Abstract
A search for energy efficient, non-herbicide weed control methods led to development of a novel electrical weeding technology. This study focuses on weed control efficiency and energy as elements of a system that would include machine vision and robotics to control escape weeds in field crops. Two pulse generation systems, one single and one multiple, were developed and evaluated at different delivered voltages and energies. Greenhouse trials using specially designed and built application and recording technology showed the application of precisely applied micro-shocks with precisely controlled direct current (DC) voltage, pulse number, pulse length and period (hereafter PMS) can kill small Lolium multiflorum Lam., Chenopodium album L., Amaranthus powellii S. Wats. and Solanum nigrum L. plants with minimal energy. Plants took as much as two weeks to die. Increasing applied energy increased effectiveness as determined by plant biomass reduction and death rate. Grasses appear difficult to control once tillering has commenced, and high voltages may destroy leaf blades but not growing points. Broadleaved plants took several days to show evidence of chlorosis which preceded senescence and death. Our results showed that 5 J is sufficient energy to bring about death or severe growth limitation in many seedlings up to 15 cm height. This is as little as 1% of the energy of, and more effective than, ultra-low energy treatments reported in other recent research. To control five herbicide resistant weeds m−2, the required energy would be about 0.25 MJ ha−1 plus transport and actuation energy for weed destruction, as compared to an optimum target of about 20–40 MJ ha−1 including transport suggested in the literature. PMS can effectively control broadleaved weed seedlings and small non-tillering grasses at a fraction of the energy required by commercially available systems. This indicates PMS has potential as a viable technology for hand-held electric weeders or as part of a site-specific robotic weeding system.
1. Introduction
Replacements for weed control chemicals are sought, driven by increasing herbicide resistance [1,2,3], consumer preferences for chemical free food [4,5,6,7,8,9], public concern about residues in water [10,11], and awareness of potential impacts of chemical treatments on soil microflora [12]. A concurrent movement is towards more conservation agriculture, systems that are typically reliant on herbicides [13,14]. Conservation agriculture is accompanied by greater quantities of ground cover in the form of crops, cover crops or crop residues. This makes mechanical weed control by tillage difficult, so non-contact methods are preferable [15].
Labour supply shortages coupled with rapid advances in robotics and vision analytics are helping push automation and systems adaptable to a robotic platform. With respect to autonomous weeding in sugarbeet, eight evaluation criteria and target values were proposed for a concept selection matrix: weed control efficiency (90% target), ability to target weeds (including under crop leaves), resolution of action (control border <5 mm), work rate (10 weeds sec−1 per row or 0.5 m s−1 operational speed), auxiliary rate (labour to assist the tool ~18 min per 2 h), energy (~2 kJ m−1 row), applicability to autonomous vehicles (<150 kg, <1 m width and height) [16], and material costs (€11,250) [17]. Of the technologies available, we believe electric weeding has most potential to address the criteria above, as part of an overall robotic solution.
Electric weeding has a long history, with the first patents in the 1890s [18,19] and has subsequently been the subject of ongoing development and patent applications [20,21,22,23,24,25]. Development in the 1970s and 1980s [22,26] competed with the advent of cost-effective translocated herbicides such as glyphosate combined with “weed wiper” application technology [27]. With increasing objections to glyphosate and increasing herbicide resistance, electrical weeding is again the subject of research and development [28,29,30]. Electric weeding plant selectivity has been achieved by physical separation, notably treating taller weeds in low-growing crops, such as bolters in sugar beet [31]. Many technology firms are active in the field, some reviving the 1980s systems, others moving to smaller robotic mounted systems [32,33,34,35,36]. Considerable research effort is developing vision systems and plant recognition algorithms to discriminate between crop plants and weeds [37,38,39,40,41]. An electric system would be useful in wet soil conditions when mechanical weeding is difficult or ineffective. It could replace herbicides in organic food production or amenity or urban settings.
The energy requirements of different weeding methods have been variously determined and reported [42,43,44,45]. Nørremark et al. [16] propose a target of 2.01 kJ m−1 of row for inter-row weeding of crops in 50 cm rows. This equates to 40.2 MJ ha−1 which is about twice Coleman et al.’s estimates of 19 MJ ha−1 [44] assuming 5 plants m−2, a reasonable assumption for post-weeding escapes [31,45].
Use of both alternating and direct current (AC and DC) electrical circuitry and spark or continuous contact applications has been reported [28,46]. Most commercially available equipment uses continuous contact application, operating at tens of kilovolts, and many amps [21,26,47,48]. This is effective but does pose a high risk to operators or accidental contacts. Treatment at these energy levels is typified by steam and sparks or flame. In dry conditions, there would be a high risk of fire, a factor that must be managed. Energy of 4 kJ–111 kJ plant−1 was reported using continuous contact in greenhouse conditions [49]. To reach the target of Nørremark et al. [16], this would allow for only one weed every 2–55 m of row length. An alternative to continuous current is some form of pulsed or spark treatment. Pulsed DC currents were shown to be more energy efficient than sinusoidal AC in creating intracellular plant tissue damage [50]. For the same input energy, the energy release in tissues was more than five times higher from pulsed DC.
A low-energy electrical weeding approach reported a short 15 kV AC pulse could “effectively destroy” a small Cerastium holosteoides Fries. plant with 304 mJ, considerably less than other systems [51]. Observing that transpiration was reduced, it was suggested that vascular bundles were destroyed. Repeating pulses reduced electrical resistance in the plants, suggested cell wall damage increased path conductivity. However, these data were obtained from very small (0.5 g, 50 mm tall, 2 mm stem diameter) plants treated on a bench, then transferred to an agar dish. Although plants were observed to be withered at 3 days post-treatment, they were not followed through to definite death. Larger Rumex japonicus plants 50 cm tall withered after being treated with 30 × 2 J pulses, but again their final death is not recorded [51]. Together these reports indicate pulsed DC shocks might control 5 small weeds m−2 with less than 5 kJ ha−1, and 5 large weeds m−2 with less than 600 kJ ha−1.
Hand-held weeding devices are used for removing low population-density weeds in amenity horticulture and those remaining after broad-acre herbicide treatments. A hand-held electric weeder was reported to successfully control Poa annua L. in golf courses [51,52]. This device applied treatments ranging from 450 J to 1000 J plant−1 which equates to 22.5–50 MJ ha−1 using Coleman’s calculations of 5 plants m−2, or 2–4 plants m−1 of row to reach the target of Nørremark et al. Treatment times ranged from 1–3 s per plant, suggesting 14–40 h ha−1 at 5 plants m−2. While there are many applications for hand-held weeding devices, there does not appear to have been any commercialisation of the technologies described, there is little evidence of further research or development, and associated patents and applications have expired [53,54,55].
Recent results from “low energy electro-physical treatment” of weeds using two systems, one 40 kV DC and one 2.2 kV AC, showed significant reductions in weed biomass, and in some cases plant death, with smaller plants of treated species being more affected by the electric treatments [28]. A range of 40 kV DC treatments applied 560 J of energy by spark or direct contact to leaf or stem of Amaranthus retroflexus L., Portulaca oleracea L., Sorghum halepense L. and Citrullus lanatus Thunb. Only direct leaf contact had significant biomass reduction on A. retroflexus and no treatments achieved 100% kill rate. Direct leaf contact and a stem-spark treatment significantly reduced biomass P.oleracea but no treatments affected S. halapense or C. lanatus. Very small (size not stated) Trifolium pratense plants, 2 weeks after seeding (WAS), were killed by the 2.2 kV AC current applying as little as 9 J but required 180 J to achieve 95% biomass reduction at 4 WAS. These doses required 1 s (9 J), 10 s (90 J) and 20 s (180 J). This implies field treatment times of about 14–280 h ha−1 (~0.6–11.6 days ha−1) at 5 plants m−2. A different approach using days-long duration, low-current DC electricity was used to kill a range of small Tamarix spp., Morus alba L. and Ulmus pumila L. shrubs and trees and Convolvulus arvensis L. vines [30] but has little relevance to field crop weed management.
The focus of our research is efficient weed control and minimum energy requirements achievable by application of precisely applied micro-shocks with precisely controlled direct current (DC) voltage, pulse number, pulse length and period (hereafter PMS) to deliver low energy electric treatments. This would make a simple battery-powered hand-held weeding system or high efficiency strip-weeders possible. In conjunction with the other factors outlined by Nørremark et al. [16] it would be suitable for deployment on low-energy autonomous field robots. It would address issues of herbicide resistance, consumer desire for chemical-free food, and avoid chemical residues in water.
Here, we report results from preliminary greenhouse trials of ultra-low energy weeding using PMS treatments applied to Lolium multiflorum Lam., Chenopodium album L., Amaranthus powellii S. Wats., and Solanum nigrum L. weed seedlings. To kill plants of apparently the same size, our treatments used approximately 1% of the energy of the recently reported low-energy DC electro-physical weed treatment method. Ultra-low energy electric weed control has not been extensively studied but has numerous potential benefits. The objectives of this study were to determine a threshold “dose” of voltage and energy for mortality, and to compare relative responses of different species. Observations, findings, and hypotheses as to the mechanism or mechanisms of plant death will direct further research to refine applications and enable a commercially viable product.
2. Materials and Methods
2.1. Equipment and Materials
Two custom PMS devices (Weda Tech, Hastings, New Zealand) were developed which generated DC pulses of up to 10 kV. The first device developed could deliver a single pulse of either 6 kV or 10 kV, releasing approximately 1.8 J and 5 J, respectively. The second device produced multiple pulses at voltages up to 7 kV, with pulse duration, pulse period, and either number of pulses or total delivered energy able to be programmed (Figure 1). The multi-pulse device was controlled using custom software running on a laptop. Each device included two 10 mm diameter aluminium electrodes; the earth was inserted 50 mm into the soil about 30–40 mm from the plant being treated and the positive electrode was held beside or on the stem of the plant. During the application of multi-pulse PMS, each application was measured with voltage, resistance and current being logged and energy calculated.
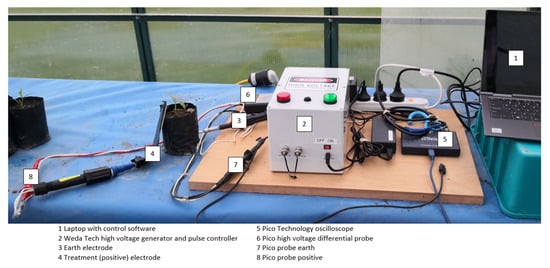
Figure 1.
Photograph showing multi-pulse DC treatment equipment including picoscope to monitor voltage and current discharge.
To determine the energy absorption split between the plant and the soil, later trials included a PicoScope 2000 (Pico Technology, St Neots, Cambridgeshire, U.K.) series oscilloscope coupled with a Pico TA044 high voltage differential probe connected to the base of the plant and the earth electrode. To achieve stable voltage throughout each application, a 50 ms interval was maintained between pulses. Total treatment times varied between 0.7 ms and 3000 ms across all trials, but active times were less than 6 ms. An electronic voltage calibration check showed strong agreement between the multi-pulse device and picoscope calculated energies (y = 1.0273x − 0.505, R2 = 0.984).
The application and monitoring equipment set voltage, pulse number, duration and period and measured the voltage, pulse number, active time and current (Figure 2).
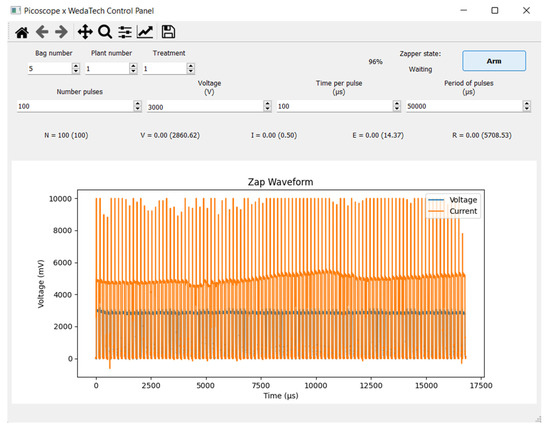
Figure 2.
Example of Weda Tech Zapper control screen shows controllable micro-shock parameters, oscilloscope trace, and measured and calculated electrical values.
The Electrical power is calculated from the standard formula:
where P is power (watts), V is voltage, and I is current (amps).
Energy transferred is calculated using the formula:
where E = energy (joules), P is power, and t is time (seconds).
Resistance is calculated using the standard formula:
where R is resistance (ohms), V is voltage, and I is current.
A series of uncontrolled preliminary tests identified doses that gave apparent plant responses around which treatments were designed. We noted that in many cases few if any symptoms were evident for about 6 days, and it could be several weeks before plant death was incontrovertible. Therefore, our experiments followed the progress of treated plants until we had confidence plants had died (fully wilted, chlorotic, roots and shoots naturally separated) or were growing healthily even if delayed relative to controls.
2.2. Plant Material and Growing Method
In all trials the growing medium was Hastings silt loam soil sieved to 5 mm. Plants were grown in 450 mL polythene bags, placed in a greenhouse and hand watered as required. Temperature and humidity were logged and showed no values considered likely to cause any negative effect. Bags were arranged in blocks and randomised within each block.
Certified Lolium multiflorum “Winter Star” seeds were sown six seeds to a bag and 12–14 days after emergence were thinned to three plants per bag, aiming to keep all plants within a similar size profile, typically with two leaves. Chenopodium album L. plants (20–30 mm high) were collected from wasteland, transplanted three per bag, and grown for a further 14 days before treatment. Amaranthus powellii S. Wats. and Solanum nigrum L. seedlings were collected from fallowed cropping areas. Thirty seedlings of each were transplanted one per bag and allowed to establish for 10 and 16 days before treatment.
2.3. Plant Measurements
Prior to treatment, L. multiflorum plants were observed for visual differences and the number of turgid green leaves and tillers, and length of the longest leaf were measured. Following treatment, measurements were repeated, and plant deaths recorded 15 days after treatment (DAT). Severely shrivelled or dead parts of leaf were discounted. For the multi-pulse equipment, at 15 DAT surviving plants were measured, harvested, and their fresh mass determined. Plants from each bag were aggregated, oven dried at 62 °C for 3 days and reweighed. The mean plant dry weight was determined for the surviving plants in each bag.
No relative growth measurements of C. album plants were made. The binary measure of effectiveness at 28 DAT was plant vitality, defined as dead or alive. A. powellii plants were monitored for 11 DAT recording the height to top growing point (±1 mm), stem diameter at 2 cm above ground (±0.01 mm), and a leaf area estimation (±0.01 cm2) using the “Easy Leaf Area Free” smartphone application (ELA Free) [56]. At 11 DAT plant status was assessed and assigned to one of three categories: healthy, collapsed or dead. For the binary logit analyses, all the plants not obviously dead were classified as alive. When S. nigrum plants were treated, in addition to recording machine energy discharge, the energy absorption by soil or plants was measured with the Picoscope by attaching to one machine electrode and the other at the base of the plant. Plants were monitored for 30 DAT recording the stem diameter (±0.01 mm) at 3 cm above ground, and a leaf area estimation (±0.01 cm2) using ELA Free. At 30 DAT plant vitality was assessed.
2.4. Treatments
Experiment 1: Single Pulses
Single pulse treatments at 6 kV and 10 kV were applied to L. multiflorum and C. album plants. The earth electrode was set 50 mm into the soil 30–60 mm from the plant base. The positive electrode was placed <5 mm from a L. multiflorum leaf 60 mm above the soil and <5 mm from the C. album stem 50 mm above the soil. The L. multiflorum experiment had three plants per bag, and the C. album experiment had one plant per bag, with four bags per treatment and the control (Table 1).

Table 1.
Trial summary table showing trial number, weed species, plant size, single electric pulse treatments applied and number of replicates.
Experiment 2: Multiple pulses
Multi-pulse treatments were applied at 3 kV and 6 kV, with the positive electrode held against the plant. For L. multiflorum the tip was set at the top of the basal sheath about 30 mm above the soil. For A. powellii and S. nigrum the tip was 50 mm above the soil. L. multiflorum had three plants in each of four bags per treatment plus the control. A. powellii and S. nigrum had one plant in each of six bags per treatment plus control (Table 2).

Table 2.
Trial summary table showing trial number, weed species, plant size, multiple electric pulse treatments applied and number of replicates.
2.5. Statistical Analysis
Data were statistically analysed using SPSS Version 28.0.1.0 (142). Where biomass differences are reported, the default for statistical comparisons of groups was the Independent Samples Kruskal–Wallis 1-way ANOVA (k samples) because almost all cases failed the ANOVA homogeneity of variance using Levene’s test. Homogenous subsets were determined from post hoc analyses and the step-wise step-down multiple comparisons. Following the recommendations of Armstrong [57] and Perneger [58], no multiple test (e.g., Bonferroni) corrections were applied. For L. multiflorum, biomass data were analysed with each bag of three grasses representing one replicate (n = 4), a sample size satisfactory for Kruskal–Wallis [59].
The dichotomous result “Dead” or “Not Dead” at the end of each experiment was analysed using binary logistic regression. Key independent factors considered for L. multiflorum modelling included set voltage, set energy, energy applied, soil moisture at treatment, and total leaf length. A. powellii and S. nigrum modelling included set voltage, set energy, energy applied, leaf number, shoot number and stem diameter. Those parameters that did not add to the model were ignored. C. album plants were assessed only for turgor and erectness. For the binary logit analysis of L. multiflorum, each individual plant was coded dead or alive and treated as a replicate (n = 12).
3. Results
Experiment 1: Single pulses
When L. multiflorum plants were treated with single 6 kV and 10 kV pulses (Trial 1), the fine leaves rapidly collapsed. In a few cases there was a smell of hot grass, and in one case steam was noted. All treated leaves were wilted and had turned grey at 1 DAT. The stem below the leaf collar was normal except for four 10 kV treated plants which had wilted to ground level, and eventually, three of these four plants died. No control plants died, showed signs of damage, or of senescence. Out of 12 plants treated with 6 and 10 kV, only two and four plants, respectively, died by the end of the trial (Table 3). Model coefficients (Chi-square, df and Sig. p), model summary (Nagelkerke R Square), percent correct prediction classification (cut value 0.500) and independent variables’ statistical significance Sig. and odds ratio Exp(B) for the trials are presented in Table 3.

Table 3.
Statistical summary table of binary logistic regression analyses of factors significantly predicting the probability of Lolium multiflorum and Chenopodium album weeds dying after receiving single pulse electric treatments.
When C. album plants were given single spark pulses (Trial 2), they slowly collapsed to lie prostrate within 30 s of treatment. After 24 h, 5 of 24 treated plants had regained turgor. At 28 DAT, those plants that recovered by 1 DAT were alive, and all but 2 of the remaining 19 plants were dead. All control plants survived and continued to grow. The treated plants showed high mortality with 9 of 12 plants treated with 6 kV and 8 of 12 plants treated with 10 kV dead by 28 DAT. Set voltage was a significant predictor of the likelihood of plants being killed by single pulse treatments, although this is confounded because the higher voltages also imparted much higher energy (Table 3).
Single-pulse treatments applied to L. multiflorum did not reliably kill all target plants but compared to controls, the Independent Samples Kruskal–Wallis Test showed that at higher energies the survivors generally had decreased biomass 15 DAT as expressed by longest leaf length, and total green leaf length (Table 4). In each case the significant difference was between the control and the 10 kV treatment.

Table 4.
Statistical Summary of Independent Samples Kruskal–Wallis Test for a range of biometric variables measured on Lolium multiflorum plants 15 DAT.
Experiment 2: Multiple pulses.
The multi-pulse equipment allowed lower energy pulses to apply the same total energy. When multiple pulse treatments were applied to L. multiflorum (Trial 3), all plants immediately but slowly collapsed, lying prostrate across the bags. All the treated plants and none of the control plants exhibited signs of wilting 1 DAT. Four DAT, 48% of treated plants exhibited leaf deformation with a concertina effect in tissue that was just inside the basal sheath when treatments were applied (Figure 3). The effect remained on plants that did not die (Figure 4). In some cases, emerging leaf tips were trapped in adjacent leaves, forming hoops as they elongated.

Figure 3.
Close-up of “concertina” leaf deformation 4 DAT on Lolium multiflorum plants treated with 5 J at 6 kV.

Figure 4.
Lolium multiflorum plants treated with 5 J at 6 kV shows characteristic leaf deformation still present 28 DAT.
The differences in total leaf number, tiller number, total leaf length, final dry mass and number of plants per bag that were alive 15 DAT between untreated control and treated plants were significant in all cases (p < 0.001) according to the Independent-Samples Kruskal–Wallis test. Post hoc pairwise comparisons showed three homogenous subsets of treatments for which the number of live plants 15 DAT was not significantly different. The 1 J treatment at 3 kV and the 1 J, 3 J and 5 J treatments at 6 kV were not significantly different to the control or each other. There was no significant difference in number of plants that were alive 15 DAT given 3 J at 3 kV or 7 J at 6 kV, or between those given 5 J or 7 J at 3 kV. Similar results with more separation were found for total plant dry mass at 15 DAT (Table 5).

Table 5.
Summary of treatments by applied voltage and energy on plant dry mass and number of live plants 15 DAT. Groups with the same letter in a column are homogenous subsets at p = 0.05 according to Kruskal–Wallis post hoc tests of pairwise comparisons.
Total plant dry weight 15 DAT shows the 3 kV treatments were more effective than 6 kV treatments of the same set energy level. No 6 kV treatments were fully effective, with 7 J treatment the best giving 62.5% death rate (Figure 5).
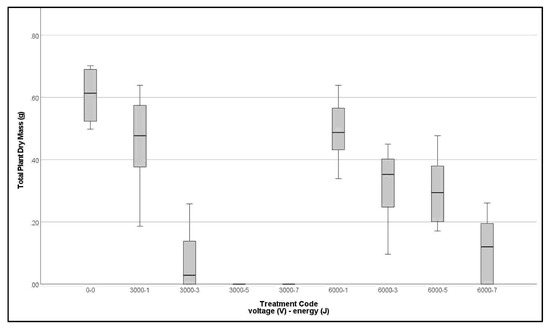
Figure 5.
Clustered boxplot of total plant dry weight per bag of Lolium multiflorum 15 days after treatment by applied voltage and target energy.
Final assessments were made 15 DAT when plants were either visibly alive and growing, or dead. Among the 3 kV treatments, 75% of plants receiving 3 J and all plants receiving 5 and 7 J died. No control plants died. Binary logit analysis showed a highly significant contribution of voltage, but the odds ratio Exp(B) indicates almost no change in effectiveness with voltage increase (Table 6, 3a). Investigation showed Exp(B) for voltage is confounded with the 0 kV control, because the 6 kV treatment was less effective than 3 kV. Analysis of only the treated plants, showed voltage to be a significant factor, with increasing voltage reducing the probability of death (Table 6, 3b).

Table 6.
Statistical summary table of binary logistic regression analyses of factors significantly predicting the probability of weeds dying after receiving multiple pulse electric treatments.
When applied to L. multiflorum plants, delivered energy at 3 kV was about 90% of the set energy (r2 = 0.97) but at 6 kV only 71% of set energy was delivered (R2 = 0.93). Thus the 7 J at 6 kV treatment only delivered about 5 J (Figure 6).
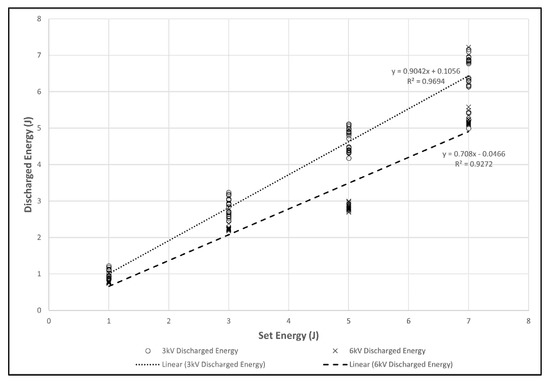
Figure 6.
Scatterplot of equipment set energy versus actual discharged energy by voltage applied to Lolium multiflorum.
All treated A. powellii plants (Trial 4) slowly collapsed after PMS were applied, except one plant treated with 1 J and one treated with 7 J which both remained erect. The 1 J plant had drooped 1 DAT but remained alive throughout. Another 1 J plant that initially drooped had recovered at 4 DAT and was healthy at the end of the trial. This plant absorbed half the energy of the rest of the 1 J treated plants. The erect 7 J plant remained healthy throughout. Electrical data show it received only a quarter of the mean energy of the rest of that treatment (Figure 7).
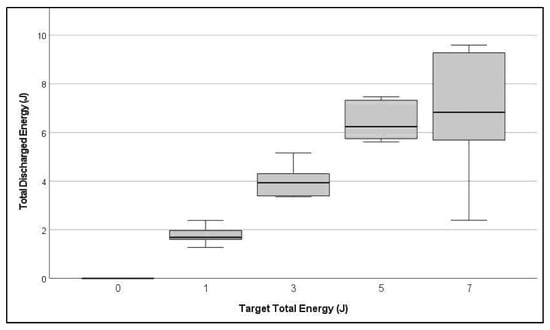
Figure 7.
Box plot of total discharged energy by target discharged energy show higher variance among the 7 J treated Amaranthus powellii plants.
Throughout the trial all A. powellii control plants remained erect and healthy and continued to grow. At 4 DAT, control plants had grown with height (166%), stem diameter (143%) and leaf area (215%) of size at treatment. Treated plants had decreased in size, with aggregated average results of height (62%), stem diameter (55%) and ELA leaf area (29%) of size at treatment. At 4 DAT, plants that collapsed after treatment showed severe chlorosis and wilting, with chlorosis beginning in interveinal leaf areas towards the tip of the leaf and spreading back over time to the stem to affect all leaves entirely (Figure 8).

Figure 8.
Amaranthus powellii leaf showing typical chlorosis following PMS treatment.
At 11 DAT, the treatments were significantly different to the control, but not to each other for stem diameter and ELA leaf area score (Table 7). At 11 DAT some plants in every treated set had died and only two of 24 were healthy. One had received only 25%, and one 48% of the energy of the rest of their treatment group. Even though some dead plants retained sections of green stem tissue, the stems had collapsed, and the hypocotyl region had decayed allowing the above ground parts to separate from roots with less than 10 mm of root attached.

Table 7.
Statistical Summary of Independent Samples Kruskal–Wallis Test for a range of biometric factors of A. powellii and S. nigrum plants 15 days after receiving multiple pulse electric treatments.
When S. nigrum plants were treated (Trial 5), the picoscope was connected between the plant base and one of the treatment electrodes. Comparing this with machine-recorded energy discharged allowed the relative energy absorption by the soil and the plant to be determined. This showed 63% (y = 0.63x + 0.06, R2 = 0.983) of discharged energy was absorbed by the above ground plant, the remainder by soil and/or roots (Figure 9).
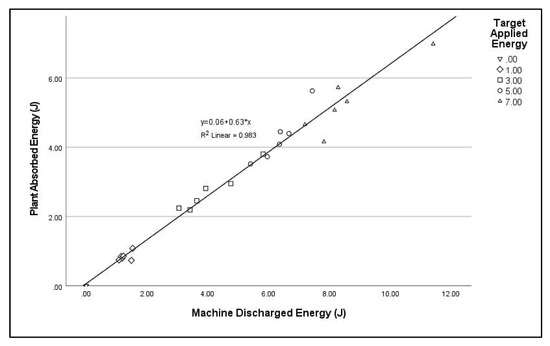
Figure 9.
Scatterplot of energy absorbed by above ground plant tissue when Solanum nigrum seedlings were treated with pulsed micro-shocks at 3.5 kV.
Control plants remained healthy and growing throughout the trial. At 8 DAT, one plant treated with 1 J and all other treated plants showed signs of main stem wilting or collapse, and curling leaves, with the degree of curling related to the energy applied, a trend that continued. At 14 DAT some treated plants remained viable with normally developing side shoots that had turgid stems and leaves. The prevalence was less as the applied energy increased. These plants were alive at the end of the trial, the rest of the treated plants were dead. At 30 DAT, final measurements were made. Treatments at 3 J or more were significantly different to the controls in all factors including final vitality, stem diameter, ELA Score and plant dry mass. Plant deaths increased as the energy applied increased with four times more plants dead when treated with 7 J than with 1 J.
4. Discussion
These experiments demonstrate the potential of PMS as an ultra-low energy treatment method for controlling a range of weed species at the seedling stage. Voltages have been kept below 10 kV, and the energy released by the equipment is low. In general, these would place the equipment used within the criteria acceptable for electric fence energisers [60,61]. The higher voltage treatments and higher individual pulse energy treatments appeared more able to impart sufficient energy to cause immediate plasmolysis of leaf tissue in fine L. multiflorum seedling leaves. This is not correlated with improved plant mortality and may reduce effectiveness. A possible explanation is that the fine leaf acts as an electrical fuse, preventing the current from impacting the rest of the plant.
The leaves of all treated L. multiflorum plants fell over within seconds of PMS application, indicating disruption of cell turgor. Wilting was not generally apparent in the sheath of the plant, but deformities originating in tissues within the sheath at time of treatment were commonly observed after the leaves emerged, the concertina effect suggesting some effect on expanding cells and the entrapped leaf tips suggesting disruption between adjacent developing leaves. While leaf deformation in tissues inside the main sheathed shoot shows PMS affected tissue development, the survival of stems and emergence of new tillers indicated insufficient energy to kill basal growing points.
Even where L. multiflorum plants are not killed, ultra-low energy treatment with PMS does have a limiting effect on growth significantly reducing growth rates for at least 2 weeks. This suggests potential to reduce competition against crop plants and may enable a strongly growing crop to get established and out-compete the grasses. There was wide variation in the growth of individual plants for any energy below 3 J. At 5 J and above all plants treated at 3 kV died, whereas many plants treated at 6 kV continued to grow, although at a reduced rate compared to the control plants. Such growth reduction may be sufficient to allow a rapidly developing crop to gain dominant competitive advantage but would not necessarily avoid weed seed bank contributions.
Several possibilities may have contributed to lower than expected energy delivery or reduced effect of a set energy level being applied. In some cases, electric arcing was observed between the positive electrode and the ground, sometimes running along the stem surface, sometimes directly through the air. This was particularly noted with 10 kV single pulses but occurred with other settings especially if the electrode was within 30 mm of the ground. In these cases, the energy discharge from the devices could be high, but the measured absorption by the plants could be very low. Multiple pulsing allows the energy of an individual pulse to be reduced but total delivered energy to be maintained. However, in these trials, longer pulse times at higher voltages (higher energy individual pulses) could still cause arcing. In the case of high energy and high voltage pulses to fine L. multiflorum leaves, this was probably due to higher resistance and lower conductance, especially when leaves collapsed. When multiple pulses were applied, especially when a longer duration was required, oscilloscope measurements indicated a probable loss of direct contact for some or all the time.
There was a demonstrated effect of UEW on broadleaved weeds even at low energy levels, with treatments significantly reducing vitality and growth relative to untreated control plants. Applying these treatments to C. album and A. powellii seedlings was largely effective, and the survival of those receiving the highest target energy treatment is assumed due to poor electrode contact. Although there was some immediate effect of treatment, the results of these trials consistently show an extended period before symptoms of senescence were expressed.
Early electrical weeding research suggested that resistive heating causes boiling within the tissue, causing the cells to rupture and the plant to die [62] although cells and vascular bundles can be damaged without boiling or burning [63]. The research reported here suggests for broadleaved plants especially that a different mechanism may be involved, as plants took some time to lose turgor and there was initially no external physical damage visible.
Those plants that drooped or collapsed did so slowly. The recovery of some plants after some hours indicates any membrane disruption was transitory and repairable. This suggests the effect was not caused by gross bursting of cells but indicates very porous rather than fully ruptured membranes.
Of particular interest is the evident onset of chlorosis and senescence, and the subsequent separation of shoot and root at the hypocotyl observed in A. powellii. The onset and progression of chlorosis indicates a physiological response was triggered in the treated plants. Plants may show no effect of treatment for several days to a week, and then take several weeks to die, usually expressing plant senescence. Broadleaved plant symptoms such as loss of leaf chlorophyll, browning and wilting of leaf and stems are similar to those associated with some herbicides. Various abiotic stresses have been reported to effect such a response through programmed cell death (PCD) triggered by pollutants, excessive light or UV radiation or environmental conditions including heat and cold [64], leading to over-production of reactive oxygen species (ROS) [65,66,67].
No treatment killed all S. nigrum plants, and death was not immediate, with several weeks passing before a confident assessment could be made. These plants were notable for being very fleshy, and for having multiple shoots at time of treatment. While the treatments of 3 J or higher killed the targeted shoot in 83% of cases, in others side shoots were not killed, or new side shoots appeared. This suggests the impact of the treatment was restricted to a section of stem and did not effectively impact the entire plant. There were significant reductions in growth associated with treatments of 3 J or over, which could potentially reduce their competitive ability against a developing crop, although this has not been assessed.
These trials demonstrate that very low energy, high voltage pulses of direct current can kill weed seedlings with far less energy than alternative weed control options, including currently available electrical weeding systems. While these trials were conducted in a laboratory setting, with precise field application the results should be replicable. This makes the system ideal for the treatment of herbicide resistant weeds that have escaped chemical or other control methods. The small energy requirement makes the system suitable for incorporation in a mobile hand-held weeding tool, or individually or in groups in a precision robotic application.
Future research will repeat these trials with other species and investigate effects of changing variables such as voltage, pulse period, pulse energy and number, plant part or parts targeted, soil conditions and time of year. Trials will be designed to develop better understanding of the mechanism of senescence and death.
Author Contributions
Conceptualization, D.J.B., K.C.H. and T.K.J.; methodology, D.J.B. and K.C.H.; validation, H.G. and K.C.H.; formal analysis, D.J.B.; investigation, D.J.B.; resources, D.J.B.; data curation, D.J.B.; writing—original draft preparation, D.J.B.; writing—review and editing, K.C.H. and H.G.; visualization, D.J.B.; supervision, K.C.H., H.G. and T.K.J.; project administration, D.J.B.; funding acquisition, T.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted as part of “Managing Herbicide Resistance”, funded by the New Zealand Ministry for Business, Innovation and Employment Endeavour Fund C10X & C10X1806, a research programme by AgResearch, Ltd., a crown research institute in New Zealand. The work contributes to material for submission as part of a PhD undertaken at Massey University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw data not contained within the article are available from Daniel Bloomer on request.
Acknowledgments
The authors acknowledge the work of Hamish Penny in developing and making available the equipment used to apply pulsed micro-shocks and record the electrical data and energies delivered. Phillipa Page helped with plant maintenance and data collection particularly during COVID-19 restrictions, and Bruce Searle provided helpful recommendations for statistical analyses.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ghanizadeh, H.; Harrington, K.C. Perspective: Root exudation of herbicides as a novel mode of herbicide resistance in weeds. Pest Manag. Sci. 2020, 76, 2543–2547. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2022. Available online: www.weedscience.com (accessed on 23 July 2022).
- Ngow, Z.; Chynoweth, R.J.; Gunnarsson, M.; Rolston, P.; Buddenhagen, C.E. A herbicide resistance risk assessment for weeds in wheat and barley crops in New Zealand. PLoS ONE 2020, 15, e0234771. [Google Scholar] [CrossRef]
- Crinnion, W.J. Organic Foods Contain Higher Levels of Certain Nutrients, Lower Levels of Pesticides, and May Provide Health Benefits for the Consumer. Environ. Med. 2010, 15, 9. [Google Scholar]
- Forbes, S.L.; Cohen, D.A.; Cullen, R.; Wratten, S.D.; Fountain, J. Consumer attitudes regarding environmentally sustainable wine: An exploratory study of the New Zealand marketplace. J. Clean. Prod. 2009, 17, 1195–1199. [Google Scholar] [CrossRef]
- Galati, A.; Schifani, G.; Crescimanno, M.; Migliore, G. “Natural wine” consumers and interest in label information: An analysis of willingness to pay in a new Italian wine market segment. J. Clean. Prod. 2019, 227, 405–413. [Google Scholar] [CrossRef]
- Hoek, A.C.; Pearson, D.; James, S.W.; Lawrence, M.A.; Friel, S. Shrinking the food-print: A qualitative study into consumer perceptions, experiences and attitudes towards healthy and environmentally friendly food behaviours. Appetite 2017, 108, 117–131. [Google Scholar] [CrossRef]
- Koch, S.; Epp, A.; Lohmann, M.; Bol, G.F. Pesticide Residues in Food: Attitudes, Beliefs, and Misconceptions among Conventional and Organic Consumers. J. Food Prot. 2017, 80, 2083–2089. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Arvola, A.; Hursti, U.-K.K.; Åberg, L.; Sjödén, P.-O. Choice of organic foods is related to perceived consequences for human health and to environmentally friendly behaviour. Appetite 2003, 40, 109–117. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Mahajan, G.; Chauhan, B.S. Nonconventional Weed Management Strategies for Modern Agriculture. Weed Sci. 2015, 63, 723–747. [Google Scholar] [CrossRef]
- Hageman, K.J.; Aebig, C.H.F.; Luong, K.H.; Kaserzon, S.L.; Wong, C.S.; Reeks, T.; Greenwood, M.; Macaulay, S.; Matthaei, C.D. Current-use pesticides in New Zealand streams: Comparing results from grab samples and three types of passive samplers. Environ. Pollut. 2019, 254, 112973. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Melander, B.; Munier-Jolain, N.; Charles, R.; Wirth, J.; Schwarz, J.; van der Weide, R.; Bonin, L.; Jensen, P.K.; Kudsk, P. European Perspectives on the Adoption of Nonchemical Weed Management in Reduced-Tillage Systems for Arable Crops. Weed Technol. 2013, 1, 231. [Google Scholar] [CrossRef]
- Reiser, D.; Sehsah, E.S.; Bumann, O.; Morhard, J.; Griepentrog, H.W. Development of an autonomous electric robot implement for intra-row weeding in vineyards. Agriculture 2019, 9, 18. [Google Scholar] [CrossRef]
- Bauer, M.V.; Marx, C.; Bauer, F.V.; Flury, D.M.; Ripken, T.; Streit, B. Thermal weed control technologies for conservation agriculture—A review. Weed Res. 2020, 60, 241–250. [Google Scholar] [CrossRef]
- Nørremark, M.; Sørensen, C.G.; Jorgensen, R.N. HortiBot: Comparison of Present and Future Phytotechnologies for Weed Control—Part III; ASABE: Portland, OR, USA, 2006. [Google Scholar]
- Sorensen, C.G.; Madsen, N.A.; Jacobsen, B.H. Organic farming scenarios: Operational analysis and costs of implementing innovative technologies. Biosyst. Eng. 2005, 91, 127–137. [Google Scholar] [CrossRef]
- Scheible, A. Apparatus for Exterminating Vegetation. US Patent 546,682, 24 September 1895. [Google Scholar]
- Sharp, A.A. Vegetation Exterminator. US Patent US492635A, 28 February 1893. [Google Scholar]
- Carr, E.R. Apparatus for Killing Weeds. US Patent US5600918A, 11 February 1997. [Google Scholar]
- Diprose, M.F. Apparatus and Method for Electrically Killing Plants. WO2016016627, 4 February 2016. [Google Scholar]
- Dykes, W.G. Plant Destruction Using Electricity. US Patent US4177603A, 1977. [Google Scholar]
- Opp, F.W.; Opp, W.H. Electric Weed-Killing Apparatus. US4428150A, 1 April 1952. [Google Scholar]
- Pluenneke, R.H.; Dykes, W.G. Method and Apparatus for Useng Electrical Current to Destroy Grasses and Weeds. 3,919,806, 18 November.
- Rona, S.A.; Valverde, B.; Mendes de Souza, D.T.; de Andrade Coutinho Filho, S. Weed Inactivation Device. EP3557750A1, 17 January 2019. Ceased. [Google Scholar]
- Diprose, M.F.; Benson, F.A. Electrical methods of killing plants. J. Agric. Eng. Res. 1984, 30, 197–209. [Google Scholar] [CrossRef]
- Diprose, M.F.; Fletcher, R.; Longden, P.C.; Champion, M.J. Use of electricity to control bolters in sugar beet (Beta vulgaris L.): A comparison of the electrothermal with chemical and mechanical cutting methods. Anwend. Von Elektrizität Zur Bekämpfung Von Schossern Zuckerrüben (Beta Vulgaris L.): Vgl. Der Elektrothermischen Methode Mit Der Chem. Und Dem Mech. Schneiden. 1985, 25, 53. [Google Scholar] [CrossRef]
- Lati, R.N.; Rosenfeld, L.; David, I.B.; Bechar, A. Power on! Low-energy electrophysical treatment is an effective new weed control approach. Pest Manag. Sci. 2021, 77, 4138–4147. [Google Scholar] [CrossRef]
- Lysakov, A.A.; Masyutina, G.V.; Rostova, A.T.; Eliseeva, A.A.; Lubentsov, V.F. Development of a Weeding Robot with Tubular Linear Electric Motors. IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012063. [Google Scholar] [CrossRef]
- Lehnhoff, E.A.; Neher, P.; Indacochea, A.; Beck, L. Electricity as an effective weed control tool in non-crop areas. Weed Res. 2022, 62, 149–159. [Google Scholar] [CrossRef]
- Vigneault, C.; Benoit, D.L.; McLaughlin, N.B. Energy aspects of weed electrocution. Rev. Weed Sci. 1990, 5, 15–26. [Google Scholar]
- McCool, C.; Beattie, J.; Firn, J.; Lehnert, C.; Kulk, J.; Bawden, O.; Russell, R.; Perez, T. Efficacy of Mechanical Weeding Tools: A Study Into Alternative Weed Management Strategies Enabled by Robotics. IEEE Robot. Autom. Lett. 2018, 3, 1184–1190. [Google Scholar] [CrossRef]
- Carley, J. Current Developments in Electrical Weeding. Lamma Show. 2021. Available online: https://www.lamma365.com/news/current-developments-in-electrical-weeding-116579 (accessed on 26 July 2022).
- Schneider, D. The Electric Weed-Zapper Renaissance—IEEE Spectrum. 2020. Available online: https://spectrum.ieee.org/the-electric-weed-zapper-renaissance (accessed on 8 August 2022).
- Vigneault, C.; Benoit, D.L. Electrical Weed Control: Theory and Applications. In Physical Control Methods in Plant Protection; Vincent, C., Panneton, B., Fleurat-Lessard, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Rootwave. RootWave Working with Small Robot Company to Create A Weed Zapping Autonomous Robot. Available online: https://rootwave.com/rootwave-working-with-small-robot-company-to-create-a-weed-zapping-autonomous-robot/ (accessed on 2 August 2021).
- Gerhards, R. Weed suppression ability and yield impact of living mulch in cereal crops. Agriculture 2018, 8, 39. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Wei, X. A review on weed detection using ground-based machine vision and image processing techniques. Comput. Electron. Agric. 2019, 158, 226–240. [Google Scholar] [CrossRef]
- Li, Y.; Al-Sarayreh, M.; Irie, K.; Hackell, D.; Bourdot, G.; Reis, M.M.; Ghamkhar, K. Identification of Weeds Based on Hyperspectral Imaging and Machine Learning. Front. Plant Sci. 2021, 11, 611622. [Google Scholar] [CrossRef] [PubMed]
- Coleman, G.; Salter, W.; Walsh, M. OpenWeedLocator (OWL): An open-source, low-cost device for fallow weed detection. Sci. Rep. 2022, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Muhammad, K.; Ahmad, I.; Ahmad, W.; Smith, M.L.; Smith, L.N.; Jain, D.K.; Wang, H.; Mehmood, I. Visual features based boosted classification of weeds for real-time selective herbicide sprayer systems. Comput. Ind. 2018, 98, 23–33. [Google Scholar] [CrossRef]
- Barber, A. New Zealand Fuel and Electricity Total Primary Energy and Life Cycle Greenhouse Gas Emission Factors; AgriLINK New Zealand Ltd.: Kumeu, New Zealand, 2010. [Google Scholar]
- Barber, A.; Lucock, D. Total Energy Indicators: Benchmarking Organic, Integrated and Conventional Sheep and Beef Farms; 06/07; The AgriBusiness Group: Lincoln, New Zealand, 2006. [Google Scholar]
- Coleman, G.R.Y.; Stead, A.; Rigter, M.P.; Xu, Z.; Johnson, D.; Brooker, G.M.; Sukkarieh, S.; Walsh, M.J. Using energy requirements to compare the suitability of alternative methods for broadcast and site-specific weed control. Weed Technol. 2019, 33, 633. [Google Scholar] [CrossRef]
- Kaufman, K.R.; Schaffner, L.W. Energy and Economics of Electrical Weed Control. Trans. ASAE 1982, 25, 297–300. [Google Scholar] [CrossRef]
- Judaev, I.V.; Brenina, T.P. The Definition of Electro Impulses Used in Weed Control. J. Agric. Sci. Belgrade 2008, 51, 37–44. [Google Scholar] [CrossRef]
- Merfield, C.N. Robotic weeding’s false dawn? Ten requirements for fully autonomous mechanical weed management. Weed Res. 2016, 56, 340–344. [Google Scholar] [CrossRef]
- Vigoureux, A. Results of trials carried out in Belgium in 1980 about killing weed beets by electric discharge. Meded. Van De Fac. Landbouwwet. Rijksuniv. Gent 1981, 46, 163–172. [Google Scholar]
- Diprose, M.F.; Hackam, R.; Benson, F.A. Weed control by high voltage electric shocks. In Proceedings of the British Crop Protection Conference-Weeds; Boots Company: Nottingham, UK, 1978; pp. 443–450. [Google Scholar]
- Baev, I.V.; Igor, Y.V. The Determination of the Most Effective Current Type for Electrical Damage of Plants. Indian J. Sci. Technol. 2017, 10, 1. [Google Scholar]
- Mizuno, A.; Tenma, T.; Yamano, N. Destruction of weeds by pulsed high voltage discharges. In Proceedings of the Conference Record of the 1990 IEEE Industry Applications Society Annual Meeting, Seattle, WA, USA, 7–12 October 1990; p. 720. [Google Scholar] [CrossRef]
- Mizuno, A.; Nagura, A.; Miyamoto, T.; Chakrabarti, A. A portable weed control device using high frequency AC voltage. In Proceedings of the Conference Record of the 1993 IEEE Industry Applications Conference Twenty-Eighth IAS Annual Meeting, Toronto, ON, Canada, 2–8 October 1993; p. 2000. [Google Scholar]
- Ejima, H.; Mizuno, A.; Nakazawa, M. Weeding and Sterilizing Device. JP2633751B2, 23 July 1997. [Google Scholar]
- Ejima, H.; Mizuno, A.; Nakazawa, M. Weeding and Sterilizing Device. JP2633750B2, 23 July 1997. [Google Scholar]
- Kimura, I.; Mizuno, A. Apparatus for Removing Weed. Patent JPH04148633A, 21 July 1992. [Google Scholar]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236. [Google Scholar] [CrossRef]
- Field, A.P. Discovering Statistics Using IBM SPSS Statistics: And Sex and Drugs and Rock ‘n’ Roll, 4th ed.; Sage: North Tyneside, UK, 2013. [Google Scholar]
- International Electrotechnical Commission. Household and Similar Electrical Appliances—Safety—Part 2-76: Particular Requirements for Electric Fence Energizers; International Electrotechnical Commission: Geneva, Switzerland, 2002; p. 43. [Google Scholar]
- Gallagher Group, L. Electric Fencing 101: Electric Fencing Systems Design, Installation & Maintenance; Gallagher North America: Riverside, MO, USA, 2022; Available online: https://store.am.gallagher.com/am/medias/Electric-Fencing-101-Manual-NA?context=bWFzdGVyfHN1cHBvcnR8NDA0NDY2NnxhcHBsaWNhdGlvbi9wZGZ8aDM5L2g2MS84ODYyMDg1NDE0OTQyL0VsZWN0cmljIEZlbmNpbmcgMTAxIE1hbnVhbCBOQXxiYzIzNzk3NWRlYjhlNWNhMDRlMTBhOGY3MmNiMjg0M2Q0MWE4ZDJlNTUwYjk5ZTc5NDMyM2IxMGIxNjYzNzc3 (accessed on 23 February 2022).
- Diprose, M.F.; Benson, F.A.; Hackam, R. Electrothermal control of weed beet and bolting sugar beet. Elektrothermische Bekämpfung Von Unkrautrüben Und Zucker-Rübenschossern 1980, 20, 311. [Google Scholar] [CrossRef]
- Eberius, M. Digital herbicides tackle weeds at the root. Outlooks Pest Manag. 2017, 28, 277–281. [Google Scholar] [CrossRef]
- Vartapetian, A.B.; Tuzhikov, A.I.; Chichkova, N.V.; Taliansky, M.; Wolpert, T.J. A plant alternative to animal caspases: Subtilisin-like proteases. Cell Death Differ. 2011, 18, 1289–1297. [Google Scholar] [CrossRef]
- Sedigheh, H.G.; Mortazavian, M.; Norouzian, D.; Atyabi, M.; Akbarzadeh, A.; Hasanpoor, K.; Ghorbani, M. Oxidative stress and leaf senescence. BMC Res. Notes 2011, 4, 477. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).