R | R | RS | 0.036 (0.01) | d | 4.2 | 0.36 (0.1) | d | 1.1 | 1.26 (0.41) | b | 37 | 0.13 (0.09) | d | 1.1 | MDRP1 |
| 12.1.037 | R | R | RS | 0.04 (0.002) | d | 4.7 | 0.31 (0.3) | d | 0.91 | 1.22 (0.8) | b | 35 | 0.09 (0.07) | d | 0.75 | MDRP1 |
| 18MGH25 | R | MR | RS | 0.06 (0.03) | d | 7.1 | 0.62 (0.33) | d | 1.8 | 0.80 (0.54) | c | 23 | 0.18 (0.11) | d | 1.5 | MDRP2 |
| 18MGH11 | R | R | HR | 0.04 (0.005) | d | 4.7 | 0.25 (0.07) | d | 0.74 | 0.47 (0.27) | c | 13 | 0.17 (0.14) | d | 1.4 | MDRP1 |
| 12.1.005 | R | R | S | 0.04 (0.03) | d | 4.7 | 0.55 (0.29) | d | 1.6 | 1.76 (1.1) | a | 50 | 0.18 (01.5) | d | 1.5 | MDRP1 |
| 12.1.183 | R | R | MR | 0.16 (0.06) | b | 19 | 0.87 (0.49) | c | 2.6 | 1.07 (0.83) | b | 31 | 0.42 (0.15) | c | 3.5 | MDRP2 |
| 18MGH19 | R | R | RS | 0.12 (0.02) | c | 14 | 1.02 (0.30) | c | 3.0 | 0.73 (1.08) | c | 21 | 0.27 (0.45) | c | 2.3 | MDRP2 |
| 18SPK6 | R | R | RS | 0.31 (0.09) | a | 36 | 1.33 (0.68) | b | 3.9 | 1.77 (0.80) | a | 51 | 0.15 (0.05) | d | 1.3 | MDRP2 |
| 12.1.299 | R | R | MR | 0.17 (0.04) | b | 20 | 0.79 (0.57) | c | 2.3 | 2.13 (0.65) | a | 61 | 0.11 (0.09) | d | 1.1 | MDRP2 |
| 12.1.312 | R | R | MR | 0.19 (0.08) | b | 22 | 0.89 (0.64) | c | 2.6 | 1.03 (0.34) | b | 29 | 0.11 (0.04) | d | 0.92 | MDRP 2 |
| 18MGF23 | R | R | MR | 0.15 (0.03) | b | 18 | 0.31 (0.12) | d | 0.91 | 1.35 (0.29) | b | 39 | 1.24 (0.53) | a | 10 | MDRP4 |
| 18SPC10 b | R | R | HR | 0.31 (0.04) | a | 36 | 0.50 (0.31) | d | 1.5 | 1.25 (1.28) | b | 36 | 0.13 (0.03) | d | 1.1 | MDRP2 |
| 12.1.165 | S | R | S | 0.27 (0.05) | a | 32 | 0.36 (0.09) | d | 1.1 | 1.47 (1.00) | b | 42 | 0.16 (0.06) | d | 1.3 | MDRP2 |
| 12.1.130 | S | HR | RS | 0.04 (0.002) | d | 4.7 | 4.40 (2.2) | a | 13 | 1.87 (0.6) | a | 53 | 0.11 (0.03) | d | 0.92 | MDRP3 |
| 18PRH9 | R | R | S | 0.03 (0.004) | d | 3.5 | 0.87 (0.45) | c | 2.6 | 0.50 (0.6) | c | 14 | 0.12 (0.03) | d | 1.0 | MDRP1 |
| Pyricularia oryzae Oryza lineage—PoO | ||||||||||||||||
| 656 | S | S | S | 0.013 (0.007) | d | 1.53 | 0.45 (0.28) | d | 1.32 | 0.06 (0.05) | d | 1.71 | 0.12 (0.1) | d | 1.0 | - |
| 421 | S | S | S | 0.004 (0.001) | d | 0.47 | 0.23 (0.08) | d | 0.68 | 0.01 (0.01) | d | 0.29 | 0.80 (0.34) | b | 6.7 | - |
| 674 | S | S | RS | 0.033 (0.03) | d | 3.88 | 0.42 (0.33) | d | 1.24 | 0.25 (0.17) | c | 7.14 | 0.09 (0.09) | d | 0.75 | - |
| 704 | S | S | MR | 0.004 (0.001) | d | 0.47 | 0.28 (0.09) | d | 0.82 | 0.05 (0.03) | d | 1.43 | 0.09 (0.06) | d | 0.75 | - |
| Pyricularia grisea—Pg | ||||||||||||||||
| 363 | R | S | S | 0.04 (0.004) | d | 4.71 | 0.26 (0.08) | d | 0.76 | 1.67 (1.4) | a | 48 | 0.11 (0.05) | d | 0.92 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicentini, S.N.C.; Moreira, S.I.; da Silva, A.G.; de Oliveira, T.Y.K.; Silva, T.C.; Assis Junior, F.G.; Krug, L.D.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Teodoro, P.E.; et al. Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy 2022, 12, 2068. https://doi.org/10.3390/agronomy12092068
Vicentini SNC, Moreira SI, da Silva AG, de Oliveira TYK, Silva TC, Assis Junior FG, Krug LD, de Paiva Custódio AA, Leite Júnior RP, Teodoro PE, et al. Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy. 2022; 12(9):2068. https://doi.org/10.3390/agronomy12092068
Chicago/Turabian StyleVicentini, Samara Nunes Campos, Silvino Intra Moreira, Abimael Gomes da Silva, Tamiris Yoshie Kiyama de Oliveira, Tatiane Carla Silva, Fabio Gomes Assis Junior, Loane Dantas Krug, Adriano Augusto de Paiva Custódio, Rui Pereira Leite Júnior, Paulo Eduardo Teodoro, and et al. 2022. "Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage" Agronomy 12, no. 9: 2068. https://doi.org/10.3390/agronomy12092068
APA StyleVicentini, S. N. C., Moreira, S. I., da Silva, A. G., de Oliveira, T. Y. K., Silva, T. C., Assis Junior, F. G., Krug, L. D., de Paiva Custódio, A. A., Leite Júnior, R. P., Teodoro, P. E., Fraaije, B., & Ceresini, P. C. (2022). Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy, 12(9), 2068. https://doi.org/10.3390/agronomy12092068








