Abstract
Several toxic pollutants are released into the atmosphere through human activities. Among these pollutants, lead (Pb) is a non-biodegradable element that can cause reduced cell division, impact negatively on the biosynthesis of photosynthetic pigments, and lower biomass accumulation, which can lead to plant death. 24-epibrassinolide (EBR) is a plant growth regulator with broad benefits on physiological functions and biochemical responses, conferring tolerance to plants against several biotic and abiotic stresses. The experiment was randomized with four treatments, two lead concentrations (0 and 200 µM Pb, described as −Pb and +Pb, respectively) and two EBR (0 and 100 nM EBR, described as −EBR and +EBR, respectively). We detected a negative impact of Pb stress in tomato plants; however, the exogenous application of EBR induced protection on leaf anatomy and photosynthetic apparatus, mitigating the Pb impacts on growth. This steroid enhances the root and leaf structures (in root tissue, the epidermis thickness; and in the leaf, palisade parenchyma, and spongy parenchyma), improving the membrane selectivity, light energy absorption, and CO2 fixation. Applying 200 µM Pb and 100 nM EBR caused an increase in superoxide dismutase, catalase, ascorbate peroxidase, and peroxidase activity (by 26%, 18%, 25%, and 20%, respectively). Moreover, the improvements obtained on photosynthetic pigments, electron transport rate, the effective quantum yield of photosystem II photochemistry, and net photosynthetic rate prove the benefits and protection of photosynthetic apparatus, resulting in increased biomass accumulation, with increases of 95%, 115%, 74%, and 92% in leaf, root, stem, and the whole plant, respectively. Taken together, our findings confirm that EBR alleviates the damages provoked by Pb stress in tomatoes.
1. Introduction
Due to fast and uncontrolled industrialization, urbanization, and intensive agriculture, the environment has been under remarkable pressure, with various toxic pollutants released into the atmosphere through human activities [1]. Heavy metals (HMs) are non-biodegradable inorganic chemical constituents and are a major threat to the environment due to their potentially cytotoxic, genotoxic, and mutagenic characteristics. Some of these HMs are not essential since they do not perform any known physiological function in plants, such as arsenic (As), cadmium (Cd), lead (Pb), chromium (Cr), and aluminum (Al) [2,3]. One of the most toxic HMs, Pb, can cause common toxic effects on plants, such as inhibition of growth, altered nutrient assimilation, low biomass accumulation, and senescence, which ultimately cause plant death [4]. Photosynthetic pathways are also affected by Pb toxicity due to disrupting chloroplast ultrastructure and obstruction of essential pigments synthesis, including chlorophyll and carotenoids, in addition to blocking the Calvin cycle and electron transport chain, which produces a shortage of carbon dioxide [5,6].
Plants under HMs toxicity usually present redox imbalance of the cell, inducing oxidative stress due to overproduction of reactive oxygen species (ROS), more specifically superoxide radical (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH−), being highly reactive, toxic, and harmful to plant metabolism [7,8]. Interestingly, Rodrigues et al. [9] described that the activities of the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and guaiacol peroxidase (POX) were stimulated after application of brassinosteroids (BRs), indicating an improvement in O2− detoxification. The SOD enzyme is part of the first line of defense of the antioxidant system in plants and is involved in the dismutation of O2− into H2O2, which will later be decomposed by the CAT, APX, and POX enzymes [10,11].
The leaf anatomy is linked to leaf mass per unit area and correlates well with plant growth rates due to its influence on photosynthesis and hence plant growth; however, HMs, including Pb, can cause damaged and altered ultrastructure of this foliar structure [12,13]. The epidermis is the protective outer layer of cells that acts as a barrier against biotic or abiotic agents and as an active interface that controls the vital exchange of gas, water, and nutrients with the environment [14,15]. Palisade and spongy parenchyma are tissues related to the photosynthetic process and formation of intercellular spaces involved with gas exchange, contributing to the influx and consequent fixation of CO2 [16].
The chlorophyll content is an important indicator of photosynthetic potential, and carotenoids are the endogenous antioxidant pigments that prevent the peroxidation of lipid membranes by the extinction of ROS [17]. Pb stress causes chloroplast disorganization and damage to its membrane, besides induced increases in chlorophyllase activity, the enzyme responsible for chlorophyll degradation [18,19]. In parallel to this, the stress induced by Pb also causes an imbalance in plastoquinone and electron transport, reducing photosynthesis, which consequently leads to a reduction in biomass [20].
BRs are polyhydroxy steroid phytohormones that perform various physiological functions, such as growth, and confer resistance to plants against various biotic and abiotic stresses [21,22]. Among all isolated and characterized, brassinolide (BL), 24-epibrassinolide (EBR), and 28-homobrassinolide (HBL) are the main bioactive BRs, and several studies are addressing the adaptive responses of plants caused by these steroids to environmental stresses [23], such as heavy metal toxicity [24], salinity [25], temperature extremes [26,27], and drought [28,29].
To overcome the harmful effects of these stresses, especially heavy metals, BRs can perform several roles, such as reducing these toxic elements’ absorption by altering cell membrane permeability and inducing a group of defensive enzymes [30]. However, some processes performed by this steroid on heavy metal stress still need further research; because of this, we hypothesized that EBR mitigates the toxic effects of heavy metals, especially Pb. Therefore, this study aimed to verify whether the exogenous application of EBR can preserve tomato plants from oxidative damages caused by excess Pb, evaluating the responses associated with leaf anatomy, antioxidant metabolism, photosynthetic apparatus, and biomass.
2. Materials and Methods
2.1. Location and Growth Conditions
The experiment was performed at the Universidade Federal Rural da Amazônia, Paragominas, Brazil (2°55′ S, 47°34′ W). The study was conducted in a greenhouse illuminated with sunlight, but with temperature and humidity controlled. The minimum, maximum, and median temperatures were 23.3, 29.2, and 25.5 °C, respectively. The relative humidity during the experimental period varied between 60% and 80% and photoperiod of 12/12.
2.2. Plants, Containers and Acclimation
Seeds of Solanum lycopersicum L. cv Santa Clara were germinated using vegetable substrate and transplanted on the 14th day into 1.2-L pots filled with a mixed substrate of sand and vermiculite at a ratio of 3:1. Plants were cultivated under semi-hydroponic conditions containing 500 mL of nutritive solution. A modified Hoagland and Arnon [31] solution was used as a source of nutrients; the ionic strength started at 50% and was modified to 100% after 2 days.
2.3. Experimental Design
The experiment was randomized with four treatments, two lead concentrations (0 and 200 µM Pb, described as −Pb and +Pb, respectively) and two EBR (0 and 100 nM EBR, described as −EBR and +EBR, respectively). Five replicates for each one of the four treatments were conducted and used in the experiment in a total of 20 experimental units, with three plants in each unit. Pb and EBR concentrations were chosen according to studies by Guedes et al. [32] and Maia et al. [33], respectively.
2.4. 24-Epibrassinolide (EBR) Preparation and Application
Fifteen-day-old seedlings were sprayed with 24-epibrassinolide (EBR) or Milli-Q water (containing a proportion of ethanol that was equally used in the preparation of the EBR solution) for 20 days (days 10−30 after the start of the experiment), being applied this steroid with intervals of 5 days. The 0 and 100 nM EBR (Sigma-Aldrich, St. Louis, MI, USA) solutions were prepared by dissolving the solute in ethanol followed by dilution with Milli-Q water [ethanol:water (v/v) = 1:10,000] [34].
2.5. Plant Nutrition and Pb Treatment
The plants received the following macro and micronutrients contained in the nutrient solution: 8.75 mM KNO3, 7.5 mM Ca(NO3)2·4H2O, 3.25 mM NH4H2PO4, 1.5 mM MgSO4·7 H2O, 62.50 µM KCl, 31.25 µM H3BO3, 2.50 μM MnSO4·H2O, 2.50 μM ZnSO4·7H2O, 0.63 μM CuSO4·5H2O, 0.63 μM NaMoO4·5H2O, and 250.0 μM NaEDTAFe·3H2O. To induce Pb stress, PbCl2 was used at concentrations of 0 and 200 µM Pb and was applied over 10 days (days 20−30 after the start of the experiment). During the study, the nutrient solutions were changed at 07:00 h at 3-day intervals, with the pH adjusted to 5.5 using HCl or NaOH. On day 30 of the experiment, physiological and morphological parameters were measured for all plants, and tissues were harvested for anatomical, biochemical, and nutritional analyses.
2.6. Determination of Pb and Nutrients
Milled samples (100 mg) of root, stem, and leaf tissues were pre-digested in conical tubes (50 mL) with 2 mL of sub-boiled HNO3. Subsequently, 8 mL of a solution containing 4 mL of H2O2 (30% v/v) and 4 mL of ultra-pure water was added and transferred to a Teflon digestion vessel in agreement with Paniz et al. [35]. Determination of Pb, Mg, K, Ca, Cu, Zn, and Mn was performed using an inductively coupled plasma mass spectrometer (model ICP-MS 7900; Agilent, Santa Clara, CA, USA).
2.7. Anatomical Measurements
Samples were collected from the middle region of the leaf limb of fully expanded leaves and roots 5 cm from the root apex. Subsequently, all collected botanical material was fixed in FAA 70 for 24 h, dehydrated in ethanol, and embedded in historesin LeicaTM (Leica, Nussloch, Germany). Transverse sections with a thickness of 5 μm were obtained with a rotating microtome (model Leica RM 2245, Leica Biosystems), stained with toluidine blue [36]. For stomatal characterization, the epidermal impression method was used according to Segatto et al. [37]. The slides were observed and photomicrographed under an optical microscope (Motic BA 310, Motic Group Co. Ltd.) coupled to a digital camera (Motic 2500, Motic Group Co., Ltd.). The images were analyzed with Motic plus 2.0, previously calibrated with a micrometer slide from the manufacturer. The leaf anatomical variables evaluated were the polar diameter of the stomata (PDS), equatorial diameter of the stomata (EDS), epidermis thickness from adaxial leaf side (ETAd), and epidermis thickness from abaxial leaf side (ETAb). In both leaf faces, the stomatal density (SD) was calculated as the number of stomata per unit area and the stomatal functionality (SF) as the ratio PDS/EDS according to Castro et al. [38]. In root samples, the root epidermis thickness (RET), root endodermis thickness (RDT), root cortex thickness (RCT), vascular cylinder diameter (VCD), and root metaxylem diameter (RMD) were measured.
2.8. Determination of Photosynthetic Pigments
The chlorophyll and carotenoid levels were determined using 40 mg of leaf tissue. The samples were homogenized in the dark with 8 mL of 90% methanol (Sigma-Aldrich™, St. Louis, MI, USA). The homogenate was centrifuged at 6000× g for 10 min at 5 °C. The supernatant was removed, and the chlorophyll a (Chl a) and b (Chl b), carotenoid (Car), and total chlorophyll (total Chl) levels were quantified using a spectrophotometer (model UV-M51; Bel Photonics) according to the methodology of Lichtenthaler and Buschmann [39].
2.9. Measurement of Chlorophyll Fluorescence
The minimal fluorescence yield of the dark-adapted state (F0), maximal fluorescence yield of the dark-adapted state (Fm), variable fluorescence (Fv), maximal quantum yield of PSII photochemistry (Fv/Fm), effective quantum yield of PSII photochemistry (ΦPSII), photochemical quenching coefficient (qP), nonphotochemical quenching (NPQ), electron transport rate (ETR), relative energy excess at the PSII level (EXC), and the ratio between the electron transport rate and net photosynthetic rate (ETR/PN) were determined using a modulated chlorophyll fluorometer (model OS5p; Opti-Sciences, Hudson, NH, USA). The chlorophyll fluorescence was measured in fully expanded leaves under light. Preliminary tests determined that the acropetal third of leaves in the middle third of the plant and that adapted to the dark for 30 min yielded the greatest Fv/Fm ratio. Therefore, this part of the plant was used for measurements. The intensity and duration of the saturation light pulse were 7500 µmol m−2.s−1 and 0.7 s, respectively.
2.10. Evaluation of Gas Exchange
The net photosynthetic rate (PN), transpiration rate (E), stomatal conductance (gs), and intercellular CO2 concentration (Ci) were evaluated using an infrared gas analyzer (model LCPro+; ADC BioScientific). These parameters were measured at the adaxial surface of fully expanded leaves that were collected from the middle region of the plant. The water-use efficiency (WUE) was estimated according to Ma et al. [40], and the instantaneous carboxylation efficiency (PN/Ci) was calculated using the formula described by Aragão et al. [41]. Gas exchange was evaluated in all plants under a constant CO2 concentration (390 μmol mol−1 CO2), photosynthetically active radiation (800 μmol photons m−2 s−1), air-flow rate (300 µmol s−1), and temperature (28 °C) in the test chamber between 10:00 and 12:00 h.
2.11. Determination of Antioxidant Enzymes, Superoxide and Soluble Proteins Level
Antioxidant enzymes (SOD, CAT, APX, and POX), superoxide, and soluble proteins were extracted from leaf tissues [42]. The extraction mixture was prepared by homogenizing 500 mg of fresh plant material in 5 mL of extraction buffer, which consisted of 50 mM phosphate buffer (pH 7.6), 1.0 mM ascorbate, and 1.0 mM EDTA. Samples were centrifuged at 14,000× g for 4 min at 3 °C, and the supernatant was collected. Quantifying the total soluble proteins was performed using the method described by Bradford [43]. Absorbance was measured at 595 nm, using bovine albumin as a standard.
2.12. Superoxide Dismutase Assay
For the SOD assay (EC 1.15.1.1), 2.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.6), 0.1 mM EDTA, 13 mM methionine (pH 7.6), 75 µM NBT, and 4 µM riboflavin was mixed with 0.2 mL of supernatant. The absorbance was then measured at 560 nm [44]. One SOD unit was defined as the amount of enzyme required to inhibit 50% of the NBT photoreduction. The SOD activity was expressed in unit mg−1 protein.
2.13. Catalase Assay
For the CAT assay (EC 1.11.1.6), 0.2 mL of supernatant and 1.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0) and 12.5 mM hydrogen peroxide were mixed, and the absorbance was measured at 240 nm [45]. The CAT activity was expressed in μmol H2O2 mg−1 protein min−1.
2.14. Ascorbate Peroxidase Assay
For the APX assay (EC 1.11.1.11), 1.8 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0), 0.5 mM ascorbate, 0.1 mM EDTA, and 1.0 mM hydrogen peroxide was mixed with 0.2 mL of supernatant, and the absorbance was measured at 290 nm [46]. The APX activity was expressed in μmol AsA mg−1 protein min−1.
2.15. Peroxidase Assay
For the POX assay (EC 1.11.1.7), 1.78 mL of a reaction mixture containing 50 mM phosphate buffer (pH 7.0) and 0.05% guaiacol was mixed with 0.2 mL of supernatant, followed by the addition of 20 µL of 10 mM hydrogen peroxide. The absorbance was then measured at 470 nm [47]. The POX activity was expressed in μmol tetraguaiacol mg−1 protein min−1.
2.16. Determination of Superoxide Concentration
To determine O2−, 1 mL of extract was incubated with 30 mM phosphate buffer [pH 7.6] and 0.51 mM hydroxylamine hydrochloride for 20 min at 25 °C. Sulphanilamide (17 mM) and 7 mM α-naphthylamine were added to the incubation mixture for 20 min at 25 °C. After the reaction, ethyl ether was added in an identical volume and centrifuged at 3000× g for 5 min. The absorbance was measured at 530 nm [48].
2.17. Extraction of Nonenzymatic Compounds
Nonenzymatic compounds (H2O2 and MDA) were extracted as described by Wu et al. [49]. Briefly, a mixture was prepared to extract H2O2 and MDA by homogenizing 500 mg of fresh leaf material in 5 mL of 5% (w/v) trichloroacetic acid. Samples were centrifuged at 15,000× g for 15 min at 3 °C to collect the supernatant.
2.18. Determination of Hydrogen Peroxide Concentration
To measure H2O2, 200 µL of supernatant and 1800 µL of the reaction mixture (2.5 mM potassium phosphate buffer [pH 7.0] and 500 mM potassium iodide) were mixed, and the absorbance was measured at 390 nm [50].
2.19. Quantification of Malondialdehyde Concentration
MDA was determined by mixing 500 µL of supernatant with 1 mL of the reaction mixture, which contained 0.5% (w/v) thiobarbituric acid in 20% trichloroacetic acid. The mixture was incubated in boiling water at 95 °C for 20 min, with the reaction terminated by placing the reaction container in an ice bath. The samples were centrifuged at 10,000× g for 10 min, and the absorbance was measured at 532 nm. The nonspecific absorption at 600 nm was subtracted from the absorbance data. The amount of MDA−TBA complex (red pigment) was calculated [51], with minor modifications and using an extinction coefficient of 155 mM−1 cm−1.
2.20. Determination of Electrolyte Leakage
Electrolyte leakage was measured according to the method of Gong et al. [52] with minor modifications. Fresh tissue (200 mg) was cut into pieces 1 cm in length and placed in containers with 8 mL of distilled deionized water. The containers were incubated in a water bath at 40 °C for 30 min, and the initial electrical conductivity of the medium (EC1) was measured. The samples were then boiled at 95 °C for 20 min to release the electrolytes. After cooling, the final electrical conductivity (EC2) was measured. The percentage of electrolyte leakage was calculated using the formula EL (%) = (EC1/EC2) × 100.
2.21. Measurements of Biomass
The biomass of roots and leaves was measured based on constant dry weights (g) after drying in a forced-air ventilation oven at 65 °C.
2.22. Data Analysis
The data were subjected to an analysis of variance, and significant differences between the means were determined using the Scott–Knott test at a probability level of 5% [53]. Standard deviations were calculated for each treatment. Statistical analysis of the data was done using R® software [54].
3. Results
3.1. Pb Content Was Minimized by EBR in Plants Exposed to Toxicity
The treatment with 200 µM of Pb caused a significant increase in the content of this metal on the analyzed tissues (Table 1). However, plants treated with Pb and EBR showed reductions of 55%, 65%, and 45% in the content of Pb in the tissues of the root, stem, and leaf, respectively, concerning the same treatment without the steroid.

Table 1.
Lead contents in tomato plants treated with EBR and subjected to Pb toxicity.
3.2. EBR Positively Modulated the Root and Leaf Structures
Toxicity caused by Pb caused reductions in RET, RDT, RCT, VCD, and RMD (Table 2 and Figure 1). Plants exposed to Pb and treated with EBR increased in RET, RDT, and RMD by 23%, 24%, and 20%, respectively. To leaf structures, Pb stress also caused decreases (Table 2 and Figure 1). However, the application of EBR in plants submitted to 200 µM Pb showed increases of 10%, 21%, 9%, and 10% in ETAd, ETAb, PPT, and SPT, respectively, when compared to equal treatment without EBR.

Table 2.
Root and leaf structures in tomato plants treated with EBR and subjected to Pb toxicity.
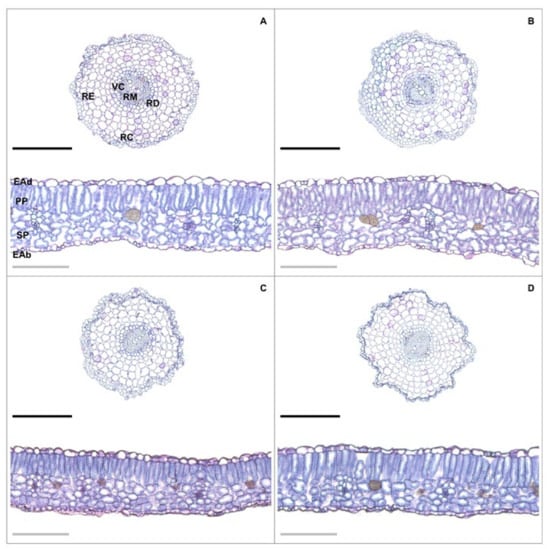
Figure 1.
Root and leaf cross sections in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. −Pb/−EBR (A), −Pb/+EBR (B), +Pb/−EBR (C), and +Pb/+EBR (D). Legends: RE = root epidermis; RC = root cortex; RD = root endodermis; VC = vascular cylinder; RM = root metaxylem; EAd = adaxial epidermis; EAb = adaxial epidermis; PP = palisade parenchyma; SP = spongy parenchyma. Black bars = 300 µm and gray bars: 150 µm.
3.3. Nutrient Contents and Metal Homeostasis Were Up-Regulated by the Steroid
Plants submitted to Pb stress showed negative changes in nutrient contents (Table 3). On the other hand, the application of EBR in plants treated with the metal promoted beneficial effects on the content of Mg, K, Cu, Zn, and Mn, with increases in the root of 30%, 19%, 41%, 16%, and 7%, respectively; stem increases of 6%, 3%, 34%, 12%, and 7%; and increases in the leaf of 14%, 19%, 41%, 18%, and 15%, compared to treatment with Pb only. The Ca content in the root and leaf was increased by 29% and 11%, respectively.

Table 3.
Nutrient contents in tomato plants treated with EBR and subjected to Pb toxicity.
3.4. An Antioxidant System Modulated by EBR
The toxicity of Pb caused an increase in the enzymatic activity of SOD, CAT, APX, and POX (Figure 2). However, the combined effect of Pb + EBR increased the performance of SOD, CAT, APX, and POX by 26%, 18%, 25%, and 20%, respectively, compared to the same treatment without the steroid.
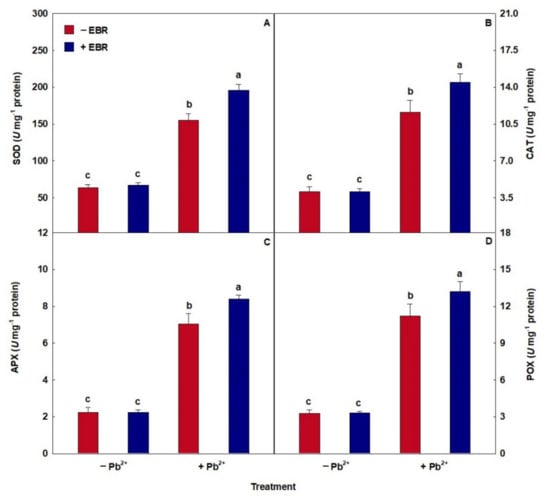
Figure 2.
(A) Activities of superoxide dismutase (SOD), (B) catalase (CAT), (C) ascorbate peroxidase (APX), and (D) peroxidase (POX) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott–Knott test (p < 0.05). Means ± SD, n = 5.
3.5. Steroid Stimulates Oxidative Damage Reduction
Treatment with Pb induced increases in oxidative compounds (Figure 3). On the other hand, the application of EBR in plants subjected to stress by Pb showed decreases of 23%, 36%, 37%, and 8% in compounds O2−, H2O2, MDA, and EL, respectively, when compared with the same treatment without EBR.
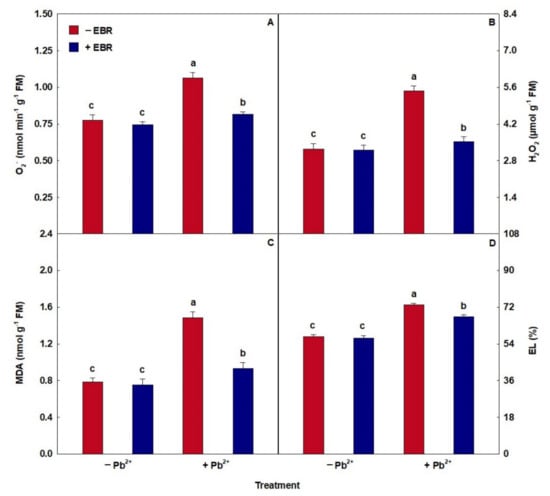
Figure 3.
(A) Superoxide (O2−), (B) hydrogen peroxide (H2O2), (C) malondialdehyde (MDA), and (D) electrolyte leakage (EL) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott–Knott test (p < 0.05). Means ± SD, n = 5.
3.6. EBR Benefited Maintenance of Membrane Integrity of Chloroplasts and Alleviated the Pb Interference on Light Capture and Gas Exchange
Plants exposed to Pb toxicity showed decreases in the values of Chl a, Chl b, Chl Total, and Car (Table 4). However, the combined treatment of Pb + 100 nM EBR promoted increases in Chl a, Chl b, Chl total, and Car of 21%, 25%, 22%, and 29%, respectively, compared to the Pb treatment. EBR instigated improvements in the capture of light energy. In chlorophyll fluorescence (Figure 4), Pb toxicity caused an increase in F0 and a reduction in Fv and Fv/Fm. However, plants exposed to Pb + EBR showed a decrease of 11% in F0 and increases of 6% and 4% in Fv and Fv/Fm, respectively, concerning the same treatment without EBR. Treatment with 200 µM Pb showed no difference in Fm in the absence or presence of EBR. Plants exposed to Pb also showed decreases in ΦPSII, qP, and ETR and addition in NPQ, EXC, and ETR/PN (Table 4). However, the Pb + EBR treatment promoted increases in ΦPSII, qP, and ETR of 24%, 11%, and 24%, respectively, and reductions of 23%, 7%, and 4% in NPQ, EXC, and ETR/PN, respectively, compared to the similar treatment without the steroid. Concerning gas exchange (Table 4), Pb caused negative effects. However, the Pb + EBR treatment stimulated increases of 29%, 18%, 26%, and 32%, respectively, in PN, gs, WUE, and PN/Ci and a decrease (5%) in Ci, when compared to plants exposed to Pb toxicity without EBR.

Table 4.
Photosynthetic pigments, chlorophyll fluorescence, gas exchange in tomato plants treated with EBR and subjected to Pb toxicity.
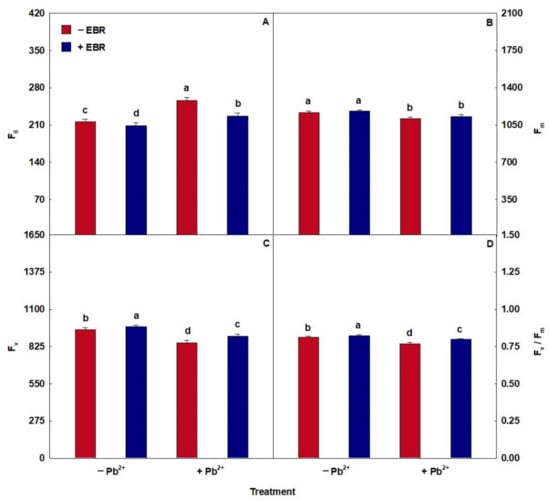
Figure 4.
(A) Minimal fluorescence yield of the dark-adapted state (F0), (B) maximal fluorescence yield of the dark-adapted state (Fm), (C) variable fluorescence (Fv), and (D) maximal quantum yield of PSII photochemistry (Fv/Fm) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott–Knott test (p < 0.05). Means ± SD, n = 5.
3.7. Limitations on Stomatal Performance Were Attenuated by EBR
Pb excess provoked negative impacts on the characteristics of the stomata (Table 5). Plants exposed to Pb toxicity and treated with 100 nM EBR showed, however, increases in SD and SI, on the adaxial face of 13% and 13%; and in abaxial, 18% and 9%, respectively. In EDS, there was a 7% and 6% reduction in the adaxial and abaxial faces, respectively, compared to the equal treatment without EBR.

Table 5.
Stomatal characteristics in tomato plants treated with EBR and subjected to Pb toxicity.
3.8. EBR Promoted Higher Biomass Accumulation
The stress caused by Pb caused a reduction in the amount of dry matter (Figure 5). In contrast, the application of EBR in plants stressed by Pb increased the values of LDM, RDM, SDM, and TDM by 95%, 115%, 74%, and 92%, respectively, relative to the same treatment in the absence of EBR.
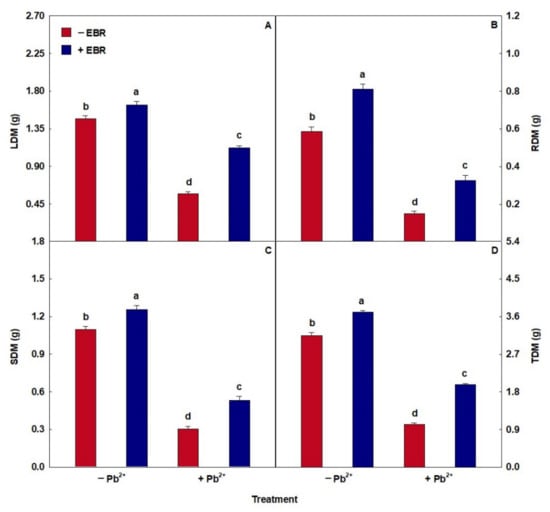
Figure 5.
(A) Leaf dry matter (LDM), (B) root dry matter (RDM), (C) stem dry matter (SDM), and (D) total dry matter (TDM) in tomato plants treated with 24-epibrassinolide (EBR) and subjected to lead (Pb) toxicity. Columns with different letters indicate significant differences from the Scott–Knott test (p < 0.05). Means ± SD, n = 5.
4. Discussion
Here, the various values we recorded in the Pb-treated plants demonstrate that the application of 200 µM of PbCl2 results in the uptake and accumulation of this metal in plants, mainly in the roots. However, the application of 100 nM EBR reduced the Pb content because this steroid is involved in regulating the electrical characteristics of membranes, cell membrane permeability, and ion transport [55], possibly inhibiting uptake and accumulation by stimulating processes that act on immobilization, sequestration, and precipitation of this heavy metal [56]. Rossato et al. [57], testing progressive Pb concentrations in Pluchea sagittalis plants, observed increases in the content of this metal in the root, stem, and leaf. Evaluating Pb stress in Acutodesmus obliquus, Talarek-Karwel et al. [58] identified a reduction in Pb content after EBR application.
EBR application alleviated the loss caused by Pb toxicity in the nutritional content, increasing the values of macronutrients (Mg, K, and Ca) and micronutrients (Cu, Zn, and Mn). These elements play essential roles in plant metabolism, being required as constituents of crucial molecules or as cofactors of various enzymatic processes. A high concentration of Pb can cause an imbalance in the absorption, assimilation, and translocation of nutrients through changes in the permeability of the plasma membrane and influence the processes involved in the transfer of these elements across the root membrane [59], which is consistent with the reduction in the root structures of plants exposed only to Pb we detected in our study. After absorption, Pb binds to the membrane and cell wall, which induces rigidity to these components and reduces cell division, inhibiting the growth of root tissues [55]. On the other hand, EBR plays an important role in signalization, regulation, and differentiation of the root tissue, inducing increments in root structures after the application of this steroid, especially in RET, RDT, and RMD, being these results clearly connected to the protection of this organ against the deleterious effects occasioned by the Pb [60,61], as well as benefits on nutritional status confirmed by the increase in the macro and micronutrient contents. Yuan et al. [62] found that the application of EBR helped to maintain the ionic balance in Cucumis sativus plants under Ca stress. Pisum sativum plants exposed to Cd stress [63] also demonstrated beneficial effects on nutritional status with EBR treatment. Santos et al. [64] researching Zn-induced stress in Glycine max, detected increases in the root structures after applying 100 nM of EBR.
Plants treated with EBR after exposure to Pb stress had increased enzyme activities (SOD, CAT, APX, and POX). The enhanced activities of the SOD, POX, and CAT enzymes are probably associated with the upregulation of gene expression of the SOD, POX, and CAT encoding genes [65]. BRs participate in the processes of gene expression, transcription, and translation in normal and stressed plants, stimulating increased activity of antioxidant enzymes in scavenging excess ROS, probably up-regulating the mRNA expression levels of Cu/Zn-SOD, Mn-SOD, CAT, APX, or specific genes, such as RBOH (respiratory burst oxidase homolog), MAPK1 (mitogen-activated protein kinase 1), and MAPK3 (mitogen-activated protein kinase 3) [66,67]. These findings are also connected with a positive balance in the nutritional status promoted by EBR since nutrients such as Zn, Cu, and Mn, which had their contents elevated after steroid application in this study, act as cofactors of SOD isozymes [68]. Zhou et al. [69] studying Vitis vinifera plants under Cu stress, detected enhanced enzyme activities with the EBR pretreatment. Similar results were described by Kohli et al. [70] with Brassica juncea seedlings under Pb stress had increased SOD, CAT, APX, and POX activities in response to EBR supplementation.
Pb toxicity induces molecular damage through the formation of ROS, such as O2− and H2O2, implying modifications to membrane structure and function, starting lipid peroxidation, and altered cell biochemical processes [71], consequently resulting in oxidative damages to membrane lipids, proteins, chloroplast pigments, enzymes, and nucleic acids [72], which was identified in this research by increases in oxidative compounds found in plants exposed to 200 µM Pb. Plants respond to excess ROS by inducing enzymatic antioxidants like SOD, CAT, APX, and POX that operate synergistically to repress metal toxicity [73]. Simultaneously, EBR works by eliminating these ROS in cells by regulating a complex antioxidant mechanism, stimulating the activity of these enzymes [74], where, in a simplified way, SOD acts as the first line of defense to counter the O2− and catalyzes the conversion of O2 to H2O2, then H2O2 can be eliminated by CAT, POX, and APX, and broken into H2O and O2 [68]. This effect of EBR was observed in our study through the reductions of O2−, H2O2, MDA, and EL triggered by the increases found in the enzymes SOD, CAT, APX, and POX. Brassica napus plants presented increases in oxidative compounds under Pb stress conditions [75]. On the other hand, in research by Kohli et al. [76] studying Brassica juncea plants exposed to Pb had reductions in O2−, H2O2, and MDA after EBR application.
Treatment with EBR in plants alleviated Pb stress on photosynthetic pigment content (Chl a, Chl b, total Chl, and Car). Photosynthetic pigments have high sensitivity to toxic metals, such as Pb, because its excess is related to the peroxidation of chloroplast membranes, which can provoke a deterioration of light-harvesting pigments and induces harm to the reaction center pigments [18,77]. Moreover, the disturbance caused by Pb on Chl content may be ascribed to the inhibition of its biosynthesis, the impaired uptake of essential ions such as Mg, Cu, and Mn, and the increased chlorophyllase (CHLASE) activity that catalyzes chlorophyll degradation [19,78]. However, EBR promotes an increase in chlorophyll content because it is directly or indirectly involved in the up-regulating Chl biosynthesis enzyme activity or the stimulation of gene expression levels related, for example, to Chl a/b binding protein-encoding [79]. In addition, this steroid attenuated these toxic effects of Pb on chlorophyll molecules through the improvements promoted in oxidative compounds and nutritional status, identified in this study by the reduction of MDA and EL and the increases in the contents of Mg, Zn, and Cu. In research by Kohli et al. [65], working with Brassica juncea plants under Pb toxicity obtained increased photosynthetic pigment content and decreased CHLASE gene expression after treatment with EBR. Similar to this study, EBR also showed positive effects on Chl a, Chl b, total Chl, and Car in a study conducted by Zhong et al. [24] with Festuca arundinacea plants subjected to Pb stress.
EBR application relieved the damage generated by Pb excess in F0, Fv, and Fv/Fm. Pb harms the efficiency of PSII photochemical reaction and electron transport chain, resulting in decreases in Fv/Fm, which may also be connected to impaired QA oxidation, further causing a decrease in electron transport from PSII to PSI [80]. The variable Fv/Fm is related to the functional state of the oxygen evolution complex and can be used as a sensitive indicator of photosynthetic performance, and when found at low levels, together with higher levels of F0, it indicates extensive photoinhibition due to environmental stresses [81]. In contrast, EBR can increase Fv and Fv/Fm and reduce F0 values, diminishing the photoinhibition effects and decreasing the dissipation of excitation energy in the antennas of photosystem II [61]. Similar to our research, Guedes et al. [32] describe increases in Fv, Fv/Fm, and decrease in F0 after application of 100 nM EBR in Oryza sativa plants exposed to 200 µM Pb. Tadaiesky et al. [82] working with Oryza sativa plants under Fe stress, reported that EBR also promoted improvements in F0, Fv, and Fv/Fm.
Plants exposed to excess Pb treated with EBR showed beneficial effects on chlorophyll fluorescence with increases in ΦPSII, qP, and ETR and decreases in NPQ, EXC, and ETR/PN. These results are related to the improvements obtained by EBR in Fv, Fv/Fm, and F0 described in this study. Pb stress conditions can cause damage to electron transport, absorption, and conversion of light energy, photochemical efficiency of the reaction center, and dissipation of excess energy in the photosynthesis process, especially in PSII activity [83]. Studies evaluating the toxicity caused by Pb suggest that the absorption and dissipation of energy within the PSII are high, while the capture and transport of electrons are reduced due to instability of Chl molecules or damage to the electron transfer system [84]. Despite that, EBR can stimulate the electron flux and improve the efficiency of the PSII, probably associated with the oxidation of QA, which receives and transfers electrons between PSII and PSI, as well as avoiding damage caused by excess energy in the reaction centers [28,85]. Alam et al. [86] related increases of 50% in ΦPSII and 81% in qP and decreases of 37% in NPQ after treatment with EBR in Glycine max seedlings under NaCl stress. Resembling our study, Santos et al. [87] evaluating seedlings of Vigna unguiculata exposed to Cd stress, found increases in ΦPSII, qP, and ETR, and reductions in NPQ, EXC, and ETR/PN after application of 100 nM EBR.
Pb toxicity causes modifications in photosynthesis due to disruption of photosynthetic pigments synthesis, injury of chloroplast ultrastructure, changes in lipid and protein composition of the thylakoid membrane, imbalance minerals uptake, restricted electron transport, inhibited activities of Calvin cycle enzymes, besides deficiency of CO2 in the result of stomatal closure [88,89]. On the other hand, plants treated with EBR and submitted to stress Pb-induced showed increases in PN, gs, WUE, and PN/Ci and a decrease in Ci. These responses may be related to the steroid’s role in promoting higher efficiency of PSII and probably better fixation of CO2 in the Calvin–Benson cycle by the RUBISCO enzyme during photosynthesis, also suggested by the increase in PN and reduction in Ci [90]. Zhou et al. [91] examining Robinia pseudoacacia seedlings under Pb stress, observed that the values of PN and gs decreased with the progressive increase in the metal. In a study with Glycine max seedlings under Mn stress, increases in PN, gs, WUE, and PN/Ci and decreased Ci after EBR application have been reported [9].
Reports have shown that the anatomical structure is altered by Pb toxicity through disturbance of external and internal tissues, which can lead to the collapse of parenchymal cells [92]. However, EBR spray revealed ameliorations on leaf structures with increases in ETAd, ETAb, PPT, and SPT. Increases in ETAd and ETAb are associated with the benefits promoted by EBR through stimulating cell division and expansion in the leaf as a strategy to avoid excessive water loss during the transpiration process, which was observed in the increase in WUE in our research [14,93]. As regards improvements in PPT and SPT, the increase is linked to the positive effects of EBR on gas exchange in this study, mainly in PN and PN/Ci, since the higher thickness of these tissues indicates better use of light energy and greater accumulation of CO2. Santos et al. [94] investigating Fe stress, verified additions on the ETAd, ETAb, PPT, and SPT variables in Glycine max seedlings treated with EBR.
The positive effects promoted by EBR found in the stomatal characteristics, through the increases in SD, EDS, and SI in plants under Pb toxicity, are correlated to the role of this steroid in improving stomatal performance. Furthermore, EBR stimulates stomata production by regulating specific proteins that act in the stomatal pathway [95]. These results are also reinforced by the fact that increased stomatal density is linked to stomatal conductance and increased intercellular spaces, thus increasing CO2 uptake and photosynthesis, which can be corroborated by increases in gs, PN and SPT, and decreases in Ci, obtained in our research with EBR application [96]. Ribeiro et al. [97] working with Eucalyptus urophylla seedlings exposed to Ni stress, described increases in SD, EDS, and SI variables after EBR treatment.
EBR positively modulates growth in Pb-stressed plants through increments in LDM, SDM, RDM, and TDM, mitigating the toxic effects of this heavy metal. Pb toxicity causes damaging effects on the growth and biomass of plants since excessive amounts in the intercellular space of this metal aggravated ultrastructural injury to leaf, often leading to cell death through disruption of chloroplasts, obstruction of chlorophyll biosynthesis, and interfering with nutrient elements transportation and photosynthetic machinery [20,98]. In contrast, as noted in the results of this work, EBR is involved in multiple roles in plants, relieving stress by restoring redox balance, stimulating pigment biosynthesis, promoting benefits in leaf anatomy, and improving chlorophyll fluorescence and gas exchange. Fariduddin et al. [99] reported increased biomass in Brassica juncea plants treated with EBR and exposed to Mn toxicity. Jan et al. [100] described biomass improvements by analyzing the effect of EBR on Solanum lycopersicon plants exposed to Cr toxicity.
5. Conclusions
This study demonstrates the interferences caused by Pb stress in tomato plants. On the other hand, our results in leaf anatomy, photosynthetic apparatus, and biomass confirm the positive action of the exogenous application of 100 nM of EBR. This steroid attenuates leaf tissue cell disruption by stimulating cell division and expansion, making this structure thicker, improving membrane selectivity, promoting greater use of light energy and, consequently, higher fixation of CO2. Additionally, EBR induced increases in the activity of antioxidant enzymes (SOD, CAT, APX, and POX), reducing membrane oxidative damages, thus preserving the chloroplast ultrastructure, being demonstrated in the reductions of oxidative compounds (O2−, H2O2, MDA, and EL) and maintenance of photosynthetic pigments. The improvements obtained in these pigments made it possible to reduce the instability of Chl molecules caused by the excess of Pb, increasing the capture and transport of electrons, improving the efficiency of PSII, and mitigating the damage caused by photoinhibition. Simultaneously, the benefits provided by EBR in the photosynthetic apparatus resulted in increments in the accumulation of biomass. Therefore, this research shows evidence of EBR’s ability to alleviate the harmful effects of Pb toxicity in tomato plants.
Author Contributions
A.K.d.S.L. was the advisor of this research, planned all phases of the research and critically revised the manuscript; C.F.M. conducted the experiment in the greenhouse and performed physiological, biochemical, and morphological determinations, wrote and edited the manuscript; B.R.S.d.S. measured anatomical parameters; B.L.B. nutritional analysis; A.B. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Universidade Federal Rural da Amazônia (UFRA/Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request to the corresponding author.
Acknowledgments
This research had financial supports from Fundação Amazônia de Amparo a Estudos e Pesquisas (FAPESPA/Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) and Universidade Federal Rural da Amazônia (UFRA/Brazil) to AKSL. In other hand, CFM was supported with scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil).
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological Approaches to Tackle Heavy Metal Pollution: A Survey of Literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2016, 6, 1–36. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead Toxicity in Plants. Braz. J. Plant Physiol. 2005, 1, 25–52. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy Metal Stress and Responses in Plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide Modulates Growth, Nodulation, Antioxidant System, and Osmolyte in Tolerant and Sensitive Varieties of Vigna Radiata under Different Levels of Nickel: A Shotgun Approach. Plant Physiol. Biochem. 2012, 57, 143–153. [Google Scholar] [CrossRef]
- Barros Junior, U.O.; Lima, M.D.R.; Alsahli, A.A.; Lobato, A.K.S. Unraveling the Roles of Brassinosteroids in Alleviating Drought Stress in Young Eucalyptus Urophylla Plants: Implications on Redox Homeostasis and Photosynthetic Apparatus. Physiol. Plant. 2021, 172, 761–784. [Google Scholar] [CrossRef]
- Rodrigues, W.S.; Pereira, Y.C.; Souza, A.L.M.; Batista, B.L.; Lobato, A.K.S. Alleviation of Oxidative Stress Induced by 24-Epibrassinolide in Soybean Plants Exposed to Different Manganese Supplies: UpRegulation of Antioxidant Enzymes and Maintenance of Photosynthetic Pigments. J. Plant Growth Regul. 2020, 39, 1425–1440. [Google Scholar] [CrossRef]
- Signorella, S.; Palopoli, C.; Ledesma, G. Rationally Designed Mimics of Antioxidant Manganoenzymes: Role of Structural Features in the Quest for Catalysts with Catalase and Superoxide Dismutase Activity. Coord. Chem. Rev. 2018, 365, 75–102. [Google Scholar] [CrossRef]
- da Silva Lobato, A.K.; Barbosa, M.A.M.; Alsahli, A.A.; Lima, E.J.A.; Da Silva, B.R.S. Exogenous Salicylic Acid Alleviates the Negative Impacts on Production Components, Biomass and Gas Exchange in Tomato Plants under Water Deficit Improving Redox Status and Anatomical Responses. Physiol. Plant. 2021, 172, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Hermle, S.; Vollenweider, P.; Gunthardt-Goerg, M.S.; McQuattie, C.J.; Matyssek, R. Leaf Responsiveness of Populus Tremula and Salix Viminalis to Soil Contaminated with Heavy Metals and Acidic Rainwater. Tree Physiol. 2007, 27, 1517–1531. [Google Scholar] [CrossRef]
- Tholen, D.; Boom, C.; Zhu, X.-G. Opinion: Prospects for Improving Photosynthesis by Altering Leaf Anatomy. Plant Sci. 2012, 197, 92–101. [Google Scholar] [CrossRef]
- Javelle, M.; Vernoud, V.; Rogowsky, P.M.; Ingram, G.C. Epidermis: The Formation and Functions of a Fundamental Plant Tissue. New Phytol. 2011, 189, 17–39. [Google Scholar] [CrossRef]
- Glover, B.J.; Airoldi, C.A.; Moyroud, E. Epidermis: Outer Cell Layer of the Plant. In eLS; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–7. ISBN 9780470015902. [Google Scholar]
- Sorin, C.; Musse, M.; Mariette, F.; Bouchereau, A.; Leport, L. Assessment of Nutrient Remobilization through Structural Changes of Palisade and Spongy Parenchyma in Oilseed Rape Leaves during Senescence. Planta 2015, 241, 333–346. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Wang, R.; Mo, G.; Luo, S.; Luo, X.; He, L.; Gonsamo, A.; Arabian, J.; Zhang, Y.; et al. The Global Distribution of Leaf Chlorophyll Content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in Photosynthetic Pigments, Protein, and Carbohydrate Metabolism in a Wild Plant Coronopus didymus L. (Brassicaceae) under Lead Stress. Acta Physiol. Plant. 2017, 39, 176. [Google Scholar] [CrossRef]
- Hussain, I.; Siddique, A.; Ashraf, M.A.; Rasheed, R.; Ibrahim, M.; Iqbal, M.; Akbar, S.; Imran, M. Does Exogenous Application of Ascorbic Acid Modulate Growth, Photosynthetic Pigments and Oxidative Defense in Okra (Abelmoschus esculentus (L.) Moench) under Lead Stress? Acta Physiol. Plant. 2017, 39, 144. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Plant-Lead Interactions: Transport, Toxicity, Tolerance, and Detoxification Mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Vardhini, B.V.; Anjum, N.A. Brassinosteroids Make Plant Life Easier under Abiotic Stresses Mainly by Modulating Major Components of Antioxidant Defense System. Front. Environ. Sci. 2015, 2, 872–876. [Google Scholar] [CrossRef]
- Vidya Vardhini, B. Modifications of Morphological and Anatomical Characteristics of Plants by Application of Brassinosteroids under Various Abiotic Stress Conditions—A Review. Plant Gene 2017, 11, 70–89. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Zulfiqar, B.; Akram, A.; Ashraf, M.Y.; Raheel, M.; Shabbir, R.N.; Hussain, R.A.; Anwar, I.; Aurangzaib, M. Understanding Brassinosteroid-Regulated Mechanisms to Improve Stress Tolerance in Plants: A Critical Review. Environ. Sci. Pollut. Res. 2017, 24, 15959–15975. [Google Scholar] [CrossRef]
- Zhong, W.; Xie, C.; Hu, D.; Pu, S.; Xiong, X.; Ma, J.; Sun, L.; Huang, Z.; Jiang, M.; Li, X. Effect of 24-Epibrassinolide on Reactive Oxygen Species and Antioxidative Defense Systems in Tall Fescue Plants under Lead Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109831. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Mir, R.A.; Alyemeni, M.N.; Ahmad, P. Combined Effects of Brassinosteroid and Kinetin Mitigates Salinity Stress in Tomato through the Modulation of Antioxidant and Osmolyte Metabolism. Plant Physiol. Biochem. 2020, 147, 31–42. [Google Scholar] [CrossRef]
- Zhang, Y.P.; He, J.; Yang, S.J.; Chen, Y.Y. Exogenous 24-Epibrassinolide Ameliorates High Temperature-Induced Inhibition of Growth and Photosynthesis in Cucumis Melo. Biol. Plant. 2014, 58, 311–318. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid Alleviates Chilling-Induced Oxidative Stress in Pepper by Enhancing Antioxidation Systems and Maintenance of Photosystem II. Acta Physiol. Plant. 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Lima, J.V.; Lobato, A.K.S. Brassinosteroids Improve Photosystem II Efficiency, Gas Exchange, Antioxidant Enzymes and Growth of Cowpea Plants Exposed to Water Deficit. Physiol. Mol. Biol. Plants 2017, 23, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Pereira, Y.C.; Rodrigues, W.S.; Lima, E.J.A.; Santos, L.R.; Silva, M.H.L.; Lobato, A.K.S. Brassinosteroids Increase Electron Transport and Photosynthesis in Soybean Plants under Water Deficit. Photosynthetica 2019, 57, 181–191. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and Physiological Functions in Plants under Heavy Metal Stress. Front. Plant Sci. 2021; in press. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil, 2nd ed.; Agricultural Experiment Station: California, CA, USA, 1950. [Google Scholar]
- Guedes, F.R.C.M.; Maia, C.F.; da Silva, B.R.S.; Batista, B.L.; Alyemeni, M.N.; Ahmad, P.; da Silva Lobato, A.K. Exogenous 24-Epibrassinolide Stimulates Root Protection, and Leaf Antioxidant Enzymes in Lead Stressed Rice Plants: Central Roles to Minimize Pb Content and Oxidative Stress. Environ. Pollut. 2021, 280, 116992. [Google Scholar] [CrossRef]
- Maia, C.F.; Silva, B.R.S.; Lobato, A.K.S. Brassinosteroids Positively Modulate Growth: Physiological, Biochemical and Anatomical Evidence Using Two Tomato Genotypes Contrasting to Dwarfism. J. Plant Growth Regul. 2018, 37, 1–14. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Paniz, F.P.; Pedron, T.; Freire, B.M.; Torres, D.P.; Silva, F.F.; Batista, B.L. Effective Procedures for the Determination of As, Cd, Cu, Fe, Hg, Mg, Mn, Ni, Pb, Se, Th, Zn, U and Rare Earth Elements in Plants and Foodstuffs. Anal. Methods 2018, 10, 4094–4103. [Google Scholar] [CrossRef]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic Staining of Plant Cell Walls by Toluidine Blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Segatto, F.B.; Bisognin, D.A.; Benedetti, M.; Costa, L.C.; Rampelotto, M.V.; Nicoloso, F.T. A Technique for the Anatomical Study of Potato Leaf Epidermis. Ciência Rural 2004, 34, 1597–1601. [Google Scholar] [CrossRef]
- Castro, E.M.; Pereira, F.J.; Paiva, R. Plant Histology: Structure and Function of Vegetative Organs, 1st ed.; UFLA Publishers: Lavras, Brazil, 2009. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 431–438. ISBN 9780471709084. [Google Scholar]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L. Photosynthesis, Transpiration, and Water Use Efficiency of Caragana Microphylla, C. Intermedia, and C. Korshinskii. Photosynthetica 2004, 42, 65–70. [Google Scholar] [CrossRef]
- Aragão, R.M.; Silva, E.N.; Vieira, C.F.; Silveira, J.A.G. High Supply of NO3−Mitigates Salinity Effects through an Enhancement in the Efficiency of Photosystem II and CO2 Assimilation in Jatropha Curcas Plants. Acta Physiol. Plant. 2012, 34, 2135–2143. [Google Scholar] [CrossRef]
- Badawi, G.H.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kawano, N.; Tanaka, K.; Tanaka, K. Enhanced Tolerance to Salt Stress and Water Deficit by Overexpressing Superoxide Dismutase in Tobacco (Nicotiana tabacum) Chloroplasts. Plant Sci. 2004, 166, 919–928. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of Nitrite Formation from Hydroxylammoniumchloride: A Simple Assay for Superoxide Dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Reactive Oxygen Metabolism in Mycorrhizal and Non-Mycorrhizal Citrus (Poncirus Trifoliata) Seedlings Subjected to Water Stress. J. Plant Physiol. 2006, 163, 1101–1110. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of Aluminium on Lipid Peroxidation, Superoxide Dismutase, Catalase, and Peroxidase Activities in Root Tips of Soybean (Glycine Max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic Acid-Induced Thermotolerance in Maize Seedlings Is Mediated by Calcium and Associated with Antioxidant Systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; Academic Internet Publishers: Moorpark, CA, USA, 2006. [Google Scholar]
- Venables, W.N.; Smith, D.M. R Core Team: An Introduction to R; R Core Team Publishers: USA, 2021; Available online: https://www.studypool.com/documents/8034079/an-introduction-to-r-author-w-n-venables-d-m-smith-the-r-core-team (accessed on 21 August 2022).
- Dalyan, E.; Yüzbaşıoğlu, E.; Akpınar, I. Effect of 24-Epibrassinolide on Antioxidative Defence System Against Lead-Induced Oxidative Stress in The Roots of Brassica juncea L. Seedlings. Russ. J. Plant Physiol. 2018, 65, 570–578. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined Effect of 24-Epibrassinolide and Salicylic Acid Mitigates Lead (Pb) Toxicity by Modulating Various Metabolites in Brassica juncea L. Seedlings. Protoplasma 2018, 255, 11–24. [Google Scholar] [CrossRef]
- Rossato, L.V.; Nicoloso, F.T.; Farias, J.G.; Cargnelluti, D.; Tabaldi, L.A.; Antes, F.G.; Dressler, V.L.; Morsch, V.M.; Schetinger, M.R.C. Effects of Lead on the Growth, Lead Accumulation and Physiological Responses of Pluchea Sagittalis. Ecotoxicology 2012, 21, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Talarek-Karwel, M.; Bajguz, A.; Piotrowska-Niczyporuk, A. 24-Epibrassinolide Modulates Primary Metabolites, Antioxidants, and Phytochelatins in Acutodesmus Obliquus Exposed to Lead Stress. J. Appl. Phycol. 2020, 32, 263–276. [Google Scholar] [CrossRef]
- Khan, F.; Hussain, S.; Tanveer, M.; Khan, S.; Hussain, H.A.; Iqbal, B.; Geng, M. Coordinated Effects of Lead Toxicity and Nutrient Deprivation on Growth, Oxidative Status, and Elemental Composition of Primed and Non-Primed Rice Seedlings. Environ. Sci. Pollut. Res. 2018, 25, 21185–21194. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Sá e Melo Marques, T.C.L.L.; de Oliveira Gonçalves Nogueira, M.; de Castro, E.M.; Soares, Â.M. Ecophysiological and Anatomical Changes Due to Uptake and Accumulation of Heavy Metal in Brachiaria Decumbens. Sci. Agric. 2011, 68, 566–573. [Google Scholar] [CrossRef]
- Pereira, Y.C.; da Silva, F.R.; da Silva, B.R.S.; Cruz, F.J.R.; Marques, D.J.; da Silva Lobato, A.K. 24-Epibrassinolide Induces Protection against Waterlogging and Alleviates Impacts on the Root Structures, Photosynthetic Machinery and Biomass in Soybean. Plant Signal. Behav. 2020, 15, 1805885. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhu, S.; Shu, S.; Sun, J.; Guo, S. Regulation of 2,4-Epibrassinolide on Mineral Nutrient Uptake and Ion Distribution in Ca(NO3)2 Stressed Cucumber Plants. J. Plant Physiol. 2015, 188, 29–36. [Google Scholar] [CrossRef]
- Jan, S.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Siddique, K.H.; Ahmad, P. Interactive Effect of 24-Epibrassinolide and Silicon Alleviates Cadmium Stress via the Modulation of Antioxidant Defense and Glyoxalase Systems and Macronutrient Content in Pisum sativum L. Seedlings. BMC Plant Biol. 2018, 18, 146. [Google Scholar] [CrossRef]
- Santos, L.R.; da Silva, B.R.S.; Pedron, T.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide Improves Root Anatomy and Antioxidant Enzymes in Soybean Plants Subjected to Zinc Stress. J. Soil Sci. Plant Nutr. 2020, 20, 105–124. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Bali, S.; Arora, S.; Sharma, A.; Kaur, R.; Bhardwaj, R. Modulation of Antioxidative Defense Expression and Osmolyte Content by Co-Application of 24-Epibrassinolide and Salicylic Acid in Pb Exposed Indian Mustard Plants. Ecotoxicol. Environ. Saf. 2018, 147, 382–393. [Google Scholar] [CrossRef]
- Xia, X.-J.; Wang, Y.-J.; Zhou, Y.-H.; Tao, Y.; Mao, W.-H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.-Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumar, A.; Bhardwaj, R. Plant Steroidal Hormone Epibrassinolide Regulate–Heavy Metal Stress Tolerance in Oryza sativa L. by Modulating Antioxidant Defense Expression. Environ. Exp. Bot. 2016, 122, 1–9. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huo, S.; Wang, L.; Meng, J.; Zhang, Z.; Xi, Z. Exogenous 24-Epibrassinolide Alleviates Oxidative Damage from Copper Stress in Grape (Vitis vinifera L.) Cuttings. Plant Physiol. Biochem. 2018, 130, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Interaction of 24-Epibrassinolide and Salicylic Acid Regulates Pigment Contents, Antioxidative Defense Responses, and Gene Expression in Brassica Juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018, 25, 15159–15173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prasad, M.N.V.; Sytar, O. Lead Toxicity, Defense Strategies and Associated Indicative Biomarkers in Talinum Triangulare Grown Hydroponically. Chemosphere 2012, 89, 1056–1065. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and Lead Interactive Effects on Oxidative Stress and Antioxidative Responses in Rice Seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive Oxygen Species and Heavy Metal Stress in Plants: Impact on the Cell Wall and Secondary Metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids Protect Photosynthesis and Antioxidant System of Eggplant Seedlings from High-Temperature Stress. Acta Physiol. Plant. 2014, 36, 251–261. [Google Scholar] [CrossRef]
- Ali, B.; Mwamba, T.M.; Gill, R.A.; Yang, C.; Ali, S.; Daud, M.K.; Wu, Y.; Zhou, W. Improvement of Element Uptake and Antioxidative Defense in Brassica Napus under Lead Stress by Application of Hydrogen Sulfide. Plant Growth Regul. 2014, 74, 261–273. [Google Scholar] [CrossRef]
- Kohli, S.K.; Bali, S.; Tejpal, R.; Bhalla, V.; Verma, V.; Bhardwaj, R.; Alqarawi, A.A.; Abd_Allah, E.F.; Ahmad, P. In-Situ Localization and Biochemical Analysis of Bio-Molecules Reveals Pb-Stress Amelioration in Brassica juncea L. by Co-Application of 24-Epibrassinolide and Salicylic Acid. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Hou, X.; Han, H.; Cai, L.; Liu, A.; Ma, X.; Zhou, C.; Wang, G.; Meng, F. Pb Stress Effects on Leaf Chlorophyll Fluorescence, Antioxidative Enzyme Activities, and Organic Acid Contents of Pogonatherum Crinitum Seedlings. Flora 2018, 240, 82–88. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, S.; Wang, C.; Lu, J. Effects of Pb on the Oxidative Stress and Antioxidant Response in a Pb Bioaccumulator Plant Vallisneria Natans. Ecotoxicol. Environ. Saf. 2012, 78, 28–34. [Google Scholar] [CrossRef]
- Li, J.; Yang, P.; Kang, J.; Gan, Y.; Yu, J.; Calderón-Urrea, A.; Lyu, J.; Zhang, G.; Feng, Z.; Xie, J. Transcriptome Analysis of Pepper (Capsicum annuum) Revealed a Role of 24-Epibrassinolide in Response to Chilling. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, M.N.V. Lead-Induced Toxicity and Interference in Chlorophyll Fluorescence in Talinum Triangulare Grown Hydroponically. Photosynthetica 2015, 53, 66–71. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, Y.J.; Xu, X.L.; Jin, M.; An, L.Z.; Zhang, H. Effect of 24-Epibrassinolide on Drought Stress-Induced Changes in Chorispora Bungeana. Biol. Plant. 2012, 56, 192–196. [Google Scholar] [CrossRef]
- Tadaiesky, L.B.A.; Silva, B.R.S.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Trigger Tolerance to Iron Toxicity in Rice. Physiol. Plant. 2020, 171, 371–387, Published, ppl.13230. [Google Scholar] [CrossRef]
- Huang, X.H.; Zhu, F.; Yan, W.D.; Chen, X.Y.; Wang, G.J.; Wang, R.J. Effects of Pb and Zn Toxicity on Chlorophyll Fluorescence and Biomass Production of Koelreuteria Paniculata and Zelkova Schneideriana Young Plants. Photosynthetica 2019, 57, 688–697. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Homvisasevongsa, S.; Suksamrarn, A. Comparative Effects of Brassinosteroid and Brassinosteroid Mimic on Improving Photosynthesis, Lipid Peroxidation, and Rice Seed Set under Heat Stress. J. Plant Growth Regul. 2015, 34, 320–331. [Google Scholar] [CrossRef]
- Alam, P.; Albalawi, T.H.; Altalayan, F.H.; Bakht, M.A.; Ahanger, M.A.; Raja, V.; Ashraf, M.; Ahmad, P. 24-Epibrassinolide (EBR) Confers Tolerance against NaCl Stress in Soybean Plants by up-Regulating Antioxidant System, Ascorbate-Glutathione Cycle, and Glyoxalase System. Biomolecules 2019, 9, 640. [Google Scholar] [CrossRef]
- Santos, L.R.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Mitigate Cadmium Toxicity in Cowpea Plants. Photosynthetica 2018, 56, 591–605. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M.; Tabassam, Q.; Hussain, M.; Firdous, H. Lead (Pb)-Induced Regulation of Growth, Photosynthesis, and Mineral Nutrition in Maize (Zea mays L.) Plants at Early Growth Stages. Biol. Trace Elem. Res. 2011, 144, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Nas, F.S. The Effect of Lead on Plants in Terms of Growing and Biochemical Parameters: A Review. MOJ Ecol. Environ. Sci. 2018, 3. [Google Scholar] [CrossRef]
- Cunha, L.F.S.; Oliveira, V.P.; Nascimento, A.W.S.; Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; Silva Lobato, A.K. Leaf Application of 24-epibrassinolide Mitigates Cadmium Toxicity in Young Eucalyptus Urophylla Plants by Modulating Leaf Anatomy and Gas Exchange. Physiol. Plant. 2020. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, Z.; Ma, J.; Yang, L.; Wei, Y. The Effects of Lead Stress on Photosynthetic Function and Chloroplast Ultrastructure of Robinia Pseudoacacia Seedlings. Environ. Sci. Pollut. Res. 2017, 24, 10718–10726. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Oniosun, B.; Akpoilih, O.A.; Adeseko, A.; Arowo, D.O. Anatomical Changes, Osmolytes Accumulation and Distribution in the Native Plants Growing on Pb-Contaminated Sites. Environ. Geochem. Health 2021, 43, 1537–1549. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Vanhoutte, I.; Boudolf, V.; Betti, C.; Dhondt, S.; Coppens, F.; Mylle, E.; Maes, S.; González-García, M.-P.; Caño-Delgado, A.I.; et al. Brassinosteroid Production and Signaling Differentially Control Cell Division and Expansion in the Leaf. New Phytol. 2013, 197, 490–502. [Google Scholar] [CrossRef]
- dos Santos, L.R.; de Sousa Paula, L.; Pereira, Y.C.; da Silva, B.R.S.; Batista, B.L.; Alsahli, A.A.; da Silva Lobato, A.K. Brassinosteroids-Mediated Amelioration of Iron Deficiency in Soybean Plants: Beneficial Effects on the Nutritional Status, Photosynthetic Pigments and Chlorophyll Fluorescence. J. Plant Growth Regul. 2021, 40, 1803–1823. [Google Scholar] [CrossRef]
- Oliveira, V.P.; Lima, M.D.R.; Silva, B.R.S.; Batista, B.L.; Lobato, A.K.S. Brassinosteroids Confer Tolerance to Salt Stress in Eucalyptus Urophylla Plants Enhancing Homeostasis, Antioxidant Metabolism and Leaf Anatomy. J. Plant Growth Regul. 2019, 38, 557–573. [Google Scholar] [CrossRef]
- Pereira, M.P.; de Almeida Rodrigues, L.C.; Corrêa, F.F.; de Castro, E.M.; Ribeiro, V.E.; Pereira, F.J. Cadmium Tolerance in Schinus Molle Trees Is Modulated by Enhanced Leaf Anatomy and Photosynthesis. Trees 2016, 30, 807–814. [Google Scholar] [CrossRef]
- Ribeiro, A.T.; de Oliveira, V.P.; de Oliveira Barros Junior, U.; da Silva, B.R.S.; Batista, B.L.; da Silva Lobato, A.K. 24-Epibrassinolide Mitigates Nickel Toxicity in Young Eucalyptus Urophylla S.T. Blake Plants: Nutritional, Physiological, Biochemical, Anatomical and Morphological Responses. Ann. For. Sci. 2020, 77. [Google Scholar] [CrossRef]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy Metal-Induced Oxidative Damage, Defense Reactions, and Detoxification Mechanisms in Plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Ahmed, M.; Mir, B.A.; Yusuf, M.; Khan, T.A. 24-Epibrassinolide Mitigates the Adverse Effects of Manganese Induced Toxicity through Improved Antioxidant System and Photosynthetic Attributes in Brassica Juncea. Environ. Sci. Pollut. Res. 2015, 22, 11349–11359. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Noman, A.; Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. 24-Epibrassinolide Alleviates the Injurious Effects of Cr(VI) Toxicity in Tomato Plants: Insights into Growth, Physio-Biochemical Attributes, Antioxidant Activity and Regulation of Ascorbate–Glutathione and Glyoxalase Cycles. J. Plant Growth Regul. 2020, 39, 1587–1604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).