Differential Responses to Yellow-Rust Stress Assist in the Identification of Candidate Wheat (Triticum aestivum L.) Genotypes for Resistance Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Breeding Material, Experimental Design and Study Location
2.2. Collection of Data
2.3. Yellow Rust Co-Efficient of Infection
2.4. Morphological, Physiological and Yield Component Parameters
2.5. Determination of Chlorophyll and Carotenoids Content
- Chlorophyll-A (CHA) = (12.7 × OD663) − (2.69 × OD645) × 1000 mL × Shoot fresh weight (g)
- Chlorophyll-B (CHB) = (22.9 × OD645) − (4.69 × OD663) × 1000 mL × Shoot fresh weight (g)
- Total-Chlorophyll (TCH) = (2.02 × OD643) + (8.02 × OD663) × 1000 mL × Shoot fresh weight (g)
- Carotenoid content (CAD) = OD480 × 4
2.6. Statistical Analysis
2.6.1. Statistical Analysis of YR CI Data
2.6.2. Estimation of General and Specific Combining Abilities
2.6.3. Variances Components Estimation
2.6.4. Cluster Analysis for Classification of Genotypes
2.6.5. Among Group Variation of Hierarchical Classes
2.6.6. Factor and Correlation Analysis
3. Results
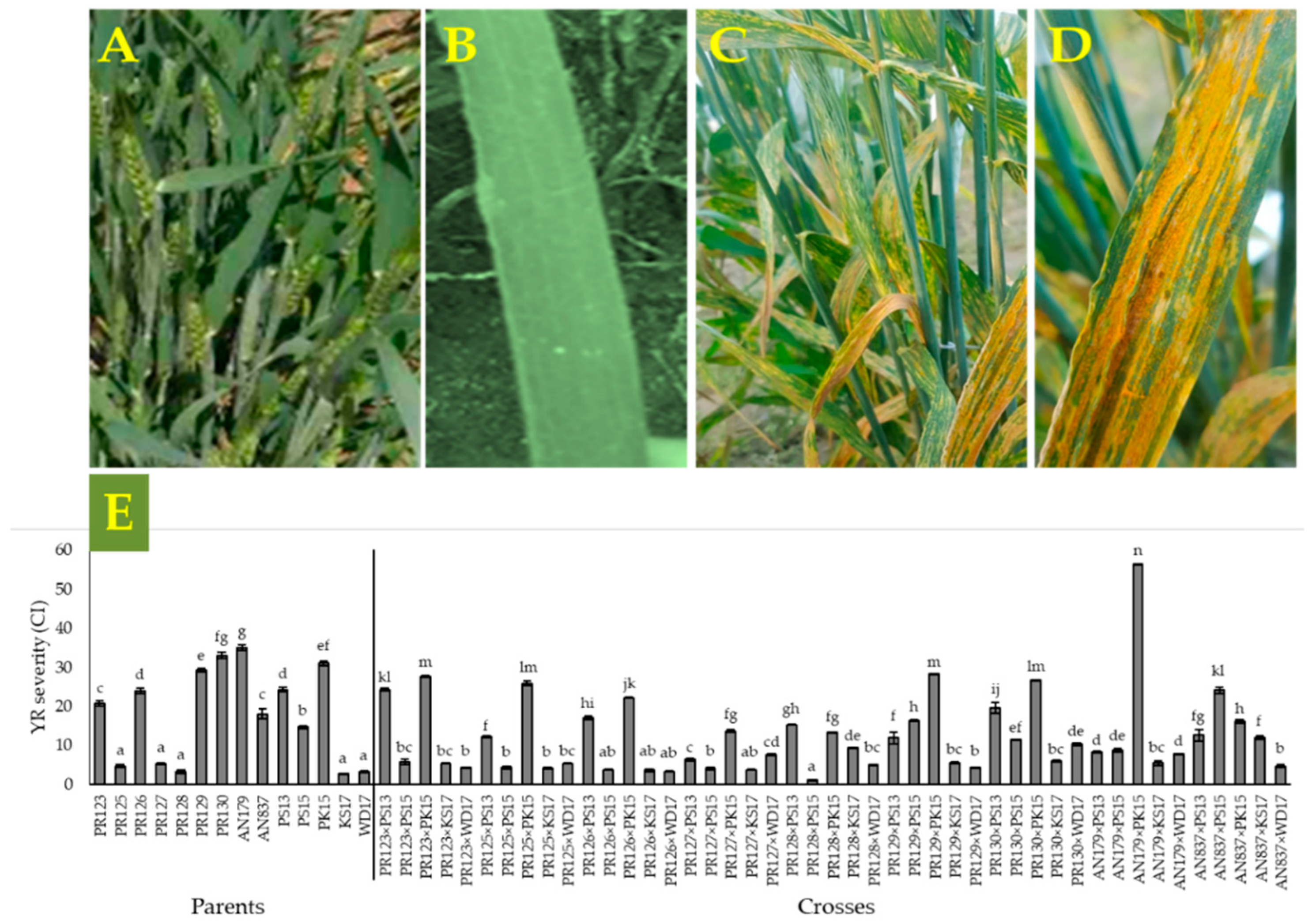
3.1. Yellow Rust Co-Efficient of Infection
3.2. Diversity among Genotypes
3.3. General Combining Ability Effects (GCA)
3.4. Specific Combining Ability Effects (SCA)
3.5. Variances and Broad Sense Heritability Estimation
3.6. Classification of Parents and Crosses
3.7. YR-Stress Significantly Affects Wheat Parameters under Study
3.8. Association Study through Principal Component Analysis
3.9. Correlation among Different Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (yellow) rust caused by Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Gebrewahid, T.W.; Zhang, P.; Zhou, Y.; Yan, X.; Xia, X.; He, Z.; Liu, D.; Li, Z. QTL mapping of adult plant resistance to stripe rust and leaf rust in a Fuyu 3/Zhengzhou 5389 wheat population. Crop J. 2020, 8, 655–665. [Google Scholar] [CrossRef]
- Chen, X. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Canad. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Mabrouk, O.I.; Fahim, M.A.; Omara, R.I. The Impact of Wheat Yellow Rust on Quantitative and Qualitative Grain Yield Losses under Egyptian Field Conditions. Egypt. J. Phytopathol. 2022, 50, 1–19. [Google Scholar] [CrossRef]
- Mishra, C.; Kumar, S.; Gupta, V.; Tiwari, V.; Sharma, I. Utilization of chlorophyll content index (CCI) to infer yellow rust severity in wheat (Triticum aestivum L.). J. Appl. Nat. Sci. 2015, 7, 38–42. [Google Scholar] [CrossRef]
- Simón, M.R.; Börner, A.; Struik, P.C. Fungal Wheat Diseases: Etiology, Breeding, and Integrated Management; Frontiers Media SA: Lausanne, Switzerland, 2021; Volume 12, p. 671060. [Google Scholar]
- Bouvet, L.; Holdgate, S.; James, L.; Thomas, J.; Mackay, I.J.; Cockram, J. The evolving battle between yellow rust and wheat: Implications for global food security. Theoret. Appl. Genet. 2021, 135, 741–753. [Google Scholar] [CrossRef]
- Sysoeva, M.I.; Markovskaya, E.F.; Shibaeva, T.G. Plants under continuous light: A review. Plant Stress 2010, 4, 5–17. [Google Scholar]
- Vergara-Diaz, O.; Kefauver, S.C.; Elazab, A.; Nieto-Taladriz, M.T.; Araus, J.L. Grain yield losses in yellow-rusted durum wheat estimated using digital and conventional parameters under field conditions. Crop J. 2015, 3, 200–210. [Google Scholar] [CrossRef]
- Singh, A.; Knox, R.; DePauw, R.; Singh, A.; Cuthbert, R.; Campbell, H.; Shorter, S.; Bhavani, S. Stripe rust and leaf rust resistance QTL mapping, epistatic interactions, and co-localization with stem rust resistance loci in spring wheat evaluated over three continents. Theor. Appl. Genet. 2014, 127, 2465–2477. [Google Scholar] [CrossRef]
- Abdulbagiyeva, S.; Zamanov, A.; Talai, J.; Allahverdiyev, T. Effect of rust disease on photosynthetic rate of wheat plant. J. Agric. Sci. Technol. B 2015, 5, 486–490. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.E.; Cui, J.M.; Su, Y.Q.; Yuan, S.; Yuan, M.; Zhang, H.Y. Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front. Plant Sci. 2015, 6, 779. [Google Scholar] [CrossRef]
- Suvi, W.T.; Shimelis, H.; Laing, M.; Mathew, I.; Shayanowako, A.I. Determining the combining ability and gene action for rice yellow mottle virus disease resistance and agronomic traits in rice (Oryza sativa L.). Agronomy 2020, 11, 12. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, S.; Sain, R. Heterosis studies for yield and its components in bread wheat over environments. Hereditas 2004, 141, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Jagger, L.; Newell, C.; Berry, S.; MacCormack, R.; Boyd, L. The genetic characterisation of stripe rust resistance in the German wheat cultivar Alcedo. Theor. Appl. Genet. 2011, 122, 723–733. [Google Scholar] [CrossRef]
- Kempthorne, O. An introduction to Genetic Statistics, John Wily and Nordskog; Chapman and Hall. Ltd.: London, UK, 1957. [Google Scholar]
- Joshi, S.; Sharma, S.; Singhania, D.; Sain, R. Genetic analysis of quantitative and quality traits under varying environmental conditions in bread wheat. Wheat Inform. Ser. (Jpn.) 2002, 95, 5–10. [Google Scholar]
- Acquaah, G. Principles of Plant Genetics and Breeding, 2nd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Pospíšilová, J. Schlegel, RHJ: Dictionary of Plant Breeding; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Pathan, A.K.; Park, R.F. Evaluation of seedling and adult plant resistance to leaf rust in European wheat cultivars. Euphytica 2006, 149, 327–342. [Google Scholar] [CrossRef]
- Peterson, R.F.; Campbell, A.; Hannah, A. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Canad. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Chowdhry, A.; Khan, I.; Bakhsh, A. Combining ability studies in bread wheat. Pak. J. Bot 1987, 19, 75–80. [Google Scholar]
- Dabholkar, A. Elements of Biometrical Genetics; revised And Enlarged Edition; Concept publishing company: New Delhi, India, 1999. [Google Scholar]
- SPSS. IBM SPSS Advanced Statistics 23.0; IBM Corporation: Armonk, NY, USA, 2014. [Google Scholar]
- Bridges, C.C., Jr. Hierarchical cluster analysis. Psychol. Rep. 1966, 18, 851–854. [Google Scholar] [CrossRef]
- Verma, J.P. One-Way ANOVA: Comparing means of more than two samples. In Data Analysis in Management with SPSS Software; Springer: Berlin/Heidelberg, Germany, 2013; pp. 221–254. [Google Scholar]
- Abdi, H.; Williams, L.J. Tukey’s honestly significant difference (HSD) test. Encycl. Res. Des. 2010, 3, 1–5. [Google Scholar]
- Liu, R.; Kuang, J.; Gong, Q.; Hou, X. Principal component regression analysis with SPSS. Comp. Method. Programs Biomed. 2003, 71, 141–147. [Google Scholar] [CrossRef]
- Van de Velden, M.; Kiers, H.A. Rotation in correspondence analysis. J. Classif. 2005, 22, 251–271. [Google Scholar] [CrossRef]
- Suhr, D.D. Principal component analysis vs. exploratory factor analysis. SUGI 30 Proc. 2005, 203, 230. [Google Scholar]
- Case, A.J.; Naruoka, Y.; Chen, X.; Garland-Campbell, K.A.; Zemetra, R.S.; Carter, A.H. Mapping stripe rust resistance in a Brundage×Coda winter wheat recombinant inbred line population. PLoS ONE 2014, 9, e91758. [Google Scholar] [CrossRef]
- Saeed, M.; Ullah, F.; Shah, L.; Ahmad, W.; Ali, M.; Munsif, F.; Zubair, A.; Ibrahim, M.; Shah, S.M.A.; Uddin, H. Identification of Three Novel QTLs Associated with Yellow Rust Resistance in Wheat (Triticum aestivum L.) Anong-179/Khaista-17 F2 Population. Sustainability 2022, 14, 7454. [Google Scholar] [CrossRef]
- Saeed, M.; Khalil, I.H. Combining ability and narrow-sense heritability in wheat (Triticum aestivum L.) under rainfed environment. Sarhad J. Agric. 2017, 33, 22–29. [Google Scholar] [CrossRef]
- Ahmad, E.; Akhtar, M.; Badoni, S.; Jaiswal, J. Combining ability studies for seed yield related attributes and quality parameters in bread wheat (Triticum aestivum L.). J. Genet. Genom. Plant Breed. 2017, 1, 21–27. [Google Scholar]
- Blambert, L.; Mallet, B.; Humeau, L.; Pailler, T. Reproductive patterns, genetic diversity and inbreeding depression in two closely related Jumellea species with contrasting patterns of commonness and distribution. Ann. Bot. 2016, 118, 93–103. [Google Scholar] [CrossRef]
- Verma, S.; Maurya, R.; Maurya, S. Prediction of gene action and combining ability for yield and quality traits in F1 and F2 generations of wheat (Triticum aestivum L.). Tropical Plant Res. 2016, 3, 449–459. [Google Scholar]
- Singh, D.P.; Singh, A.K.; Singh, A. Chapter 16—Population improvement. In Plant Breeding and Cultivar Development; Academic Press: Cambridge, MA, USA, 2021; pp. 305–330. [Google Scholar]
- Begna, T. Combining ability and heterosis in plant improvement. Open J. Plant Sci. 2021, 6, 108–117. [Google Scholar]
- Gulnaz, S.; Zulkiffal, M.; Sajjad, M.; Ahmed, J.; Musa, M.; Abdullah, M.; Ahsan, A.; Rehman, A. Identifying Pakistani wheat landraces as genetic resources for yield potential, heat tolerance and rust resistance. Int. J. Agric. Biol. 2019, 21, 520–526. [Google Scholar]
- Brinton, J.; Uauy, C. A reductionist approach to dissecting grain weight and yield in wheat. J. Integ. Plant Biol. 2019, 61, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.W.; Yang, L.; Yousaf, M.I.; Sami, A.; Mei, X.D.; Shah, L.; Rehman, S.; Xue, L.; Si, H.; Ma, C. Effects of heat stress on growth, physiology of plants, yield and grain quality of different spring wheat (Triticum aestivum L.) genotypes. Sustainability 2021, 13, 2972. [Google Scholar] [CrossRef]
- Farokhzadeh, S.; Fakheri, B.A.; Zinati, Z.; Tahmasebi, S. New selection strategies for determining the traits contributing to increased grain yield in wheat (Triticum aestivum L.) under aluminum stress. Genet. Resourc. Crop Evol. 2021, 68, 2061–2073. [Google Scholar] [CrossRef]
- Ashfaq, M.; Khan, A.S.; Ali, Z. Association of morphological traits with grain yield in wheat (Triticum aestivum L.). Int. J. Agric. Biol. 2003, 5, 262–264. [Google Scholar]
- Frih, B.; Oulmi, A.; Guendouz, A.; Bendada, H.; Selloum, S. Statistical analysis of the relationships between yield and yield components in some durum wheat (Triticum durum desf.) genotypes growing under semi-arid conditions. Int. J. Bio-Resour. Stress Manag. 2021, 12, 385–392. [Google Scholar] [CrossRef]
- Ali, Y.; Khan, A.A.; Ijaz, M.; Aatif, H.M.; Rahim, A.; Ahmad, S.; Sabir, W.; Naseer, S.; Rafiq, M. Characterization of environmental conditions conducive for leaf rust and genetic diversity on wheat crosses based upon physiomorphic traits. Pak. J. Phytopathol. 2021, 33, 125–136. [Google Scholar] [CrossRef]
- Xie, Q.; Sparkes, D.L. Dissecting the trade-off of grain number and size in wheat. Planta 2021, 254, 3. [Google Scholar] [CrossRef]



| S. No. | Parentage | Abbr. | S. No. | Parentage | Abbr. | S. No. | Parentage | Abbr. | |
|---|---|---|---|---|---|---|---|---|---|
| Parents | 1 | PR123 | L1 | 6 | PR129 | L6 | 11 | PS15 | T2 |
| 2 | PR125 | L2 | 7 | PR130 | L7 | 12 | PK15 | T3 | |
| 3 | PR126 | L3 | 8 | AN179 | L8 | 13 | KS17 | T4 | |
| 4 | PR127 | L4 | 9 | AN837 | L9 | 14 | WD17 | T5 | |
| 5 | PR128 | L5 | 10 | PS13 | T1 | ||||
| Crosses | 1 | PR123 × PS13 | L1 × T1 | 16 | PR127 × PS13 | L4 × T1 | 31 | PR130 × PS13 | L7 × T1 |
| 2 | PR123 × PS15 | L1 × T2 | 17 | PR127 × PS15 | L4 × T2 | 32 | PR130 × PS15 | L7 × T2 | |
| 3 | PR123 × PK15 | L1 × T3 | 18 | PR127 × PK15 | L4 × T3 | 33 | PR130 × PK15 | L7 × T3 | |
| 4 | PR123 × K17 | L1 × T4 | 19 | PR127 × KS17 | L4 × T4 | 34 | PR130 × KS17 | L7 × T4 | |
| 5 | PR123 × WD17 | L1 × T5 | 20 | PR127 × WD17 | L4 × T5 | 35 | PR130 × WD17 | L7 × T5 | |
| 6 | PR125 × PS13 | L2 × T1 | 21 | PR128 × PS13 | L5 × T1 | 36 | AN179 × PS13 | L8 × T1 | |
| 7 | PR125 × PS15 | L2 × T2 | 22 | PR128 × PS15 | L5 × T2 | 37 | AN179 × PS15 | L8 × T2 | |
| 8 | PR125 × PK15 | L2 × T3 | 23 | PR128 × PK15 | L5 × T3 | 38 | AN179 × PK15 | L8 × T3 | |
| 9 | PR125 × KS17 | L2 × T4 | 24 | PR128 × KS17 | L5 × T4 | 39 | AN179 × KS17 | L8 × T4 | |
| 10 | PR125 × WD17 | L2 × T5 | 25 | PR128 × WD17 | L5 × T5 | 40 | AN179 × WD17 | L8 × T5 | |
| 11 | PR126 × PS13 | L3 × T1 | 26 | PR129 × PS13 | L6 × T1 | 41 | AN837 × PS13 | L9 × T1 | |
| 12 | PR126 × PS15 | L3 × T2 | 27 | PR129 × PS15 | L6 × T2 | 42 | AN837 × PS15 | L9 × T2 | |
| 13 | PR126 × PK15 | L3 × T3 | 28 | PR129 × PK15 | L6 × T3 | 43 | AN837 × PK15 | L9 × T3 | |
| 14 | PR126 × KS17 | L3 × T4 | 29 | PR129 × KS17 | L6 × T4 | 44 | AN837 × KS17 | L9 × T4 | |
| 15 | PR126 × WD17 | L3 × T5 | 30 | PR129 × WD17 | L6 × T5 | 45 | AN837 × WD17 | L9 × T5 |
| Source of Var. | Degree of Freedom | Mean Square | F. Calculated-Value |
|---|---|---|---|
| Replicates (r) | (r − 1) = 2 | M1/M3 | |
| Lines (L) | L − 1 = 8 | MSq1 | σ2e + r(co-var. F-S − 2 × co-var. H-S) + (rT × co-var. H-S) |
| Testers (T) | T − 1 = 4 | MSq2 | σ2e + r(co-var. F-S − 2 × co-var. H-S) + (rL × co-var. H-S) |
| L × T | (L − 1)(T − 1) = 32 | MSq3 | σ2e + r(co-var. F-S − 2 × co-var. H-S) |
| Error | (r − 1)(L + T + LT) − 1 = 116 | MSq4 | σ2e |
| Total | (r)(L + T + LT) − L = 162 |
| MSE (Optimal) | SOV | DF | CTV | NDVI | PHT | FLA | TPP | SPL | PDL | GPS | TGW | GYD | CH A | CH B | TCH | CAD |
| Replicates | 2 | 0.53 ns | 0.031 ns | 25.8 ns | 25.3 ns | 3.3 ns | 1.6 ns | 7.8 ns | 57.2 ns | 11.5 ns | 612.6 ns | 1.07 ns | 0.94 ns | 3.64 ns | 0.04 ns | |
| Lines (L) | 8 | 1.50 ** | 0.036 ** | 379.8 ** | 26.6 ** | 3.8 ** | 4.5 ** | 111.3 ** | 219.0 ** | 126.7 ** | 41.3 ** | 196.7 ** | 56.6 ** | 454.5 ** | 7.8 ** | |
| Testers (T) | 4 | 0.14 ** | 0.081 ** | 500.3 ** | 15.4 ** | 3.2 ** | 2.4 ** | 168.9 ** | 178.0 ** | 57.3 ** | 98.2 ** | 60.2 ** | 27.9 ** | 166.0 ** | 4.7 ** | |
| L × T | 32 | 0.51 ns | 0.009 ** | 40.4 ** | 3.8 ** | 2.5 ** | 0.3 ** | 21.9 ** | 55.6 ** | 55.2 ** | 50.3 ** | 30.7 ** | 10.4 ** | 68.9 ** | 1.3 ** | |
| Error | 116 | 0.75 | 0.0001 | 9.0 | 26.6 | 1.2 | 0.12 | 2.2 | 18.47 | 10.78 | 21.50 | 0.7 | 0.2 | 0.8 | 0.01 | |
| MSE (YR-Stress) | SOV | DF | CTV | NDVI | PHT | FLA | TPP | SPL | PDL | GPS | TGW | GYD | CHA | CHB | TCH | CAD |
| Replicates | 2 | 1.06 ns | 0.022 ns | 41.8 ns | 38.7 ns | 7.47 | 1.24 ns | 9.1 ns | 106.8 ns | 48.7 ns | 1252.6 ns | 0.83 ns | 0.17 ns | 0.59 ns | 0.03 ns | |
| Lines (L) | 8 | 12.4 ** | 0.050 ** | 179.6 ** | 211.1 ** | 1.88 ** | 3.66 ** | 90.1 ** | 140.2 ** | 87.3 ** | 84.3 ** | 134.0 ** | 40.2 ** | 314.9 ** | 6.4 ** | |
| Testers (T) | 4 | 5.3 ** | 0.005 ** | 413.3 ** | 122.5 ** | 1.57 ** | 1.93 ** | 136.8 ** | 113.9 ** | 39.5 ** | 200.4 ** | 68.7 * | 19.3 ** | 157.8 ** | 3.9 ** | |
| L × T | 32 | 7.72 ** | 0.001 ** | 65.60 ** | 30.08 ** | 1.22 * | 0.24 ** | 17.77 ** | 35.59 ** | 38.05 ** | 102.7 ** | 23.85 ** | 7.06 ** | 50.08 ** | 1.02 ** | |
| Error | 116 | 1.36 | 0.022 | 6.97 | 7.77 | 1.10 | 0.15 | 1.76 | 10.51 | 13.92 | 45.42 | 0.52 | 0.18 | 0.93 | 0.03 |
| Condition | Type | CTV | PHT | FLA | TPP | SPL | PDL | GPS | TGW | GYD | CHA | CHB | TCH | CAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal | Lines | PR125 | PR125 | PR123 | ||||||||||
| PR126 | PR126 | |||||||||||||
| Testers | PS15 | PS15 | KS17 | PS15 | PS13 | PS15 | ||||||||
| Overall | Lines | PR130 | PR128 | PR123 | PR123 | PR128 | PR123 | PR127 | PR128 | PR128 | PR128 | PR128 | ||
| AN837 | PR129 | AN179 | PR126 | PR129 | PR126 | AN179 | AN179 | AN179 | AN179 | AN179 | ||||
| PR130 | PR128 | AN837 | AN837 | AN837 | AN837 | AN837 | ||||||||
| PR130 | ||||||||||||||
| Testers | PS13 | PS15 | PK15 | PS15 | PS13 | PS13 | PS13 | PS13 | ||||||
| KS17 | KS17 | KS17 | KS17 | KS17 | ||||||||||
| WD17 | WD17 | WD17 | WD17 | WD17 | WD17 | |||||||||
| YR-stress | Lines | PR126 | PR123 | PR128 | ||||||||||
| PR130 | AN179 | |||||||||||||
| AN837 | ||||||||||||||
| Testers | PK15 | WD17 | KS17 | WD17 |
| Condition | CTV | NDVI | PHT | FLA | TPP | SPL | PDL | GPS | TGW | GYD | CH A | CH B | TCH | CAD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal | L7 × T4 | L5 × T2 | L5 × T3 | L2 × T5 | L3 × T1 | L3 × T1 | L1 × T4 | L2 × T3 | ||||||
| L9 × T2 | L7 × T2 | L5 × T5 | L4 × T2 | L8 × T4 | L8 × T1 | L5 × T3 | ||||||||
| L9 × T1 | L8 × T5 | L8 × T5 | L6 × T3 | |||||||||||
| Overall | L2 × T4 | L1 × T4 | L2 × T3 | L1 × T2 | L2 × T4 | L2 × T4 | L1 × T4 | L5 × T3 | L3 × T4 | L1 × T2 | L1 × T3 | L1 × T3 | L1 × T3 | L1 × T3 |
| L2 × T2 | L5 × T3 | L3 × T1 | L3 × T5 | L3 × T1 | L6 × T4 | L4 × T3 | L1 × T3 | L2 × T1 | L2 × T1 | L2 × T1 | L2 × T1 | |||
| L2 × T4 | L8 × T1 | L8 × T1 | L5 × T1 | L8 × T1 | L6 × T1 | L2 × T4 | L3 × T2 | L2 × T3 | L3 × T2 | L3 × T2 | ||||
| L3 × T2 | L5 × T3 | L8 × T4 | L8 × T2 | L5 × T3 | L3 × T4 | L3 × T2 | L3 × T4 | L3 × T4 | ||||||
| L3 × T3 | L9 × T4 | L9 × T1 | L9 × T5 | L4 × T3 | L3 × T4 | L4 × T2 | L4 × T3 | |||||||
| L4 × T1 | L9 × T5 | L5 × T3 | L4 × T3 | L4 × T3 | L5 × T4 | |||||||||
| L5 × T2 | L5 × T4 | L5 × T3 | L5 × T4 | L6 × T5 | ||||||||||
| L5 × T4 | L6 × T5 | L5 × T4 | L6 × T5 | L9 × T2 | ||||||||||
| L6 × T4 | L7 × T3 | L6 × T4 | L7 × T3 | L9 × T4 | ||||||||||
| L6 × T5 | L8 × T1 | L6 × T5 | L9 × T2 | |||||||||||
| L7 × T1 | L9 × T2 | L7 × T3 | L9 × T4 | |||||||||||
| L8 × T1 | L9 × T4 | L9 × T2 | ||||||||||||
| L8 × T3 | L9 × T4 | |||||||||||||
| L9 × T5 | ||||||||||||||
| YR-stress | L5 × T1 | L3 × T4 | L4 × T3 | L8 × T5 | L9 × T2 | L2 × T4 | L2 × T3 | L8 × T4 | L7 × T1 | |||||
| L5 × T3 | L5 × T5 | L9 × T5 | ||||||||||||
| L6 × T2 | L7 × T1 | |||||||||||||
| L7 × T5 | L9 × T2 | |||||||||||||
| L8 × T3 |
| Traits | σ2gca (L) | σ2gca (T) | σ2gca (Av.) | σ2sca (C) | σ2A | σ2D | σ2gca/σ2sca | (σ2sca/σ2gca)1/2 | H2BS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Variances estimation (optimal) | CTV (°C) | 0.07 | 0.02 | 0.03 | 0.08 | 0.03 | 0.08 | 0.19 | 0.44 | 0.30 |
| NDVI % | 0.018 | 0.027 | 0.02 | 0.03 | 0.05 | 0.029 | 0.83 | 0.91 | 0.90 | |
| FLA (cm2) | 14.58 | 4.13 | 7.86 | 8.91 | 15.72 | 8.91 | 0.88 | 0.94 | 0.76 | |
| TPP | 0.09 | 0.03 | 0.05 | 0.43 | 0.10 | 0.43 | 0.11 | 0.34 | 0.40 | |
| SPL (cm) | 0.28 | 0.08 | 0.15 | 0.06 | 0.30 | 0.06 | 2.63 | 1.62 | 0.86 | |
| PDL (cm) | 5.96 | 5.44 | 5.63 | 6.59 | 11.25 | 6.59 | 0.85 | 0.92 | 0.90 | |
| GPS | 10.90 | 4.53 | 6.81 | 12.38 | 13.61 | 12.38 | 0.55 | 0.74 | 0.68 | |
| TGW(g) | 4.76 | 0.08 | 1.75 | 14.82 | 3.50 | 14.82 | 0.12 | 0.34 | 0.69 | |
| GYP (g) | 1.23 | 3.62 | 1.89 | 19.09 | 3.78 | 19.09 | 0.10 | 0.31 | 0.78 | |
| PHT(cm) | 22.63 | 17.03 | 19.03 | 10.48 | 38.06 | 10.48 | 1.82 | 1.35 | 0.91 | |
| CHA | 11.07 | 1.09 | 4.65 | 10.01 | 9.31 | 10.01 | 0.47 | 0.68 | 0.90 | |
| CHB | 3.08 | 0.65 | 1.52 | 3.38 | 3.04 | 3.38 | 0.45 | 0.67 | 0.95 | |
| TCH | 25.70 | 3.60 | 11.49 | 22.7 | 22.99 | 22.7 | 0.51 | 0.71 | 0.96 | |
| CAD | 0.44 | 0.128 | 0.24 | 0.41 | 0.48 | 0.41 | 0.58 | 0.76 | 0.90 | |
| Variances estimation (YR-Stress) | CTV (°C) | 0.31 | 0.09 | 0.19 | 2.12 | 0.11 | 2.12 | 0.03 | 0.16 | 0.62 |
| NDVI % | 0.011 | 0.017 | 0.029 | 0.02 | 0.03 | 0.017 | 0.85 | 0.92 | 0.95 | |
| FLA (cm2) | 12.07 | 3.42 | 0.88 | 7.43 | 13.02 | 7.43 | 0.88 | 0.94 | 0.77 | |
| TPP | 0.04 | 0.013 | 0.024 | 0.23 | 0.048 | 0.23 | 0.10 | 0.32 | 0.48 | |
| SPL (cm) | 0.23 | 0.06 | 0.12 | 0.05 | 0.24 | 0.05 | 2.54 | 1.59 | 0.89 | |
| PDL (cm) | 4.83 | 4.41 | 4.56 | 5.34 | 9.12 | 5.34 | 0.85 | 0.92 | 0.92 | |
| GPS | 6.97 | 2.90 | 4.36 | 8.36 | 8.71 | 8.36 | 0.52 | 0.72 | 0.71 | |
| TGW(g) | 3.28 | 0.05 | 1.21 | 8.04 | 2.41 | 8.04 | 0.15 | 0.39 | 0.51 | |
| GYP (g) | −0.60 | 1.77 | 0.93 | 11.05 | 1.85 | 11.05 | 0.08 | 0.29 | 0.65 | |
| PHT(cm) | 7.60 | 12.88 | 10.99 | 19.54 | 21.99 | 19.54 | 0.56 | 0.75 | 0.91 | |
| CHA | 7.34 | 1.66 | 3.69 | 7.78 | 7.38 | 7.78 | 0.47 | 0.69 | 0.97 | |
| CHB | 2.21 | 0.45 | 1.08 | 2.29 | 2.16 | 2.29 | 0.48 | 0.70 | 0.95 | |
| TCH | 17.66 | 3.99 | 8.87 | 16.38 | 17.74 | 16.38 | 0.54 | 0.74 | 0.98 | |
| CAD | 0.36 | 0.11 | 0.20 | 0.33 | 0.39 | 0.33 | 0.59 | 0.77 | 0.97 |
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Traits | Optimal | YR-Stress | F-Value | ||||||||
| Class-1 | Class-2 | Class-1 | Class-2 | Class-3 | |||||||
| CTV | 18.6 ± 0.17 a | 18.7 ± 0.17 ab | 20.6 ± 0.59 b | 20.4 ± 0.77 ab | 20.9 ± 1.08b | 4.6 ** | |||||
| NDVI | 0.7 ± 0.02 bc | 0.8 ± 0.02 c | 0.5 ± 0.02 a | 0.6 ± 0.02 a | 0.6 ± 0.02 ab | 14.2 *** | |||||
| PLH | 87.5 ± 4.41 ab | 104.3 ± 3.01 b | 84.3 ± 5.19 ab | 75.4 ± 6.27 a | 93.6 ± 4.65 ab | 3.6 * | |||||
| FLA | 37.1 ± 2.60 | 34.9 ± 3.01 | 36.7 ± 2.45 | 27.1 ± 1.71 | 33.6 ± 3.27 | 1.4 ns | |||||
| PDL | 31.2 ± 1.58 ab | 38.0 ± 2.09 b | 30.8 ± 0.46 ab | 24.6 ± 3.45 a | 35.8 ± 2.22 b | 5.8 ** | |||||
| TPP | 6.9 ± 0.50 b | 6.9 ± 0.50 b | 6.0 ± 0.34 ab | 4.6 ± 0.64 a | 5.4 ± 0.51 ab | 3.0 * | |||||
| SPL | 10.9 ± 0.46 bc | 11.5 ± 0.47 c | 9.4 ± 0.21 b | 7.4 ± 0.31 a | 9.8 ± 0.20 bc | 10.1 *** | |||||
| GPS | 51.3 ± 2.73 bc | 57.8 ± 5.15 c | 43.2 ± 1.55 ab | 31.1 ± 0.87 a | 49.3 ± 2.38 bc | 7.4 ** | |||||
| TGW | 45.3 ± 1.84 b | 42.0 ± 3.66 bc | 35.5 ± 0.80 ab | 45.4 ± 1.06 b | 31.9 ± 1.09 a | 7.8 *** | |||||
| GYP | 30.2 ± 2.79 b | 34.6 ± 3.97 b | 25.9 ± 1.10 ab | 15.6 ± 3.72 a | 28.0 ± 3.31 ab | 3.8 * | |||||
| CHA | 6.53 ± 0.78 a | 15.7 ± 0.99 b | 4.7 ± 0.62 a | 8.2 ± 2.73 a | 13.6 ± 1.44 b | 19.0 *** | |||||
| CHB | 3.8 ± 0.52 a | 8.6 ± 1.10 c | 2.6 ± 0.40 a | 4.8 ± 1.05 ab | 7.4 ± 0.93 bc | 11.9 *** | |||||
| TCH | 10.1 ± 1.14 a | 24.3 ± 1.76 b | 7.2 ± 0.81 a | 13.0 ± 3.27 a | 21.0 ± 2.26 b | 22.0 *** | |||||
| CAD | 1.6 ± 0.31 | 2.5 ± 0.38 | 1.4 ± 0.35 | 2.1 ± 0.58 | 2.2 ± 0.44 | 1.4 ns | |||||
| |||||||||||
| Traits | Optimal | YR-Stress | F-value | ||||||||
| Class-1 | Class-2 | Class-3 | Class-1 | Class-2 | Class-3 | Class-4 | |||||
| CTV | 18.4 ± 0.09 a | 18.5 ± 0.08 a | 18.8 ± 0.58a | 20.9 ± 0.27 b | 20.1 ± 0.57 ab | 21.5 ± 0.82 b | 20.3 ± 1.02 ab | 15.6 *** | |||
| NDVI | 0.6 ± 0.01 c | 0.6 ± 0.02 c | 0.5 ± 0.01b | 0.5 ± 0.01 b | 0.5 ± 0.01 b | 0.5 ± 0.02 b | 0.3 ± 0.01 a | 25.3 *** | |||
| PLH | 102.0 ± 1.16 b | 101.8 ± 2.01 b | 87.6 ± 2.14a | 91.2 ± 0.98 a | 93.0 ± 1.71 ab | 92.3 ± 3.53 ab | 84.5 ± 3.76 a | 12.7 *** | |||
| FLA | 38.8 ± 0.99 | 35.8 ± 1.26 | 35.8 ± 1.63 | 35.0 ± 0.86 | 33.3 ± 3.32 | 33.1 ± 1.29 | 32.6 ± 1.48 | 2.8 ns | |||
| TPP | 7.3 ± 0.16 d | 7.0 ± 0.30 cd | 5.8 ± 0.31 bc | 5.1 ± 0.11 ab | 4.5 ± 0.13 ab | 5.2 ± 0.31 ab | 4.0 ± 0.21 a | 31.4 *** | |||
| SPL | 12.1 ± 0.10 d | 11.4 ± 0.16 cd | 11.5 ± 0.22 cd | 10.8 ± 0.09 abc | 10.9 ± 0.16 bc | 10.0 ± 0.18 a | 10.3 ± 0.19 ab | 25.7 *** | |||
| PDL | 36.7 ± 0.69 b | 35.4 ± 1.04 b | 25.1 ± 0.47 a | 32.7 ± 0.54 b | 33.3 ± 3.12 b | 32.2 ± 0.83 b | 22.6 ± 0.42 a | 15.0 *** | |||
| GPS | 52.2 ± 0.92 e | 51.4 ± 1.34 de | 33.5 ± 2.66 ab | 41.6 ± 0.69 c | 44.8 ± 1.91 cd | 39.4 ± 1.24 bc | 26.8 ± 2.13 a | 37.6 *** | |||
| TGW | 46.0 ± 0.79 bc | 46.2 ± 1.12 bc | 61.1 ± 6.40 d | 38.2 ± 0.66 a | 36.3 ± 1.06 a | 39.7 ± 1.14 ab | 50.7 ± 5.31 c | 20.9 *** | |||
| CHA | 5.8 ± 0.29 a | 13.3 ± 1.33 b | 15.3 ± 0.08 b | 4.5 ± 0.27 a | 8.2 ± 0.51 a | 13.5 ± 1.00 b | 13.2 ± 0.17 b | 43.3 *** | |||
| CHB | 2.5 ± 0.22 ab | 6.7 ± 0.71 d | 6.3 ± 0.01 cd | 1.8 ± 0.15 a | 4.2 ± 0.50 bc | 6.7 ± 0.56 d | 4.8 ± 0.23 cd | 32.3 *** | |||
| TCH | 8.3 ± 0.47 ab | 20.0 ± 1.95 c | 21.7 ± 0.08 c | 6.4 ± 0.38 a | 12.3 ± 0.61 b | 20.2 ± 1.38 c | 18.1 ± 0.37 c | 45.9 *** | |||
| CAD | 0.9 ± 0.07 ab | 2.6 ± 0.22 c | 2.9 ± 0.04 c | 0.8 ± 0.06 a | 1.5 ± 0.31 b | 2.7 ± 0.11 c | 2.6 ± 0.06 c | 46.8 *** | |||
| GYP | 42.9 ± 0.95 d | 41.9 ± 1.83 d | 28.3 ± 2.03 c | 29.0 ± 0.76 c | 28.1 ± 1.72 ab | 36.2 ± 2.55 cd | 19.8 ± 1.42 a | 31.0 *** | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Ahmad, W.; Ibrahim, M.; Khan, M.; Ullah, F.; Bari, A.; Ali, S.; Shah, L.; Ali, M.; Munsif, F.; et al. Differential Responses to Yellow-Rust Stress Assist in the Identification of Candidate Wheat (Triticum aestivum L.) Genotypes for Resistance Breeding. Agronomy 2022, 12, 2038. https://doi.org/10.3390/agronomy12092038
Saeed M, Ahmad W, Ibrahim M, Khan M, Ullah F, Bari A, Ali S, Shah L, Ali M, Munsif F, et al. Differential Responses to Yellow-Rust Stress Assist in the Identification of Candidate Wheat (Triticum aestivum L.) Genotypes for Resistance Breeding. Agronomy. 2022; 12(9):2038. https://doi.org/10.3390/agronomy12092038
Chicago/Turabian StyleSaeed, Muhammad, Waqas Ahmad, Muhammad Ibrahim, Majid Khan, Farhan Ullah, Abdul Bari, Sartaj Ali, Liaqat Shah, Murad Ali, Fazal Munsif, and et al. 2022. "Differential Responses to Yellow-Rust Stress Assist in the Identification of Candidate Wheat (Triticum aestivum L.) Genotypes for Resistance Breeding" Agronomy 12, no. 9: 2038. https://doi.org/10.3390/agronomy12092038
APA StyleSaeed, M., Ahmad, W., Ibrahim, M., Khan, M., Ullah, F., Bari, A., Ali, S., Shah, L., Ali, M., Munsif, F., Zubair, A., Shah, S. M. A., Lu, J., Si, H., & Ma, C. (2022). Differential Responses to Yellow-Rust Stress Assist in the Identification of Candidate Wheat (Triticum aestivum L.) Genotypes for Resistance Breeding. Agronomy, 12(9), 2038. https://doi.org/10.3390/agronomy12092038




