Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa hispida)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of Chlorophyll and Carotenoid Contents
2.3. Metabolite Measurements and Data Analysis
2.4. Transcriptome Profiling through RNA-Seq
2.5. Validation of Gene Expression Level with Real-Time Quantitative PCR (qPCR)
3. Results
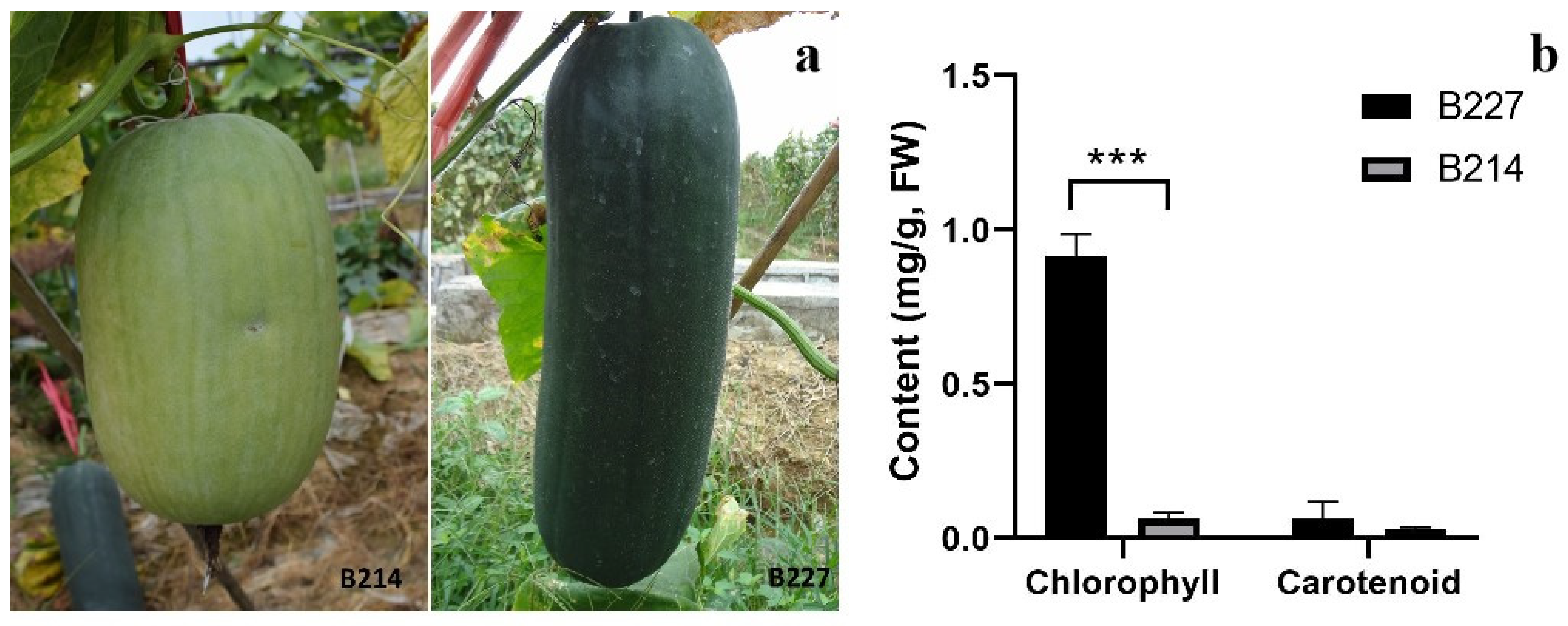
3.1. Phenotypic Differences between B214 and B227 in Pericarp Color
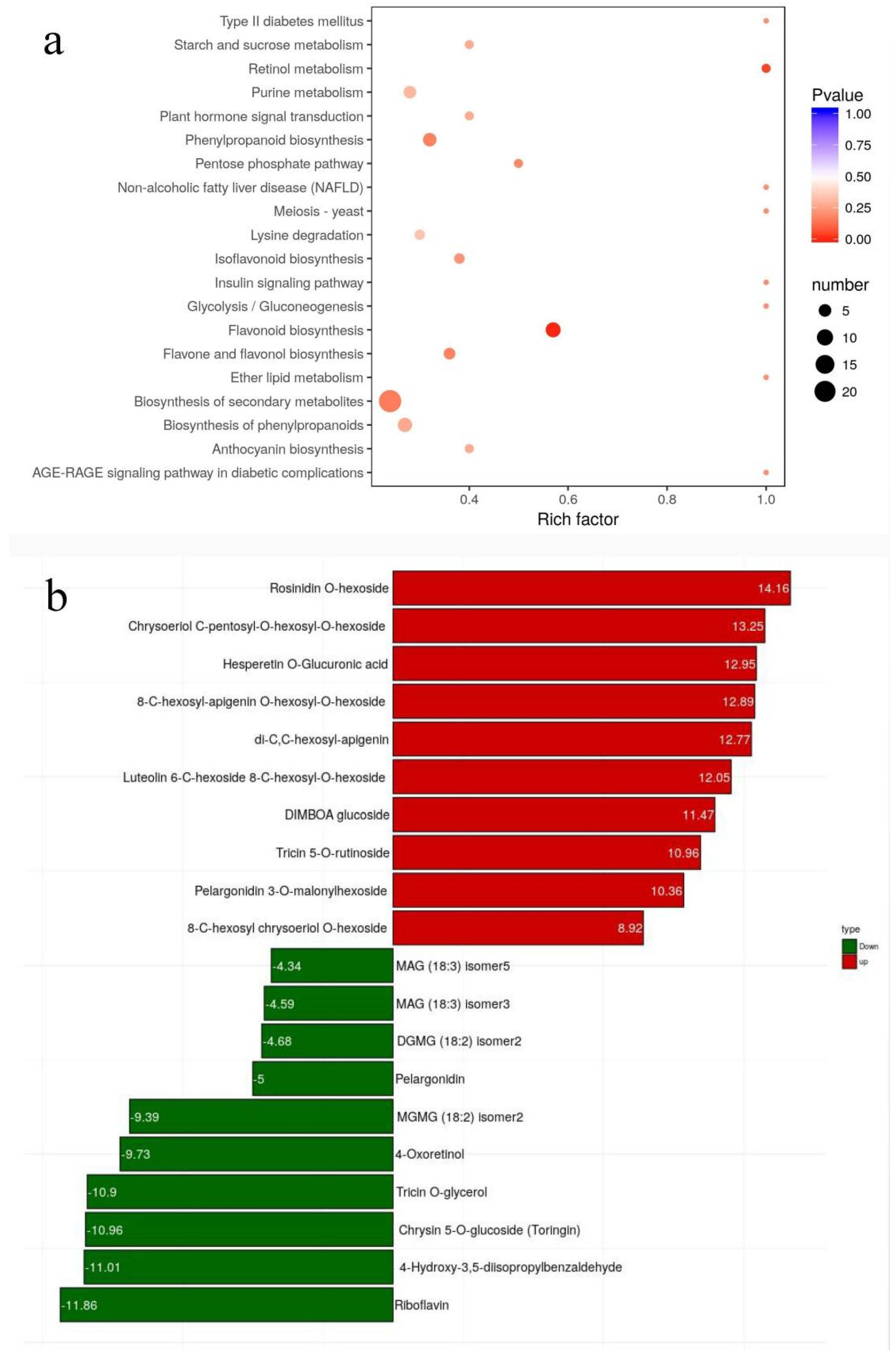
3.2. Metabolic Differences between Mature Pericarp of B214 and B227
3.3. DAMs Associated with Flavonoid Biosynthesis Pathway
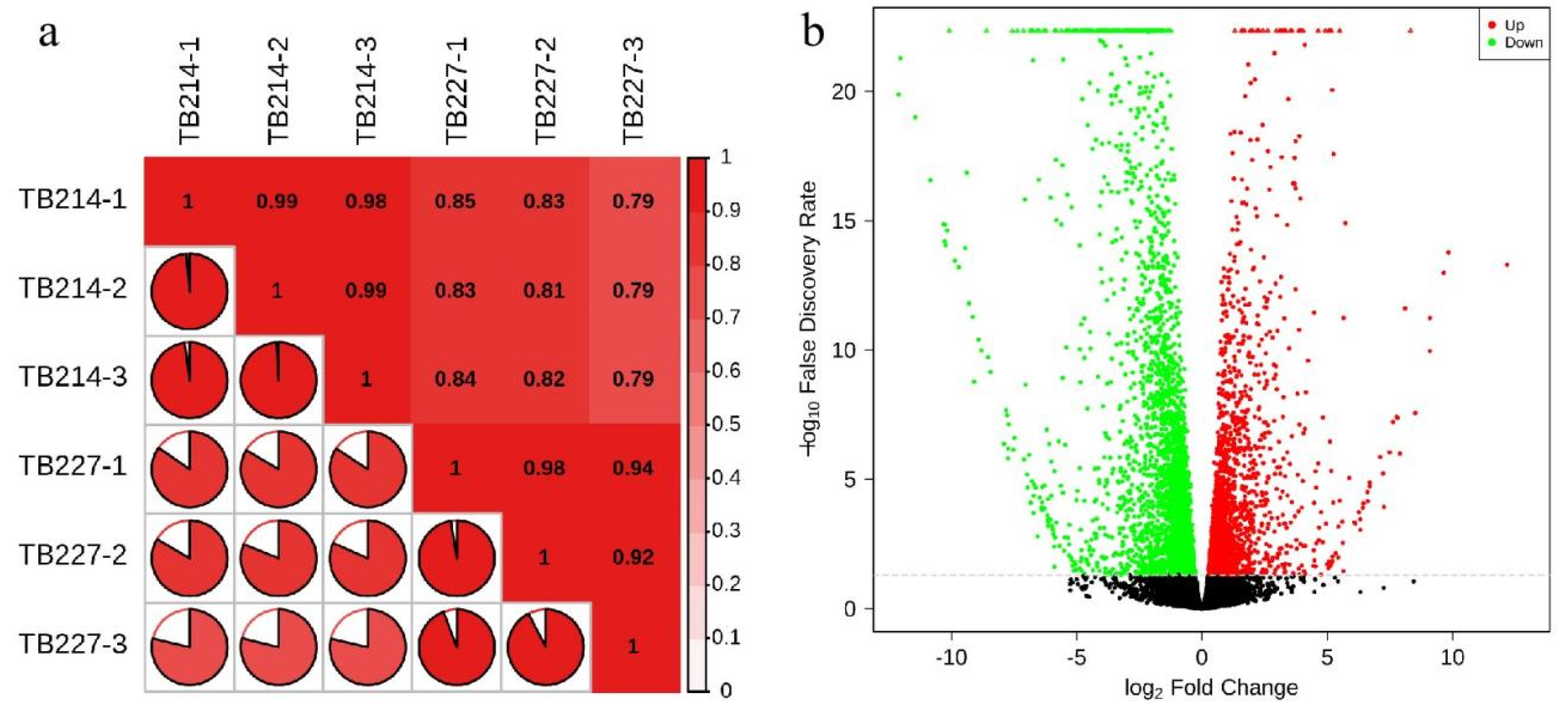
3.4. Transcriptomic Analysis
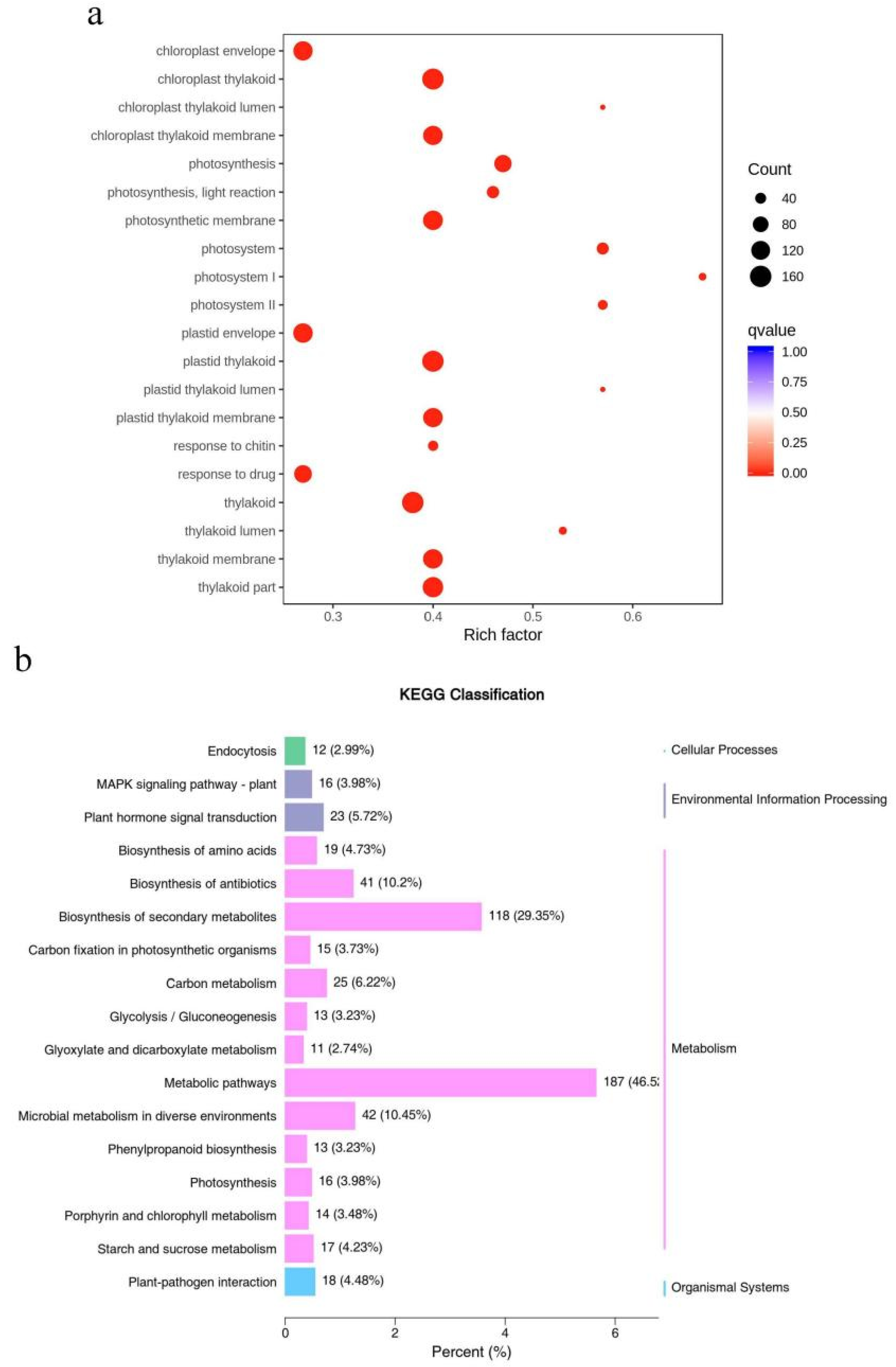
3.5. Functional Classification of DEGs
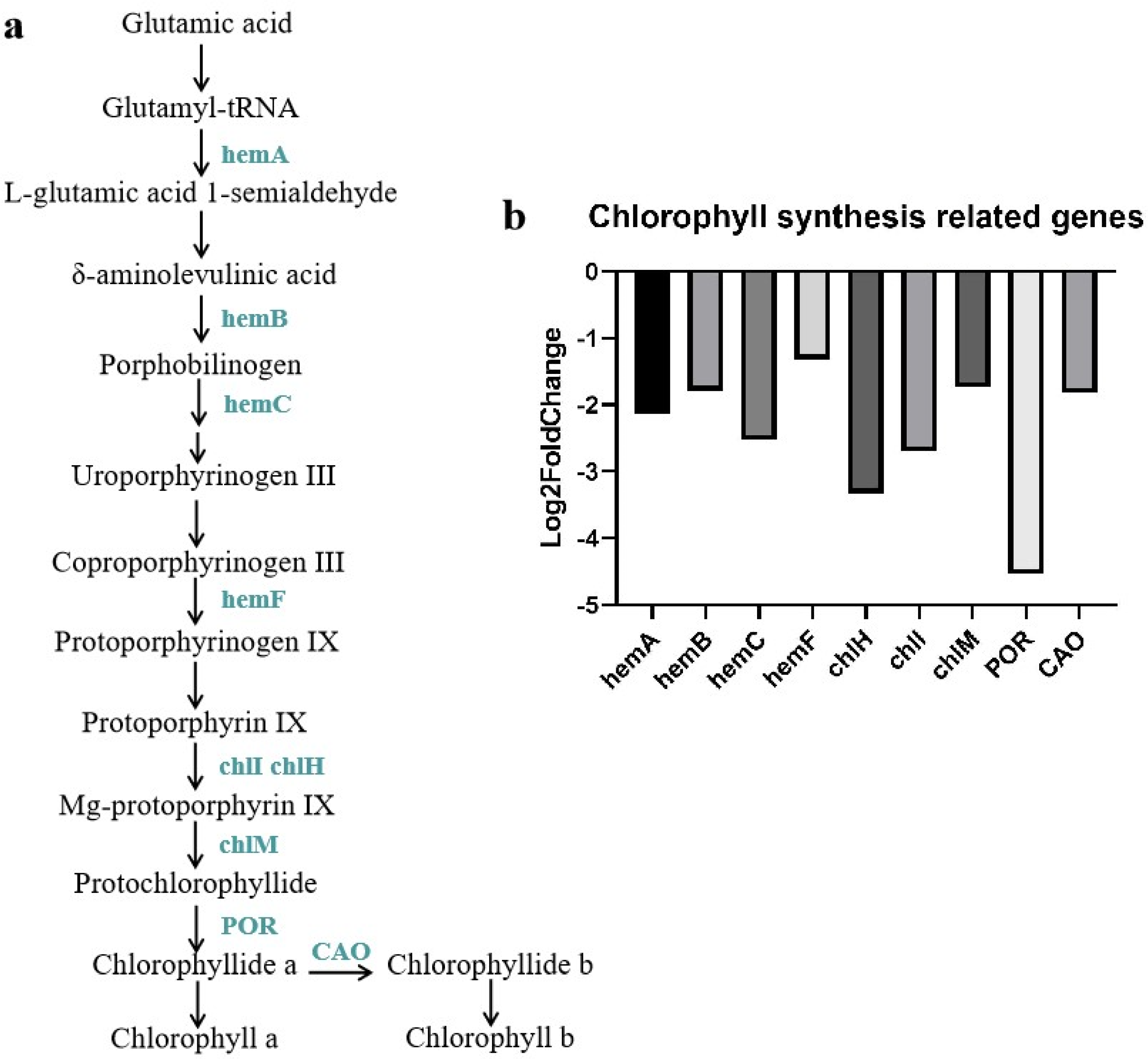
3.6. DEGs Associated with Chlorophyll Biosynthesis Pathway
3.7. DEGs Associated with Photosynthesis
3.8. DEGs Associated with Flavonoid Biosynthesis Pathway
3.9. Expressions of bHLH and MYB Transcription Factors
3.10. Real-Time PCR Validation of Transcriptomic Data
3.11. Combined Analysis of Transcriptome and Metabolome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, R.N.; Nayar, S.L.; Chopra, K. Glossary of Indian Medicinal Plants; Central Institute of Scientific and Industrial Research (CSIR): New Delhi, India, 1956; pp. 35–36. [Google Scholar]
- Warrier, P.K.; Nambiar, V.; Ramankutty, C. Indian Medicinal Plants; Orient Longman Limited: Kottakkal, India, 1993; p. 261. [Google Scholar]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Hu, B.; Qin, Y.-H.; Zhao, J.-T.; Wang, H.-C.; Hu, G.-B. Transcriptomic analysis of Litchi chinensis pericarp during maturation with a focus on chlorophyll degradation and flavonoid biosynthesis. BMC Genom. 2015, 16, 225. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiao, J.; Liang, X.; Liu, J.; Meng, H.; Chen, S.; Li, Y.; Cheng, Z. Map-based cloning, identification and characterization of the w gene controlling white immature fruit color in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2016, 129, 1247–1256. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Dong, X.; Wang, J.-K.; Xia, J.-H.; Xie, F.; Zhang, Y.; Yao, X.; Xu, Y.-J.; Wan, Z.-J. Fine Mapping and Candidate Gene Prediction for White Immature Fruit Skin in Cucumber (Cucumis sativus L.). Int. J. Mol. Sci. 2018, 19, 1493. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Takos, A.M.; Jaffé, F.W.; Jacob, S.R.; Bogs, J.; Robinson, S.P.; Walker, A.R. Light-Induced Expression of a MYB Gene Regulates Anthocyanin Biosynthesis in Red Apples. Plant Physiol. 2006, 142, 1216–1232. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Han, X.; Li, J.; Gao, Y.; Richards, C.M.; Zhang, C.; Tian, Y.; Liu, G.; Gul, H.; et al. A high-quality apple genome assembly reveals the association of a retrotransposon and red fruit colour. Nat. Commun. 2019, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Dwamena, C.; Thrimawithana, A.H.; Dejnoprat, S.; Lewis, D.; Espley, R.V.; Allan, A.C. A kiwifruit (Actinidia deliciosa ) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol. 2019, 221, 309–325. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Li, M.; Wang, J.; Yang, Y.; Zhang, X.; Yan, G.; Zhang, H.; Liu, J.; Zhang, K. The R2R3 MYB transcription factor PavMYB10.1 involves in anthocyanin biosynthesis and determines fruit skin colour in sweet cherry (Prunus avium L.). Plant Biotechnol. J. 2016, 14, 2120–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, G.; Ming, M.; Allan, A.C.; Gu, C.; Li, L.; Wu, X.; Wang, R.; Chang, Y.; Qi, K.; Zhang, S.; et al. Map-based cloning of the pear gene MYB114 identifies an interaction with other transcription factors to coordinately regulate fruit anthocyanin biosynthesis. Plant J. Cell Mol. Biol. 2017, 92, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Hong, M.; Zhang, Y.; Zu, F.; Wen, J.; Yi, B.; Ma, C.; Shen, J.; Tu, J.; et al. A Large Insertion in bHLH Transcription Factor BrTT8 Resulting in Yellow Seed Coat in Brassica rapa. PLoS ONE 2012, 7, e44145. [Google Scholar] [CrossRef] [PubMed]
- Ben Zvi, M.M.; Shklarman, E.; Masci, T.; Kalev, H.; Debener, T.; Shafir, S.; Ovadis, M.; Vainstein, A. PAP1 transcription factor enhances production of phenylpropanoid and terpenoid scent compounds in rose flowers. New Phytol. 2012, 195, 335–345. [Google Scholar] [CrossRef]
- Kim, D.-H.; Park, S.; Lee, J.-Y.; Ha, S.-H.; Lim, S.-H. Enhancing Flower Color through Simultaneous Expression of the B-peru and mPAP1 Transcription Factors under Control of a Flower-Specific Promoter. Int. J. Mol. Sci. 2018, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, D.J.; Condon, B.D.; Thyssen, G.; Naoumkina, M.; Madison, C.A.; Reynolds, M.; Delhom, C.D.; Fang, D.D.; Li, P.; McCarty, J. The GhTT2_A07 gene is linked to the brown colour and natural flame retardancy phenotypes of Lc1 cotton (Gossypium hirsutum L.) fibres. J. Exp. Bot. 2016, 67, 5461–5471. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, Y.; Vainstein, A.; Chen, S.; Ma, H. Regulation of Fig (Ficus carica L.) Fruit Color: Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway. Front. Plant Sci. 2017, 8, 1990. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Liu, Y.; Qi, Y.; Jiao, S.; Tian, F.; Jiang, L.; Wang, Y. Transcriptome sequencing and metabolite analysis reveals the role of delphinidin metabolism in flower colour in grape hyacinth. J. Exp. Bot. 2014, 65, 3157–3164. [Google Scholar] [CrossRef]
- Lv, Y.; Amanullah, S.; Liu, S.; Zhang, C.; Liu, H.; Zhu, Z.; Zhang, X.; Gao, P.; Luan, F. Comparative Transcriptome Analysis Identified Key Pathways and Genes Regulating Differentiated Stigma Color in Melon (Cucumis melo L.). Int. J. Mol. Sci. 2022, 23, 6721. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, M.; Luo, C.; Li, J.; Gong, H.; Zheng, X.; Liu, X.; Luo, J.; Wu, H. Metabolome and Transcriptome Analyses of Cucurbitacin Biosynthesis in Luffa (Luffa acutangula). Front. Plant Sci. 2022, 13, 886870. [Google Scholar] [CrossRef]
- Rhee, S.J.; Seo, M.; Jang, Y.J.; Cho, S.; Lee, G.P. Transcriptome profiling of differentially expressed genes in floral buds and flowers of male sterile and fertile lines in watermelon. BMC Genom. 2015, 16, 914. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.-J.; Jang, Y.J.; Park, J.-Y.; Ryu, J.; Lee, G.P. Virus-induced gene silencing for in planta validation of gene function in cucurbits. Plant Physiol. 2022, kiac363. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liu, W.; Xie, D.; Peng, Q.; He, X.; Lin, Y.; Liang, Z. High-density genetic map construction and gene mapping of pericarp color in wax gourd using specific-locus amplified fragment (SLAF) sequencing. BMC Genom. 2015, 16, 1035. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography-Mass Spectrometry, XCMS, and Chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Wang, S.; Tu, H.; Wan, J.; Chen, W.; Liu, X.; Luo, J.; Xu, J.; Zhang, H. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Götz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Bo, K.; Wei, S.; Wang, W.; Miao, H.; Dong, S.; Zhang, S.; Gu, X. QTL mapping and genome-wide association study reveal two novel loci associated with green flesh color in cucumber. BMC Plant Biol. 2019, 19, 243. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wen, C.; Weng, Y. Fine mapping of the pleiotropic locus B for black spine and orange mature fruit color in cucumber identifies a 50 kb region containing a R2R3-MYB transcription factor. Theor. Appl. Genet. 2013, 126, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Zhang, X. Molecular basis of cucumber fruit domestication. Curr. Opin. Plant Biol. 2019, 47, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Nat. Genet. 2019, 51, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, A.; Sasaki, K.; Nashima, K.; Oda-Yamamizo, C.; Hirashima, M.; Sumitomo, K. Transcriptome analysis in petals and leaves of chrysanthemums with different chlorophyll levels. BMC Plant Biol. 2017, 17, 202. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shi, N.; An, X.; Liu, C.; Fu, H.; Cao, L.; Feng, Y.; Sun, D.; Zhang, L. Candidate Genes for Yellow Leaf Color in Common Wheat (Triticum aestivum L.) and Major Related Metabolic Pathways according to Transcriptome Profiling. Int. J. Mol. Sci. 2018, 19, 1594. [Google Scholar] [CrossRef]
- Hao, N.; Du, Y.; Li, H.; Wang, C.; Wang, C.; Gong, S.; Zhou, S.; Wu, T. CsMYB36 is involved in the formation of yellow green peel in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2018, 131, 1659–1669. [Google Scholar] [CrossRef]
- Wu, Q.; Tian, J.; Li, P.-C.; Zhang, H.-J.; Feng, C.-Y.; Li, S.-S.; Yin, D.-D.; Xu, W.-Z.; Wang, L.-S. Relationship between the flavonoid composition and flower colour variation in Victoria. Plant Biol. 2018, 20, 674–681. [Google Scholar] [CrossRef]
- Gao, J.; Ren, R.; Wei, Y.; Jin, J.; Ahmad, S.; Lu, C.; Wu, J.; Zheng, C.; Yang, F.; Zhu, G. Comparative Metabolomic Analysis Reveals Distinct Flavonoid Biosynthesis Regulation for Leaf Color Development of Cymbidium sinense ‘Red Sun’. Int. J. Mol. Sci. 2020, 21, 1869. [Google Scholar] [CrossRef]
- Liao, J.; Zang, J.; Yuan, F.; Liu, S.; Zhang, Y.; Li, H.; Piao, Z.; Li, H. Identification and analysis of anthocyanin components in fruit color variation in Schisandra chinensis. J. Sci. Food Agric. 2016, 96, 3213–3219. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, J.; Chen, X.; Shi, X.; Wang, H.; Fu, Q. Comprehensive Analysis of Transcriptome and Metabolome Reveals the Flavonoid Metabolic Pathway Is Associated with Fruit Peel Coloration of Melon. Molecules 2021, 26, 2830. [Google Scholar] [CrossRef]
- Han, S.J.; Ryu, S.N.; Trinh, H.T.; Joh, E.H.; Jang, S.Y.; Han, M.J.; Kim, D.H. Metabolism of Cyanidin-3-O-Beta-D-Glucoside Isolated from Black Colored Rice and Its Antiscratching Behavioral Effect in Mice. J. Food Sci. 2019, 74, H253–H258. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Hoshino, A.; Kikuchi, Y.; Okuhara, H.; Ono, E.; Tanaka, Y.; Fukui, Y.; Saito, N.; Nitasaka, E.; Noguchi, H.; et al. Japanese morning glory dusky mutants displaying reddish-brown or purplish-gray flowers are deficient in a novel glycosylation enzyme for anthocyanin biosynthesis, UDP-glucose: Anthocyanidin 3-O-glucoside-2″-O-glucosyltransferase, due to 4-bp insertions in the Gene. Plant J. 2005, 42, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Vimolmangkang, S.; Soria-Guerra, R.E.; Rosales-Mendoza, S.; Zheng, D.; Lygin, A.V.; Korban, S.S. Ectopic Expression of Apple F3′H Genes Contributes to Anthocyanin Accumulation in the Arabidopsis tt7 Mutant Grown Under Nitrogen Stress. Plant Physiol. 2010, 153, 806–820. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ke, H.; Keting, H.; Qiaoyan, X.; Silan, D. Flower Colour Modification of Chrysanthemum by Suppression of F3’H and Overexpression of the Exogenous Senecio cruentus F3′5′H Gene. PLoS ONE 2013, 8, e74395. [Google Scholar] [CrossRef]
- Nishizaki, Y.; Sasaki, N.; Yasunaga, M.; Miyahara, T.; Okamoto, E.; Okamoto, M.; Hirose, Y.; Ozeki, Y. Identification of the glucosyltransferase gene that supplies the p-hydroxybenzoyl-glucose for 7-polyacylation of anthocyanin in delphinium. J. Exp. Bot. 2014, 65, 2495–2506. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Fang, Z.-Z.; Ye, X.-F.; Pan, S.-L. Identification of candidate genes involved in anthocyanin accumulation in the peel of jaboticaba (Myrciaria cauliflora) fruits by transcriptomic analysis. Gene 2018, 676, 202–213. [Google Scholar] [CrossRef]
- Nakayama, M.; Koshioka, M.; Kondo, T.; Imizu, K. Flavone C-Glucosides Responsible for Yellow Pigmentation Induced by Low Temperature in Bracts of Zantedeschia aethiopica. Nat. Prod. Commun. 2015, 10, 425–427. [Google Scholar] [CrossRef]
- Qi, T.; Wang, J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.; Song, S.; Xie, D. Regulation of Jasmonate-Induced Leaf Senescence by Antagonism between bHLH Subgroup IIIe and IIId Factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Fan, M.; Zhang, X.; Gao, H.; Huang, H.; Wu, D.; Guo, H.; Xie, D. The bHLH Subgroup IIId Factors Negatively Regulate Jasmonate-Mediated Plant Defense and Development. PLoS Genet. 2013, 9, e1003653. [Google Scholar] [CrossRef] [Green Version]

| Sample | Raw Reads | Clean Reads | Clean Base | Q20 | Q30 | GC Content |
|---|---|---|---|---|---|---|
| B214-1 | 48,005,504 | 47,722,880 | 7.12 G | 97.96% | 94.72% | 43.89% |
| B214-2 | 47,993,264 | 47,625,966 | 7.11 G | 97.68% | 94.11% | 43.85% |
| B214-3 | 47,999,846 | 47,690,334 | 7.11 G | 97.88% | 94.56% | 44.11% |
| B227-1 | 48,009,350 | 47,703,118 | 7.12 G | 97.89% | 94.58% | 44.09% |
| B227-2 | 47,976,656 | 47,650,708 | 7.11 G | 97.85% | 94.48% | 44.25% |
| B227-3 | 47,984,830 | 47,682,834 | 7.12 G | 97.91% | 94.61% | 44.55% |
| ID | Gene | B227 | B214 | log2 Fold Change | p-Value | Regulated |
|---|---|---|---|---|---|---|
| evm.TU.Contig37.39 | LHCA3 | 15,775 | 1488 | −3.406 | 9.18262 × 10−27 | down |
| evm.TU.Contig644.1 | LHCB1 | 49,230 | 46 | −10.084 | 2.07354 × 10−254 | down |
| evm.TU.Contig224.65 | LHCB2 | 54 | 8 | −2.835 | 4.07974 × 10−8 | down |
| evm.TU.Contig377.7 | LHCB3 | 4102 | 6 | −9.444 | 2.51075 × 10−16 | down |
| evm.TU.Contig169.75 | LHCB5 | 25,570 | 340 | −6.232 | 2.32559 × 10−101 | down |
| evm.TU.Contig512.42 | LHCB6 | 7911 | 184 | −5.43 | 4.79903 × 10−47 | down |
| ID | Gene | B227 | B214 | log2 Fold Change | p Value | Regulated |
|---|---|---|---|---|---|---|
| evm.TU.Contig115.34 | CHS | 442 | 936 | 1.081 | 5.77 × 10−8 | up |
| evm.TU.Contig705.13 | ANR | 13 | 0 | −6.141 | 9.24 × 10−5 | down |
| evm.TU.Contig87.39 | F3′5′H | 311 | 90 | −1.782 | 4.42 × 10−8 | down |
| evm.TU.Contig87.40 | F3′H | 397 | 22 | −4.149 | 4.73 × 10−11 | down |
| evm.TU.Contig211.255 | FLS | 287 | 72 | −1.989 | 0.000249 | down |
| evm.TU.Contig416.27 | GT5 | 37 | 10 | −1.84 | 0.000831 | down |
| evm.TU.Contig26.80 | GT7 | 707 | 289 | −1.292 | 1.02 × 10−5 | down |
| evm.TU.Contig112.25 | GT7 | 2966 | 1289 | −1.203 | 0.000574 | down |
| ID | Gene | B227 | B214 | log2 Fold Change | p Value | Regulated |
|---|---|---|---|---|---|---|
| evm.TU.Contig112.11 | bHLH95 | 0 | 5 | 4.746 | 0.014 | up |
| evm.TU.Contig164.13 | bHLH154 | 8 | 290 | 5.197 | 1.03 × 10−22 | up |
| evm.TU.Contig179.20 | bHLH14 | 68 | 4 | −4.1 | 1.33 × 10−6 | down |
| evm.TU.Contig209.54 | bHLH14 | 44 | 6 | −2.968 | 0.0013 | down |
| evm.TU.Contig234.152 | bHLH041 | 11 | 1 | −4.088 | 0.0095 | down |
| evm.TU.Contig329.8 | bHLH92 | 618 | 20 | −4.968 | 1.34 × 10−36 | down |
| evm.TU.Contig391.9 | bHLH35 | 81 | 0 | −8.801 | 3.85 × 10−12 | down |
| evm.TU.Contig88.51 | bHLH25 | 19 | 1 | −4.295 | 0.0002 | down |
| ID | Gene | B227 | B214 | log2 Fold Change | p Value | Regulated |
|---|---|---|---|---|---|---|
| evm.TU.Contig10.51 | MYB30 | 1 | 187 | 8.115 | 7.25 × 10−14 | up |
| evm.TU.Contig105.117 | MYB14 | 112 | 12 | −3.218 | 1.38 × 10−11 | down |
| evm.TU.Contig13.239 | MYB124 | 2613 | 484 | −2.434 | 5.13 × 10−19 | down |
| evm.TU.Contig177.101 | MYB3 | 22 | 734 | 5.105 | 3.01 × 10−65 | up |
| evm.TU.Contig198.7 | MYB4 | 3 | 17 | 2.312 | 0.004023 | up |
| evm.TU.Contig216.35 | MYB38 | 85 | 16 | −2.45 | 6.35 × 10−9 | down |
| evm.TU.Contig328.27 | MYB15 | 11 | 1 | −3.109 | 0.008564 | down |
| evm.TU.Contig356.67 | MYB86 | 128 | 541 | 2.079 | 1.61 × 10−15 | up |
| evm.TU.Contig493.41 | MYB14 | 41 | 6 | −2.852 | 0.00111 | down |
| evm.TU.Contig57.22 | MYB62 | 21 | 1 | −3.991 | 0.002206 | down |
| evm.TU.Contig88.71 | MYB84 | 6 | 0 | −5.016 | 0.009042 | down |
| evm.TU.Contig96.61 | MYB16 | 3 | 96 | 4.824 | 2.3 × 10−9 | up |
| evm.TU.Contig98.10 | MYB2 | 182 | 20 | −3.17 | 1.13 × 10−6 | down |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Sun, P.; Liu, W.; Xie, D.; Wang, M.; Peng, Q.; Sun, Q.; Jiang, B. Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa hispida). Agronomy 2022, 12, 2045. https://doi.org/10.3390/agronomy12092045
Yan J, Sun P, Liu W, Xie D, Wang M, Peng Q, Sun Q, Jiang B. Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa hispida). Agronomy. 2022; 12(9):2045. https://doi.org/10.3390/agronomy12092045
Chicago/Turabian StyleYan, Jinqiang, Piaoyun Sun, Wenrui Liu, Dasen Xie, Min Wang, Qingwu Peng, Qingming Sun, and Biao Jiang. 2022. "Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa hispida)" Agronomy 12, no. 9: 2045. https://doi.org/10.3390/agronomy12092045
APA StyleYan, J., Sun, P., Liu, W., Xie, D., Wang, M., Peng, Q., Sun, Q., & Jiang, B. (2022). Metabolomic and Transcriptomic Analyses Reveal Association of Mature Fruit Pericarp Color Variation with Chlorophyll and Flavonoid Biosynthesis in Wax Gourd (Benincasa hispida). Agronomy, 12(9), 2045. https://doi.org/10.3390/agronomy12092045




