The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis sativus L.) in High-Wire Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. The Method of Choosing the Leaves to Be Investigated
2.3. Light and Transmission Electron Microscopy Investigations
2.4. Confocal Laser Scanning Microscopy Investigation and Colocalisation Analysis
- Plan-apochromat objective 63× was used to reduce chromatic shift;
- Images were acquired by sequential scanning to minimalise bleed-through effect;
- To avoid saturation, the brightness of red and green channels was adjusted by gain to avoid saturated pixels;
- To improve the accuracy of the measurements, the background of images was reduced by an appropriate adjustment of the gain and offset of the detector, three-times filtered (line filtered) to minimalise randomness in fluorescence intensity;
- Chloroplast regions with well visible grana in their upper view were selected as regions of interest (ROIs) to perform Pearson correlation coefficient (r) measurements.
Visualisation of Colocalisation
2.5. SPAD Index
2.6. Chlorophyll Fluorescence
2.7. Analysis of Chosen Components in the Leaves
2.7.1. Dry Matter
2.7.2. Chlorophyll and Carotenoid Content
2.7.3. Total Soluble Solids and Total Sugars
2.7.4. Nitrate Reductase Activity
2.8. Statistical Analysis
3. Results
3.1. Leaf Structure
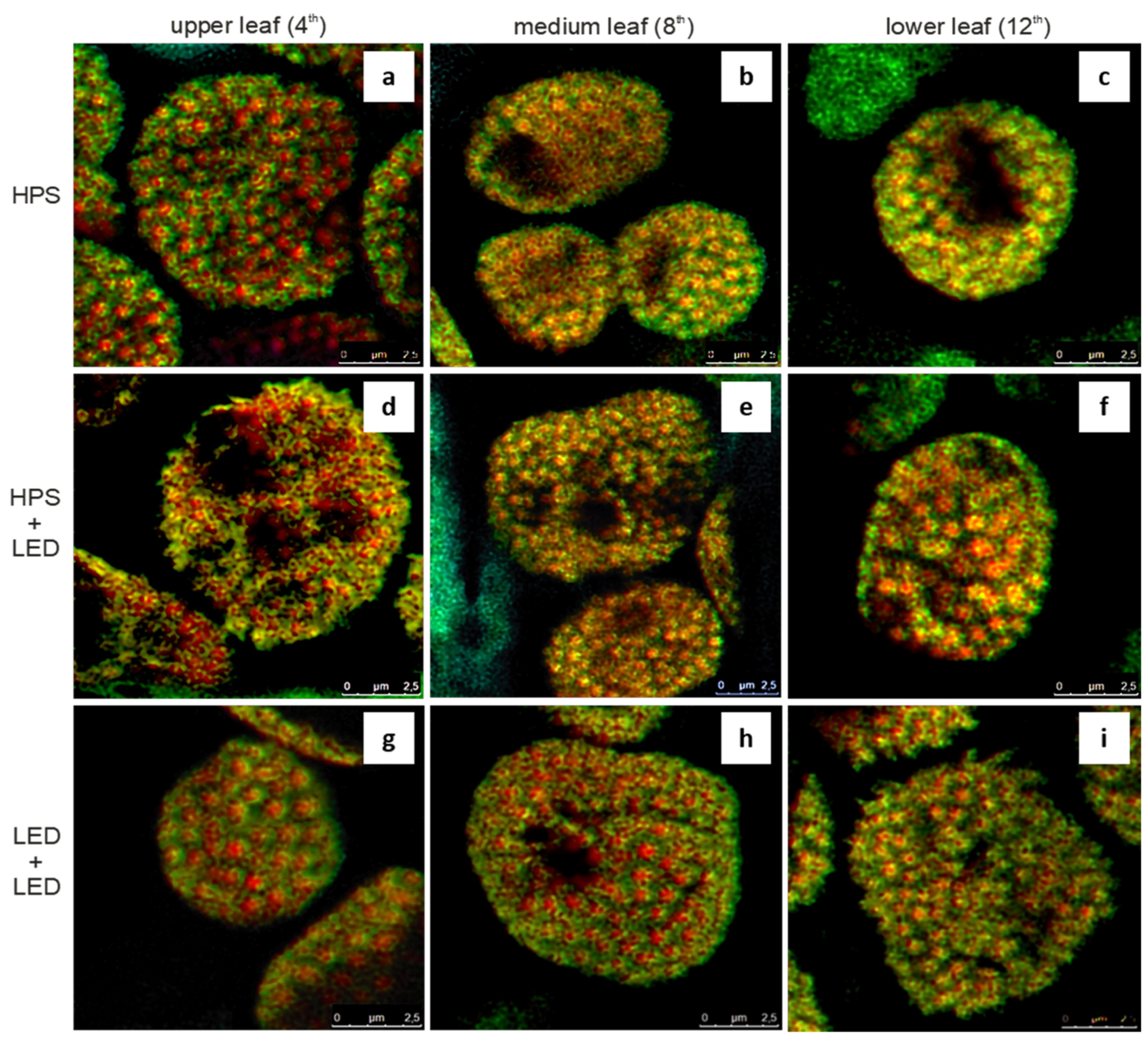
3.2. Colocalisation of Chlorophyll and Carotenoid Fluorescence Signals
3.3. Statistics for Data from the Confocal Microscope
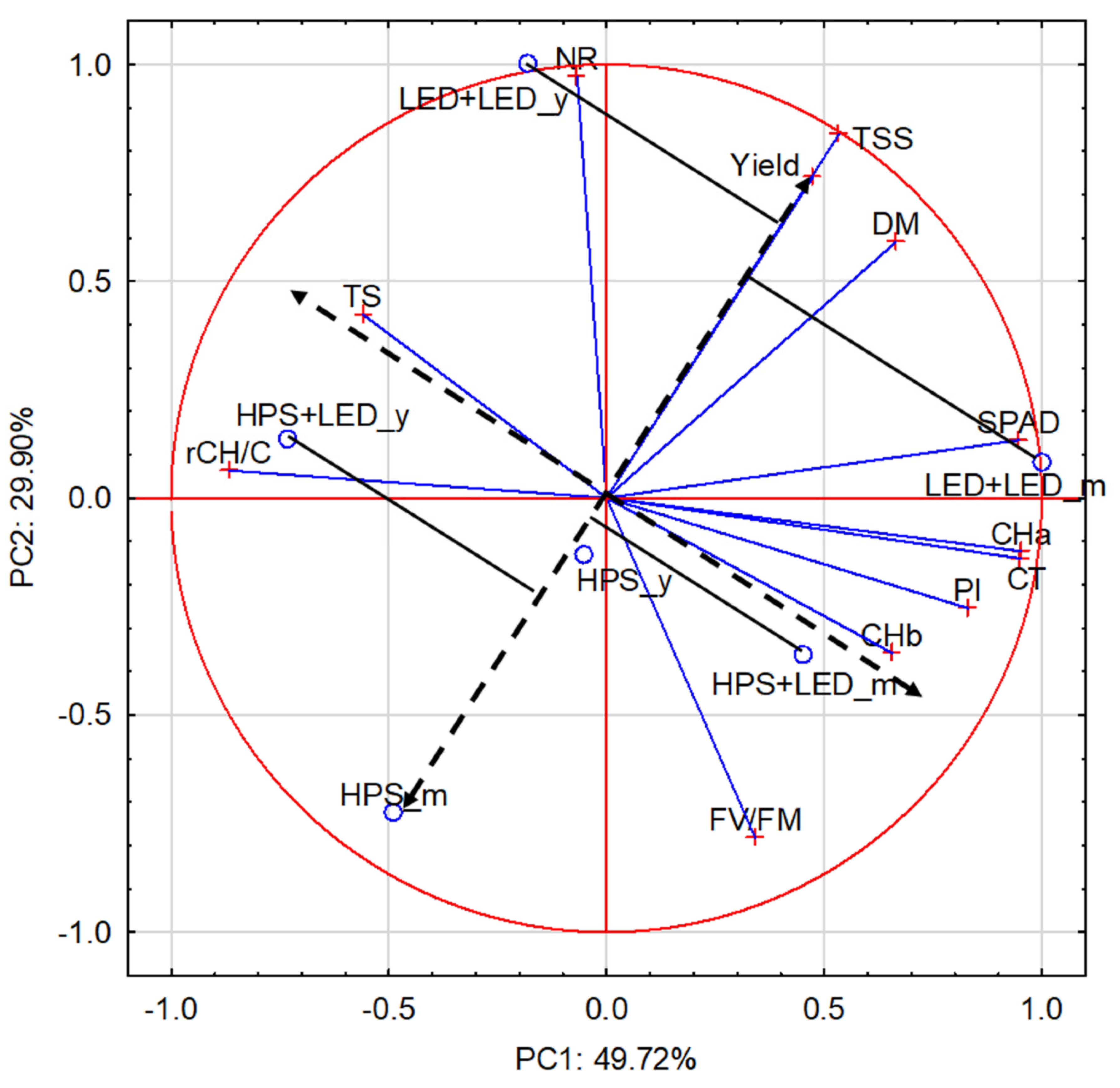
3.4. The Principal Component Analysis for the Relationships between Experiment Factors, Leaf Quality Parameters and Fruit Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 August 2022).
- Powierzchnia Uprawy i Produkcja Warzyw Pod Osłonami w 2020 r. Available online: https://www.podoslonami.pl/uprawy-i-odmiany/warzywa/powierzchnia-uprawy-i-produkcja-warzyw-pod-oslonami-2020-r/ (accessed on 12 August 2022).
- Statistics Poland. Production of Agricultural and Horticultural Crops in 2021. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/agricultural-and-horticultural-crops/production-of-agricultural-and-horticultural-crops-in-2021,2,6.html (accessed on 12 August 2022).
- Murad, H.; Nyc, M.A. Evaluating the potential benefits of cucumbers for improved health and skin care. J. Aging Res. Clin. Pract. 2016, 5, 139–141. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rayalu, S. Health beneficial effects of cucumber. In Cucumber Economic Values and Its Cultivation and Breeding, 1st ed.; Wang, H., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- De Bossoreille de Ribou, S.; Douam, F.; Hamant, O.; Frohlich, M.W.; Negrutiu, I. Plant science and agricultural productivity: Why are we hitting the yield ceiling? Plant Sci. 2013, 210, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Papadopoulos, A.P.; Khosla, S. Development of a high-wire cucumber production system on raised-troughs with supplemental lighting for year-round production. Acta Hortic. 2007, 761, 337–340. [Google Scholar] [CrossRef]
- Hovi, T.; Näkkilä, J.; Tahvonen, R. Interlighting improves production of year-round cucumber. Sci. Hortic. 2004, 102, 283–294. [Google Scholar] [CrossRef]
- Hovi-Pekkanen, T.; Tahvonen, R. Effects of interlighting on yield and external fruit quality in year-round cultivated cucumber. Sci. Hortic. 2008, 116, 152–161. [Google Scholar] [CrossRef]
- Guo, X.; Hao, X.; Zheng, J.M.; Little, C.; Khosla, S. Response of greenhouse mini-cucumber to different vertical spectra of LED lighting under overhead high pressure sodium and plasma lighting. Acta Hortic. 2016, 1134, 87–94. [Google Scholar] [CrossRef]
- Särkkä, L.E.; Jokinen, K.; Ottosen, C.-O.; Kaukoranta, T. Effects of HPS and LED lighting on cucumber leaf photosynthesis, light quality penetration and temperature in the canopy, plant morphology and yield. Agric. Food Sci. 2017, 26, 101–109. [Google Scholar] [CrossRef]
- Ouzounis, T.; Rosenqvist, E. Spectral effects of artificial light on plant physiology and secondary metabolism: A Review. HortScience 2015, 50, 1128–1135. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Gómez, C.; Izzo, L.G. Increasing efficiency of crop production with LEDs. AIMS Agric. Food 2018, 3, 135–153. [Google Scholar] [CrossRef]
- Kumar, K.G.S.; Hao, X.; Khosla, S.; Guo, X.; Bennett, N. Comparison of HPS lighting and hybrid lighting with top HPS and intracanopy LED lighting for high-wire mini-cucumber production. Acta Hortic. 2016, 1134, 111–118. [Google Scholar] [CrossRef]
- Hasan, M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of Calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B Biol. 2009, 96, 30–37. [Google Scholar] [CrossRef]
- Trouwborst, G.; Hogewoning, S.W.; van Kooten, O.; Harbinson, J.; van Ieperen, W. Plasticity of photosynthesis after the ‘red light syndrome’ in cucumber. Environ. Exp. Bot. 2016, 121, 75–82. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on the photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2016, 55, 85–95. [Google Scholar] [CrossRef]
- Zou, J.; Zhou, C.; Xu, H.; Cheng, R.; Yang, Q.; Li, T. The effect of artificial solar spectrum on growth of cucumber and lettuce under controlled environment. J. Integr. Agric. 2020, 19, 2027–2034. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Liu, X.-Y.; Xu, Z.-G.; Jiao, X.-L. Effects of light intensity on leaf microstructure and growth of rape seedlings cultivated under a combination of red and blue LEDs. J. Integr. Agric. 2017, 16, 97–105. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest and postharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar] [CrossRef]
- Pavlova, T.; Stamatovska, V.; Dimov, I.; Nakov, G.; Kalevska, T. Carotenoids in foods. ARTTE 2018, 6, 321–328. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Sakalauskienė, S.; Samuolienė, G.; Jankauskienė, J.; Viršilė, A.; Novičkovas, A.; Sirtautas, R.; Miliauskienė, J.; Vaštakaitė, V.; Dabašinskas, L.; et al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015, 173, 600–606. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Liu, L.; Shao, Z.; Zhang, M.; Wang, Q. Regulation of carotenoid metabolism in tomato. Mol. Plant 2015, 8, 28–39. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 468–485. [Google Scholar] [CrossRef]
- Choudhury, N.K.; Behera, R.K. Photoinhibition of photosynthesis: Role of carotenoids in photoprotection of chloroplast constituents. Photosynthetica 2001, 39, 481–488. [Google Scholar] [CrossRef]
- Gall, A.; Berera, R.; Alexandre, M.T.A.; Pascal, A.A.; Bordes, L.; Mendes-Pinto, M.M.; Andrianambinintsoa, S.; Stoitchkova, K.V.; Marin, A.; Valkunas, L.; et al. Molecular adaptation of photoprotection: Triplet states in light-harvesting proteins. Biophys. J. 2011, 101, 934–942. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.C.; Pons, T.L.; Groeneveld, H.W.; Azinheira, H.G.; Nunes, M.A. Photosynthetic acclimation of high light conditions in mature leaves of Coffea arabica L.: Role of xanthophylls, quenching mechanisms and nitrogen nutrition. Austr. J. Plant Physiol. 2000, 27, 43–51. [Google Scholar] [CrossRef]
- Bartley, G.E.; Scolnik, P.A. Plant carotenoids: Pigments for photoprotection, visual attraction, and human health. Plant Cell 1995, 7, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, K.; Gajc-Wolska, J.; Mirgos, M.; Geszprych, A.; Kowalczyk, W.; Sieczko, L.; Niedzińska, M.; Gajewski, M. Mineral nutrients needs of cucumber and its yield in protected winter cultivation, with HPS and LED supplementary lighting. Sci. Hortic. 2020, 265, 109217. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde–glutaraldehyde fixative of high osmolality for use in eletron microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- Landmann, L. Deconvolution improves colocalization analysis of multiple fluorochromes in 3D confocal data sets more than filtering techniques. J. Microsc. 2002, 208, 134–147. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determination of total carotenoids and chlorophyll a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Klepacka, M. (Ed.) Analiza Żywności, 1st ed.; Fundacja Rozwój SGGW: Warszawa, Poland, 1996; pp. 44–47. [Google Scholar]
- Bar-Akiva, A.; Sagiv, J.; Leshem, J. Nitrate reductase activity as an indicator for assessing the nitrogen requirement of grass crops. J. Sci. Food Agric. 1970, 21, 405–407. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Song, J.X.; Meng, Q.W.; Du, W.F.; He, D.X. Effects of light quality on growth and development of cucumber seedlings in controlled environment. Int. J. Agric. Biol. Eng. 2017, 10, 312–318. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Front. Plant Sci. 2021, 12, 709313. [Google Scholar] [CrossRef] [PubMed]
- Hamedalla, A.M.; Ali, M.M.; Ali, W.M.; Ahmed, M.A.A.; Kaseb, M.O.; Kalaji, H.M.; Gajc-Wolska, J.; Yousef, A.F. Increasing the performance of cucumber (Cucumis sativus L.) seedlings by LED illumination. Sci. Rep. 2022, 12, 852. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and physiological responses of cucumber seedlings to supplemental LED light under extremely low irradiance. Agronomy 2020, 10, 1698. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Sci. Hortic. 2014, 173, 92–99. [Google Scholar] [CrossRef]
- Humphrey, C.D.; Pittman, F.E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974, 49, 9–14. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Hashimoto, H.; Sugai, Y.; Uragami, C.; Gardiner, A.T.; Cogdell, R.J. Natural and artificial light-harvesting systems utilizing the functions of carotenoids. J. Photochem. Photobiol. C 2015, 25, 46–70. [Google Scholar] [CrossRef]
- Pan, X.; Li, M.; Wan, T.; Wang, L.; Jia, C.; Hou, Z.; Zhao, X.; Zhang, J.; Chang, W. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 2011, 18, 309–315. [Google Scholar] [CrossRef]
- Pospíšil, P. The role of metals in production and scavenging of reactive oxygen species in photosystem II. Plant Cell Physiol. 2014, 55, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Hideg, É.; Krieger-Liszkay, A. Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid. Redox Signal. 2013, 18, 2145–2162. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Holleboom, C.-P.; Gacek, D.A.; Liao, P.-N.; Negretti, M.; Croce, R.; Walla, P.J. Carotenoid-chlorophyll coupling and fluorescence quenching in aggregated minor PSII proteins CP24 and CP29. Photosynth. Res. 2015, 124, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A.; Samuolienė, G.; Jankauskienė, J.; Kasiulevičiūtė-Bonakėrė, A.; Bliznikas, Z.; Novičkovas, A.; Breivė, K.; Žukauskas, A. The effect of light-emitting diodes lighting on cucumber transplants and after-effect on yield. Zemdirb.-Agric. 2009, 96, 102–118. [Google Scholar]
- Wojciechowska, R.; Kołton, A.; Długosz-Grochowska, O.; Żupnik, M.; Grzesiak, W. The effect of LED lighting on photosynthetic parameters and weight of lamb’s lettuce (Valerianella locusta). Folia Hortic. 2013, 25, 41–47. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef]
- Ptushenko, V.V.; Avercheva, O.V.; Bassarskaya, E.M.; Berkovich, Y.A.; Erokhin, A.N.; Smolyanina, S.O.; Zhigalova, T.V. Possible reasons of a decline in growth of Chinese cabbage under a combined narrowband red and blue light in comparison with illumination by high-pressure sodium lamp. Sci. Hortic. 2015, 194, 267–277. [Google Scholar] [CrossRef]
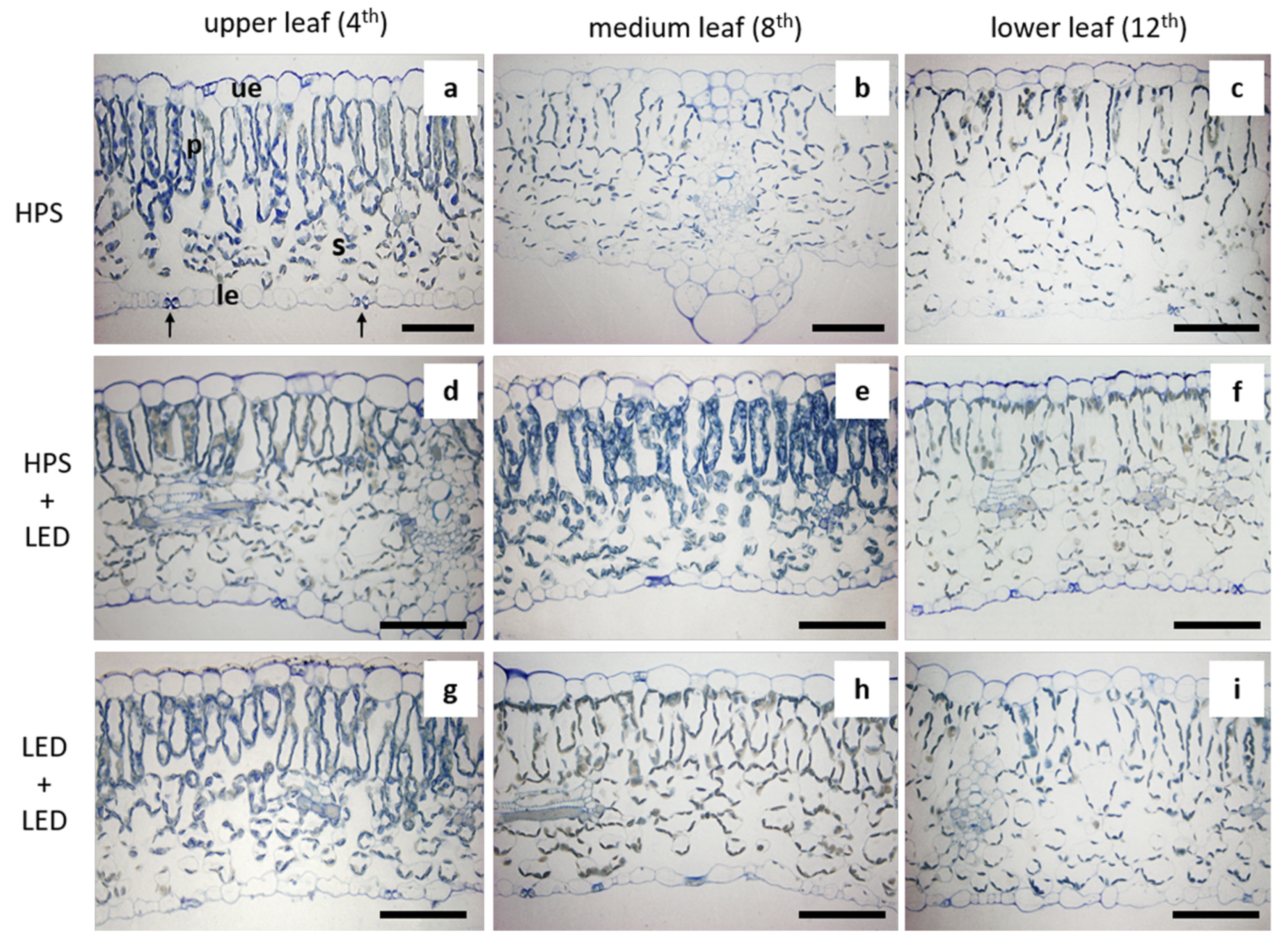

| Supplemental Lighting | Leaf | Palisade Parenchyma | Spongy Parenchyma | ||||
|---|---|---|---|---|---|---|---|
| Number of Cell Layers | Cell Length (µm) | Cell Width (µm) | Number of Cell Layers | Cell Length (µm) | Cell Width (µm) | ||
| HPS | upper (4th) | 1 | 42.0 ± 1.9 b | 9.6 ± 0.8 b | 3–4 | 12.7 ± 2.1 e | 17.9 ± 1.1 bc |
| medium (8th) | 1 | 44.1 ± 4.4 b | 12.7 ± 1.2 ab | 4 | 16.5 ± 0.9 de | 17.2 ± 1.8 bc | |
| lower (12th) | 1 | 41.3 ± 0.8 b | 11.6 ± 0.4 b | 4 | 15.2 ± 1.9 e | 21.1 ± 1.1 ab | |
| HPS + LED | upper (4th) | 1 | 45.0 ± 1.8 b | 11.1± 1.7 b | 3–4 | 17.6 ± 2.7 cde | 13.5 ± 0.3 cd |
| medium (8th) | 1 | 44.3 ± 0.6 b | 12.4 ± 1.3 b | 4 | 23.1 ± 3.6 bcd | 22.8 ± 3.4 a | |
| lower (12th) | 1 | 55.5 ± 3.3 a | 17.1 ± 2.0 a | 4 | 24.6 ± 3.7 bc | 22.8 ± 0.1 a | |
| LED + LED | upper (4th) | 1 | 39.5 ± 1.6 b | 12.4± 2.3 b | 3–4 | 15.1 ± 2.8 e | 12.1 ± 2.1 d |
| medium (8th) | 1 | 55.3 ± 2.0 a | 12.4 ± 2.0 b | 3 | 34.2 ± 2.7 a | 21.0 ± 0.8 ab | |
| lower (12th) | 1 | 56.2 ± 4.0 a | 13.9 ± 0.9 ab | 3 | 26.3 ± 3.1 b | 19.6 ± 1.1 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, K.; Sieczko, L.; Borucki, W.; Sujkowska-Rybkowska, M.; Mirgos, M.; Niedzińska, M.; Bederska-Błaszczyk, M.; Kowalczyk, W.; Geszprych, A.; Gajc-Wolska, J. The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis sativus L.) in High-Wire Cultivation. Agronomy 2022, 12, 2004. https://doi.org/10.3390/agronomy12092004
Kowalczyk K, Sieczko L, Borucki W, Sujkowska-Rybkowska M, Mirgos M, Niedzińska M, Bederska-Błaszczyk M, Kowalczyk W, Geszprych A, Gajc-Wolska J. The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis sativus L.) in High-Wire Cultivation. Agronomy. 2022; 12(9):2004. https://doi.org/10.3390/agronomy12092004
Chicago/Turabian StyleKowalczyk, Katarzyna, Leszek Sieczko, Wojciech Borucki, Marzena Sujkowska-Rybkowska, Małgorzata Mirgos, Monika Niedzińska, Magdalena Bederska-Błaszczyk, Waldemar Kowalczyk, Anna Geszprych, and Janina Gajc-Wolska. 2022. "The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis sativus L.) in High-Wire Cultivation" Agronomy 12, no. 9: 2004. https://doi.org/10.3390/agronomy12092004
APA StyleKowalczyk, K., Sieczko, L., Borucki, W., Sujkowska-Rybkowska, M., Mirgos, M., Niedzińska, M., Bederska-Błaszczyk, M., Kowalczyk, W., Geszprych, A., & Gajc-Wolska, J. (2022). The Effect of LED and HPS Assimilation Lighting on Leaf Anatomy, Chlorophyll and Carotenoid Autofluorescence Signals, and Some Physiological and Chemical Leaf Traits Related to the Productivity of Cucumber (Cucumis sativus L.) in High-Wire Cultivation. Agronomy, 12(9), 2004. https://doi.org/10.3390/agronomy12092004








