Sewage Sludge Fertilization—A Case Study of Sweet Potato Yield and Heavy Metal Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Agronomic Procedure
2.3. Soil Physicochemical Properties
2.4. Heavy Metals
2.5. Statistical Analysis
3. Results
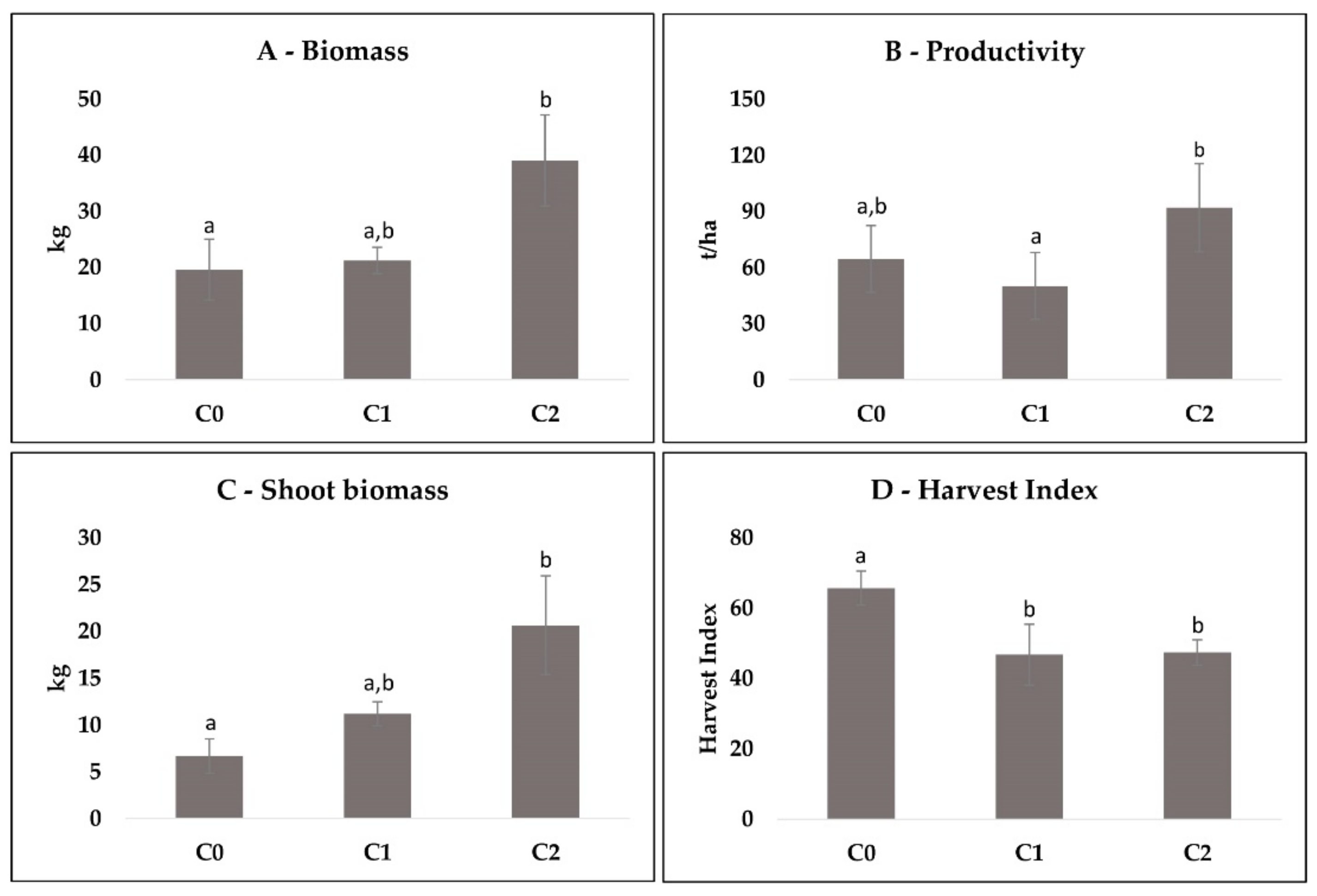
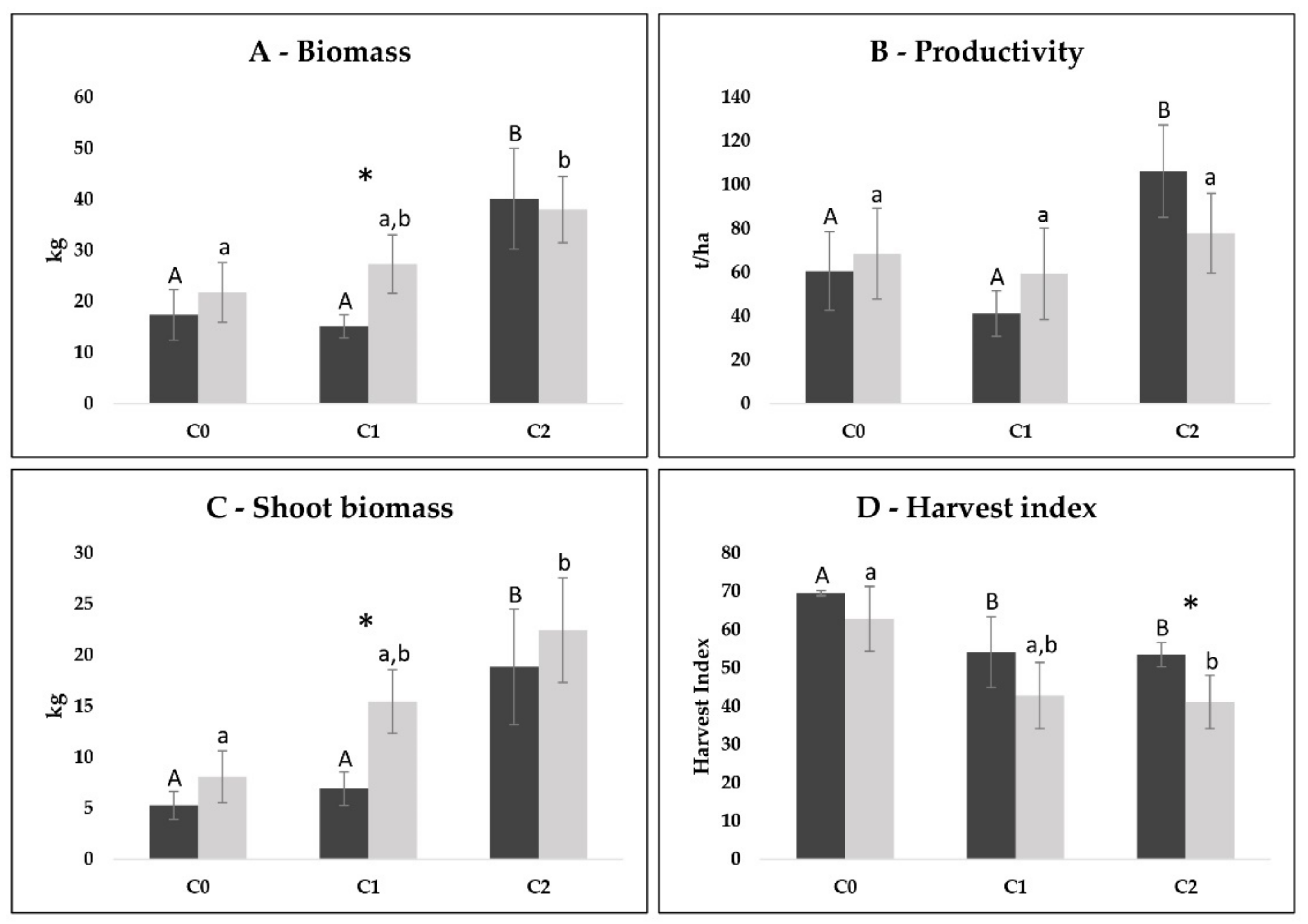
3.1. Agronomic Data per Plot
3.2. Comparison between Cycles per Plot
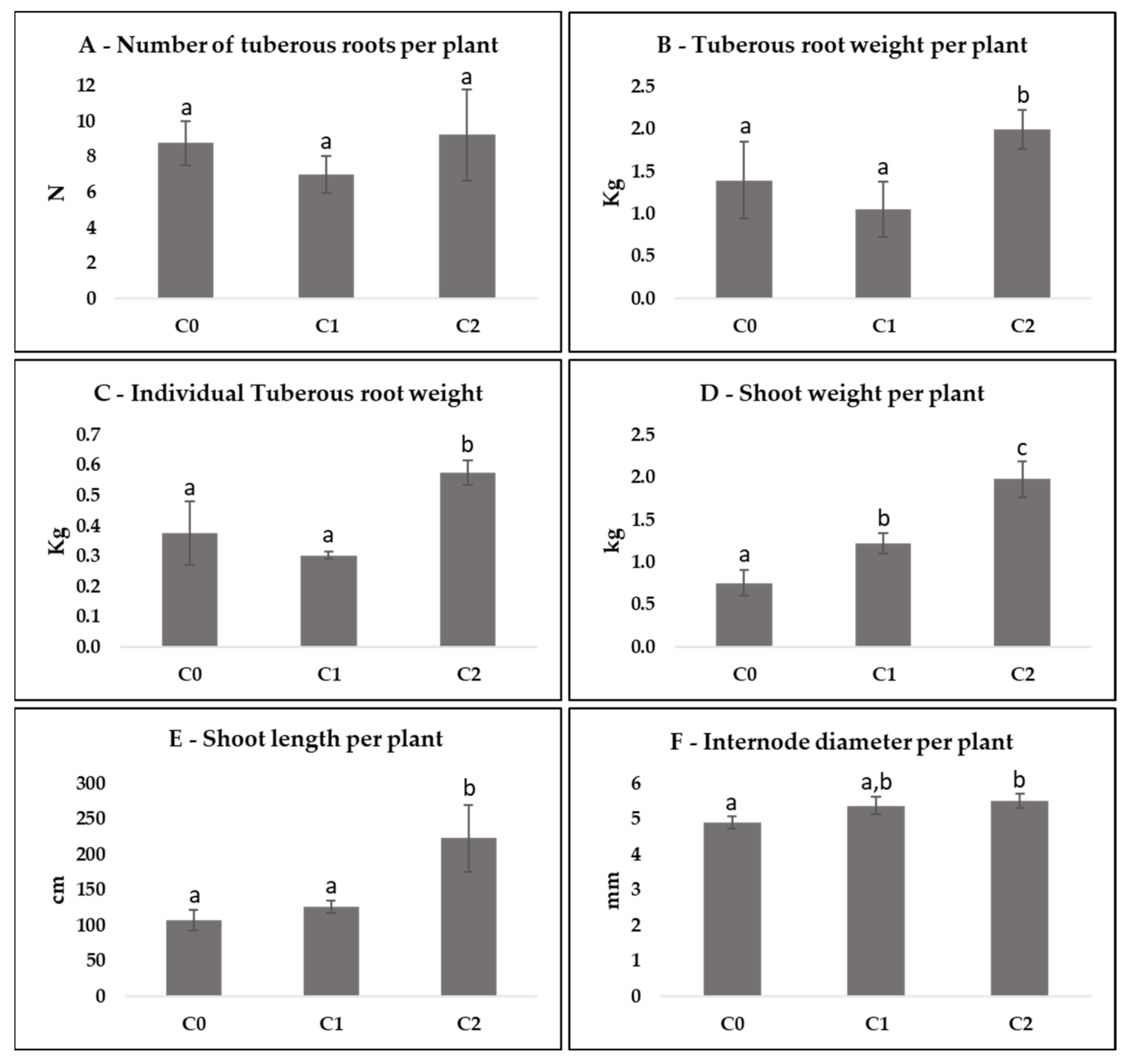
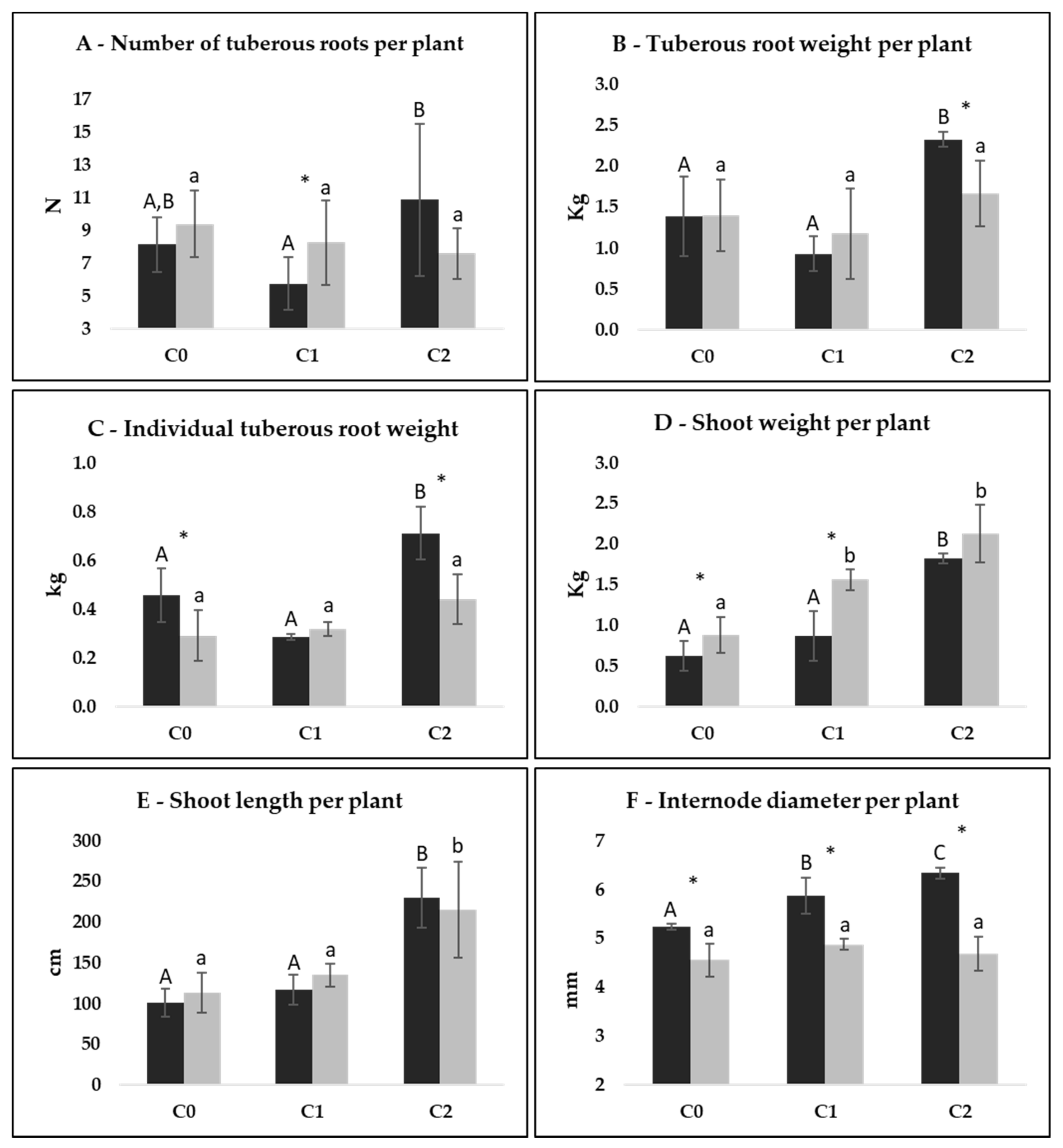
3.3. Agronomic Data per Plant
3.4. Comparison between Cycles per Plant
3.5. Soil Physicochemical Properties after SS Application
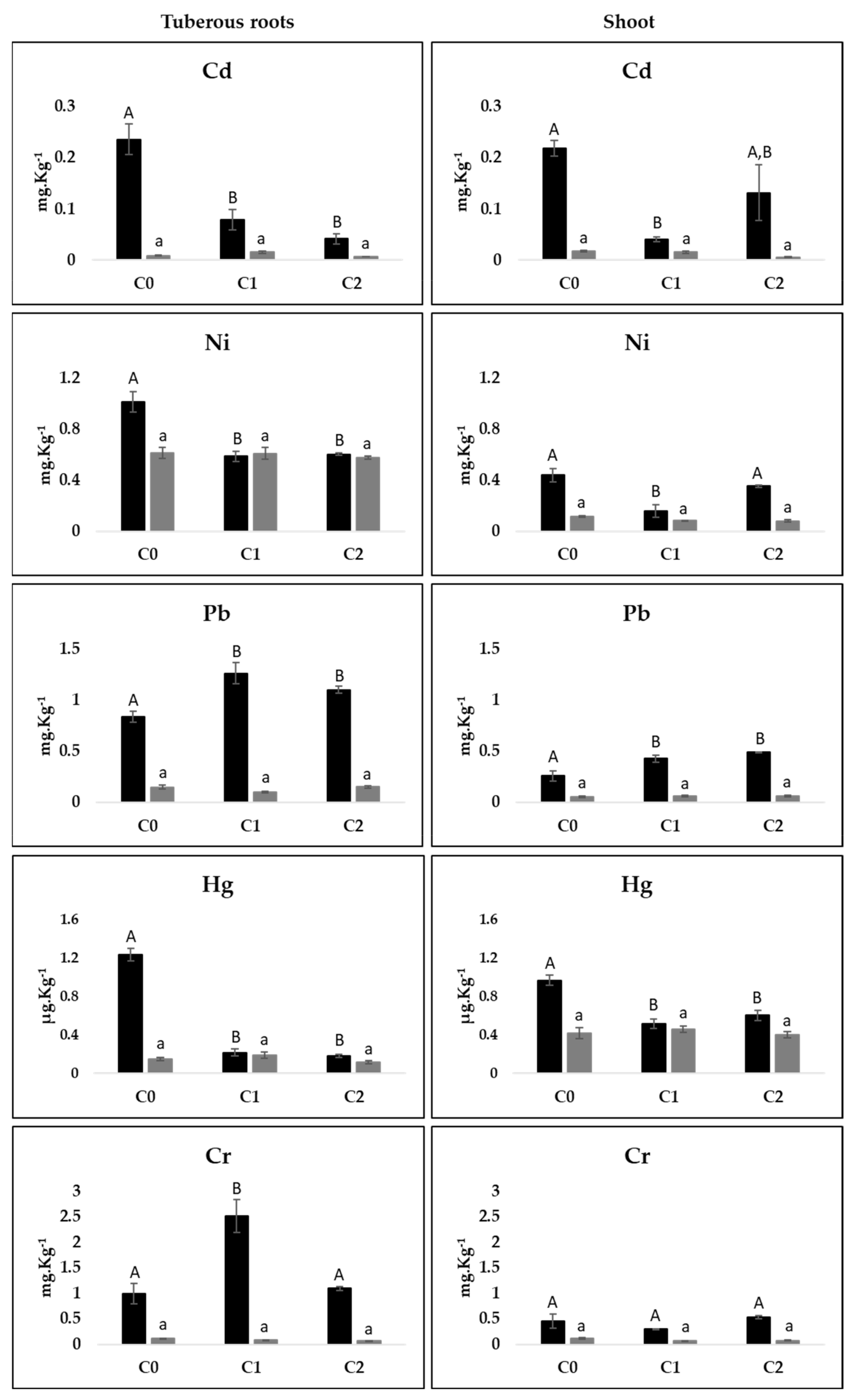
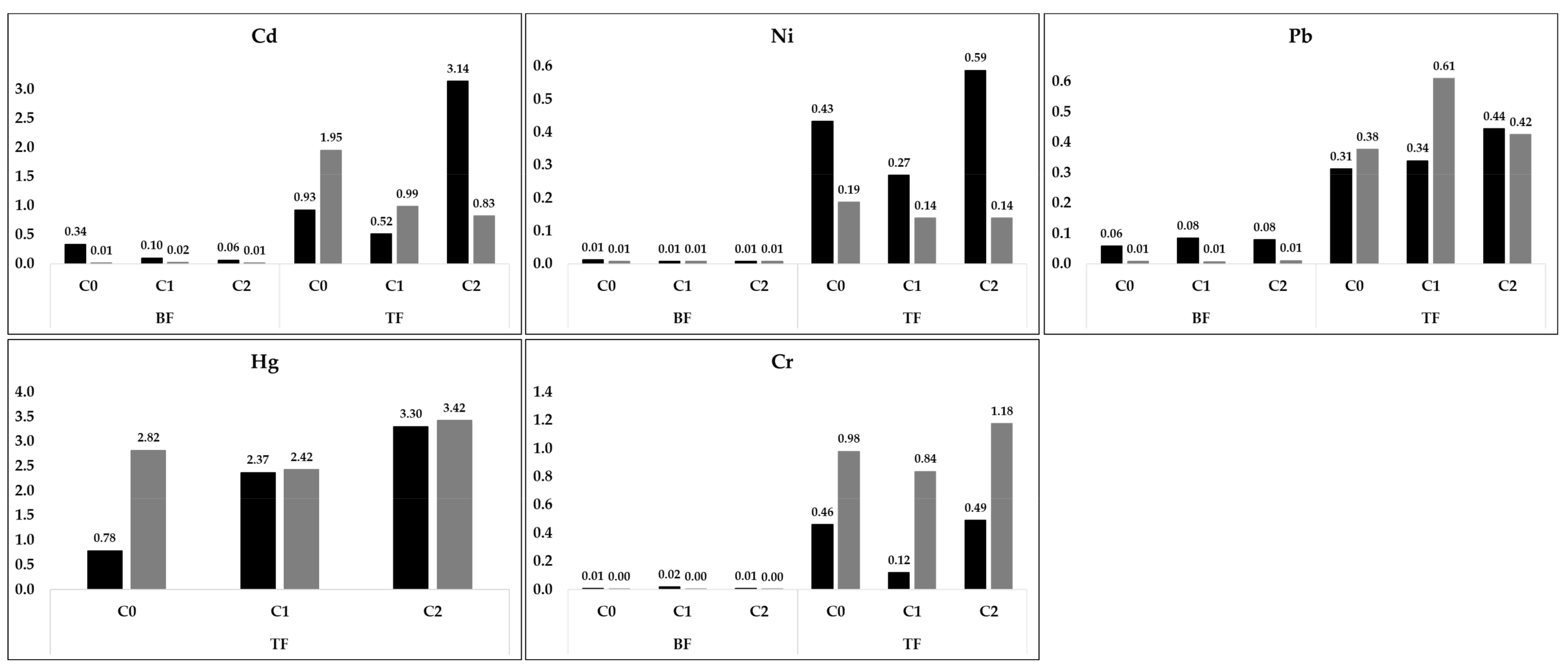
3.6. Effect of SS Application on Heavy Metals Contents
4. Discussion
4.1. Agronomic Data per Plot
4.2. Agronomic Data per Plant
4.3. Impact on Soil Physicochemical Properties
4.4. Heavy Metal Content in Soil and Sweet Potato Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Demirbas, A.; Edris, G.; Alalayah, W.M. Sludge production from municipal wastewater treatment in sewage treatment plant. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 999–1006. [Google Scholar] [CrossRef]
- Pereira, I.S.; Bamberg, A.L.; Sousa, R.O.; Monteiro, A.B.; Martinazzo, R.; Silveira, C.A.P.; Silveira, A.O. Agricultural use and pH correction of anaerobic sewage sludge with acid pH. J. Environ. Manag. 2020, 275, 111203. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Martín-Consuegra, S.; García-Navarro, F.J.; Amorós-Ortíz-Villajos, J.Á.; Pérez-de-los-Reyes, C.; Higueras, P.L. Effect of the addition of sewage sludge as a fertilizer on a sandy vineyard soil. J. Soils Sediments 2016, 16, 1360–1365. [Google Scholar] [CrossRef]
- Liew, C.S.; Kiatkittipong, W.; Lim, J.W.; Lam, M.K.; Ho, Y.C.; Ho, C.D.; Ntwampe, S.K.O.; Mohamad, M.; Usman, A. Stabilization of heavy metals loaded sewage sludge: Reviewing conventional to state-of-the-art thermal treatments in achieving energy sustainability. Chemosphere 2021, 277, 130310. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, N.; Li, L.Y. The one-stage autothermal thermophilic aerobic digestion for sewage sludge treatment: Stabilization process and mechanism. Bioresour. Technol. 2012, 104, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.; Li, W.; Shou, Z.; Yuan, H.; Lou, Z.; Zhu, N.; Cai, C. Comparison of effects of ferric nitrate additions in thermophilic, mesophilic and psychrophilic aerobic digestion for sewage sludge. J. Taiwan Inst. Chem. Eng. 2016, 67, 346–354. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, W.; Chen, J.; Wang, X.; Xing, D.; Dong, W.; Wang, H.; Wang, J. The combination of aerobic digestion and bioleaching for heavy metal removal from excess sludge. Chemosphere 2022, 290, 133231. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Pharmaceutically active compounds in sludge stabilization treatments: Anaerobic and aerobic digestion, wastewater stabilization ponds and composting. Sci. Total Environ. 2015, 503–504, 97–104. [Google Scholar] [CrossRef]
- Nges, I.A.; Liu, J. Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophilic and thermophilic conditions. Renew. Energy 2010, 35, 2200–2206. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Lee, H.S.; Zhang, H.; Alder, J.; Banaszak, J.E.; Lopez, R. Full-scale application of focused-pulsed pre-treatment for improving biosolids digestion and conversion to methane. Water Sci. Technol. 2008, 58, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Samaras, P.; Papadimitriou, C.A.; Haritou, I.; Zouboulis, A.I. Investigation of sewage sludge stabilization potential by the addition of fly ash and lime. J. Hazard. Mater. 2008, 154, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Samara, E.; Matsi, T.; Balidakis, A. Soil application of sewage sludge stabilized with steelmaking slag and its effect on soil properties and wheat growth. Waste Manag. 2017, 68, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Casado-Vela, J.; Sellés, S.; Díaz-Crespo, C.; Navarro-Pedreño, J.; Mataix-Beneyto, J.; Gómez, I. Effect of composted sewage sludge application to soil on sweet pepper crop (Capsicum annuum var. annuum) grown under two exploitation regimes. Waste Manag. 2007, 27, 1509–1518. [Google Scholar] [CrossRef]
- Suthar, S. Pilot-scale vermireactors for sewage sludge stabilization and metal remediation process: Comparison with small-scale vermireactors. Ecol. Eng. 2010, 36, 703–712. [Google Scholar] [CrossRef]
- APA. Gestão de Lamas Abrangidas pelo Decreto-Lei n.o 276/2009, Report from Portuguese Environmental Protection Agency; Diário da República: Lisbon, Portugal, 2017; pp. 7156–7160. [Google Scholar]
- Águas e Resíduos da Madeira, S.A. WWTP Porto Santo. Available online: http://www.aguasdamadeira.pt/Portals/0/Documentos/Instalações/ETAR%20da%20Ponta.pdf (accessed on 11 March 2022).
- European Commission. Protection of the Environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Communities 1986, 4, 6–12. [Google Scholar]
- FAO. Agricultural Use of Sewage. In Waste Water Treatment and Use in Agriculture, FAO Irrigation and Drawing Paper; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992; p. 47. [Google Scholar]
- European Commission. Setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 12, 5–24. [Google Scholar]
- Tang, Y.; Xie, H.; Sun, J.; Li, X.; Zhang, Y.; Dai, X. Alkaline thermal hydrolysis of sewage sludge to produce high-quality liquid fertilizer rich in nitrogen-containing plant-growth-promoting nutrients and biostimulants. Water Res. 2022, 211, 118036. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chopra, A.K. Accumulation and translocation of metals in soil and different parts of french bean (Phaseolus vulgaris L.) amended with sewage sludge. Bull. Environ. Contam. Toxicol. 2014, 92, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 2010, 36, 969–972. [Google Scholar] [CrossRef]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. J. Sci. Food Agric. 2010, 90, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 2007, 67, 2229–2240. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Y. Effects of sewage sludge compost application on crops and cropland in a 3-year field study. Chemosphere 2005, 59, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Formulation and evaluation of organo-mineral fertilizers based on sewage sludge optimized for maize and sunflower crops. Waste Manag. 2021, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Potentiality of sewage sludge-based organo-mineral fertilizer production in Poland considering nutrient value, heavy metal content and phytotoxicity for rapeseed crops. J. Environ. Manag. 2019, 248, 109283. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.C.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Lag-Brotons, A.; Gómez, I.; Navarro-Pedreño, J.; Mayoral, A.M.; Curt, M.D. Sewage sludge compost use in bioenergy production—A case study on the effects on Cynara cardunculus L energy crop. J. Clean. Prod. 2014, 79, 32–40. [Google Scholar] [CrossRef]
- Guoqing, X.; Xiuqin, C.; Liping, B.; Hongtao, Q.; Haibo, L. Absorption, accumulation and distribution of metals and nutrient elements in poplars planted in land amended with composted sewage sludge: A field trial. Ecotoxicol. Environ. Saf. 2019, 182, 109360. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rosikon, K.; Grobelak, A.; Hutchison, D.; Kacprzak, M.J. Modification of properties of energy crops under Polish condition as an effect of sewage sludge application onto degraded soil. J. Environ. Manag. 2018, 217, 509–519. [Google Scholar] [CrossRef]
- Direção-Geral Agricultura e Desenvolvimento Rural Portuguese Tradicional Products. Available online: https://tradicional.dgadr.gov.pt/pt/cat/horticolas-e-cereais/1108-batata-doce-da-madeira (accessed on 11 March 2022).
- Direcção Regional de Estatística, Agricultural Statistics. Available online: https://estatistica.madeira.gov.pt/download-now/economica/agricultura-floresta-e-pesca/prod-veg-prd-animal-pesca-pt/prod-vegetal-quadros-pt/quadros-estatisticas-anuais-pt.html (accessed on 8 February 2022).
- Fontinha, S.; Andrade, S.; Pinheiro de Carvalho, M.Â.A. Plant data survey in Porto Santo. Boletim 2020, 70, 11–18. [Google Scholar]
- Madeira, M.; Pinheiro, J.; Madruga, J.; Monteiro, F. Soils of volcanic systems in Portugal. In Soils of Volcanic Regions in Europe; Arnalds, Ó., Óskarsson, H., Bartoli, F., Buurman, P., Stoops, G., García-Rodeja, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 69–81. ISBN 978-3-540-48710-4. [Google Scholar]
- Ganança, J.F.T.; Freitas, J.G.R.; Nóbrega, H.G.M.; Rodrigues, V.; Antunes, G.; Gouveia, C.S.S.; Rodrigues, M.; Chaïr, H.; De Carvalho, M.Â.A.P.; Lebot, V. Screening for drought tolerance in thirty three taro cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 65–74. [Google Scholar] [CrossRef]
- Anislag, M. V Varietal Performance of Ipomoea batatas L. to Fertilization Strategies as Intercropped in Mulberry Trees. Int. J. Innov. Sci. Res. Technol. 2019, 4, 385–407. [Google Scholar]
- Pinheiro de Carvalho, M.Â.A.; Slaski, J.J.; dos Santos, T.M.M.; Ganança, F.T.; Abreu, I.; Taylor, G.J.; Clemente Vieira, M.R.; Popova, T.N.; Franco, E. Identification of Aluminum Resistant Genotypes Among Madeiran Regional Wheats. Commun. Soil Sci. Plant Anal. 2003, 34, 2967–2979. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 3, 29–387. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation Exchange Capacity. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar]
- Riehm, H. Die ammonium-laktatessignäure. Methods zur bestimmung der leichtloslichen phosphorsäure in karbonataligen böden. Agrochimica 1958, 3, 49–65. [Google Scholar]
- ISO 11885 (2007); Water quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standardization: Geneva, Switzerland, 2007.
- EN 1483:2007; Water Quality—Determination of Mercury—Method Using Atomic Absorption Spectrometry. European Committee for Standardization: Brussels, Belgium, 2007.
- Hansen, T.H.; de Bang, T.C.; Laursen, K.H.; Pedas, P.; Husted, S.; Schjoerring, J.K. Multielement Plant Tissue Analysis Using ICP Spectrometry. In Plant Mineral Nutrients: Methods and Protocols, Methods in Molecular Biology; Maathuis, F.J.M., Ed.; Humana Press: New Delhi, India, 2012; pp. 121–141. ISBN 978-1-62703-152-3. [Google Scholar]
- Eid, E.M.; Shaltout, K.H.; Alamri, S.A.M.; Alrumman, S.A.; Hussain, A.A.; Sewelam, N.; Ragab, G.A. Monitored Sewage Sludge Application Improves Soil Quality, Enhances Plant Growth, and Provides Evidence for Metal Remediation by Sorghum bicolor L. J. Soil Sci. Plant Nutr. 2021, 21, 2325–2338. [Google Scholar] [CrossRef]
- Da Silva, W.R.; do Nascimento, C.W.A.; da Silva, F.B.V.; de Souza, A.A.B.; Fracetto, G.G.M.; de Sá Veloso Ximenes, D.H. Effects of Sewage Sludge Stabilization Processes on Soil Fertility, Mineral Composition, and Grain Yield of Maize in Successive Cropping. J. Soil Sci. Plant Nutr. 2021, 21, 1076–1088. [Google Scholar] [CrossRef]
- Melo, W.; Delarica, D.; Guedes, A.; Lavezzo, L.; Donha, R.; de Araújo, A.; de Melo, G.; Macedo, F. Ten years of application of sewage sludge on tropical soil. A balance sheet on agricultural crops and environmental quality. Sci. Total Environ. 2018, 643, 1493–1501. [Google Scholar] [CrossRef]
- Vaca, R.; Lugo, J.; Martínez, R.; Esteller, M.V.; Zavaleta, H. Effects of sewage sludge and sewage sludge compost amendment on soil properties and Zea mays L. plants (heavy metals, quality and productivity). Rev. Int. Contam. Ambient. 2011, 27, 303–311. [Google Scholar]
- Cocarta, D.M.; Subtirelu, V.R.; Badea, A. Effect of sewage sludge application on wheat crop productivity and heavy metal accumulation in soil and wheat grain. Environ. Eng. Manag. J. 2017, 16, 1093–1100. [Google Scholar] [CrossRef]
- Eid, E.M.; Alamri, S.A.M.; Shaltout, K.H.; Galal, T.M.; Ahmed, M.T.; Brima, E.I.; Sewelam, N. A sustainable food security approach: Controlled land application of sewage sludge recirculates nutrients to agricultural soils and enhances crop productivity. Food Energy Secur. 2020, 9, e197. [Google Scholar] [CrossRef]
- Eid, E.M.; Hussain, A.A.; Taher, M.A.; Galal, T.M.; Shaltout, K.H.; Sewelam, N. Sewage Sludge Application Enhances the Growth of Corchorus olitorius Plants and Provides a Sustainable Practice for Nutrient Recirculation in Agricultural Soils. J. Soil Sci. Plant Nutr. 2020, 20, 149–159. [Google Scholar] [CrossRef]
- Stark, J.C.; Love, S.L.; Knowles, N.R. Tuber quality. In Potato Production Systems; Springer: Berlin/Heidelberg, Germany, 2020; pp. 479–497. [Google Scholar]
- Naumann, M.; Koch, M.; Thiel, H.; Gransee, A.; Pawelzik, E. The Importance of Nutrient Management for Potato Production Part II: Plant Nutrition and Tuber Quality. Potato Res. 2020, 63, 121–137. [Google Scholar] [CrossRef]
- Crawford, N.M.; Forde, B.G. Molecular and Developmental Biology of Inorganic Nitrogen Nutrition. Arab. B 2002, 1, e0011. [Google Scholar] [CrossRef] [PubMed]
- Razaq, M.; Zhang, P.; Shen, H.L.; Salahuddin. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef]
- Sokombela, A.; Eiasu, B.K.; Nyambo, P. Nitrogen and Phosphorus Fertilizers Improve Growth and Leaf Nutrient Composition of Moringa oleifera. Front. Sustain. Food Syst. 2022, 6, 861400. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Binder, D.L.; Dobermann, A.; Sander, D.H.; Cassman, K.G. Biosolids as Nitrogen Source for Irrigated Maize and Rainfed Sorghum. Soil Sci. Soc. Am. J. 2002, 66, 531–543. [Google Scholar] [CrossRef]
- Tarrasón, D.; Ojeda, G.; Ortiz, O.; Alcañiz, J.M. Differences on nitrogen availability in a soil amended with fresh, composted and thermally-dried sewage sludge. Bioresour. Technol. 2008, 99, 252–259. [Google Scholar] [CrossRef]
- Hechmi, S.; Hamdi, H.; Mokni-Tlili, S.; Ghorbel, M.; Khelil, M.N.; Zoghlami, I.R.; Benzarti, S.; Jellali, S.; Hassen, A.; Jedidi, N. Impact of urban sewage sludge on soil physico-chemical properties and phytotoxicity as influenced by soil texture and reuse conditions. J. Environ. Qual. 2020, 49, 973–986. [Google Scholar] [CrossRef]
- Kidd, P.S.; Domínguez-Rodríguez, M.J.; Díez, J.; Monterroso, C. Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 2007, 66, 1458–1467. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, S.; Pérez, D.V.; Almeida, M.R.A.; Silva, G.M.; Polidoro, J.C.; Bettiol, W. Chemical Changes and Heavy Metal Partitioning in an Oxisol Cultivated with Maize (Zea mays L.) after 5 Years Disposal of a Domestic and an Industrial Sewage Sludge. Water. Air. Soil Pollut. 2009, 203, 3–16. [Google Scholar] [CrossRef]
- Zaragüeta, A.; Enrique, A.; Virto, I.; Antón, R.; Urmeneta, H.; Orcaray, L. Effect of the long-term application of sewage sludge to a calcareous soil on its total and bioavailable content in trace elements, and their transfer to the crop. Minerals 2021, 11, 356. [Google Scholar] [CrossRef]
- Nunes, N.; Ragonezi, C.; Gouveia, C.S.S.; Pinheiro de Carvalho, M.Â.A. Review of sewage sludge as a soil amendment in relation to current international guidelines: A heavy metal perspective. Sustainability 2021, 13, 2317. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2011; Volume 213, ISBN 978-1-4419-9859-0. [Google Scholar]
- Luo, B.F.; Du, S.T.; Lu, K.X.; Liu, W.J.; Lin, X.Y.; Jin, C.W. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. J. Exp. Bot. 2012, 63, 3127–3136. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Xu, S.; Shi, H.; Wen, D.; Huang, Y.; Peng, L.; Deng, T.; Du, R.; Li, F.; et al. Increasing ammonium nutrition as a strategy for inhibition of cadmium uptake and xylem transport in rice (Oryza sativa L.) exposed to cadmium stress. Environ. Exp. Bot. 2018, 155, 734–741. [Google Scholar] [CrossRef]
- Larsson Jönsson, E.H.; Asp, H. Effects of pH and nitrogen on cadmium uptake in potato. Biol. Plant. 2013, 57, 788–792. [Google Scholar] [CrossRef]
- Cheng, Y.; Bao, Y.; Chen, X.; Yao, Q.; Wang, C.; Chai, S.; Zeng, J.; Fan, X.; Kang, H.; Sha, L.; et al. Different nitrogen forms differentially affect Cd uptake and accumulation in dwarf Polish wheat (Triticum polonicum L.) seedlings. J. Hazard. Mater. 2020, 400, 123209. [Google Scholar] [CrossRef]
- Pant, P.; Allen, M.; Tansel, B. Mercury uptake and translocation in Impatiens Walleriana plants grown in the contaminated soil from Oak Ridge. Int. J. Phytoremediation 2011, 13, 168–176. [Google Scholar] [CrossRef]
- Ellis, R.W.; Eslick, L. Variation and range of mercury uptake into plants at a mercury- contaminated abandoned mine site. Bull. Environ. Contam. Toxicol. 1997, 59, 763–769. [Google Scholar] [CrossRef]
- Muddarisna, N.; Siahaan, B.C. Application of organic matter to enhance phytoremediation of mercury contaminated soils using local plant species: A case study on small-scale gold mining locations in Banyuwangi of East Java. J. Degrad. Min. Lands Manag. 2014, 2, 251–258. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef] [PubMed]

| Properties | Agricultural Soil | Sewage Sludge | |
|---|---|---|---|
| Measured Values | Measured Values | European Norms * | |
| pH | 7.2 | 6.7 | NA |
| OM (%) | 1.42 | 74.1 | NA |
| CEC (meq/100 g) | 31.3 | ND | NA |
| NO3-N (mg·kg−1) | 3 | <0.5 | ** |
| NH4-N (mg·kg−1) | 2.3 | 6000 | ** |
| P (mg·kg−1) | 1237 | 29,000 | ** |
| K (mg·kg−1) | 960 | 10,000 | ** |
| Cd (mg·kg−1) | ND | 0.7 | 20 |
| Cu (mg·kg−1) | 10 | 110 | 1000 |
| Cr (mg·kg−1) | ND | 28 | 1000 |
| Hg (mg·kg−1) | ND | 0.3 | 16 |
| Ni (mg·kg−1) | ND | 24 | 300 |
| Pb (mg·kg−1) | ND | 21 | 750 |
| Zn (mg·kg−1) | 6 | 690 | 2500 |
| Properties | End of 1st Cycle | End of 2nd Cycle | ||||
|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C0 | C1 | C2 | |
| OM (%) | 1.48 ± 0.05 | 1.71 ± 0.16 | 1.75 ± 0.24 | 2.01 ± 0.00 | 1.91 ± 0.00 | 2.01 ± 0.00 |
| pH | 7.4 ± 0.05 a | 7.4 ± 0.05 a | 7.3 ± 0.05 b | 7.8 ± 0.00 | 7.8 ± 0.00 | 7.7 ± 0.00 |
| CEC (meq/100 g) | 33.7 ± 2.12 | 32.83 ± 3.24 | 34.57 ± 1.23 | 52.4 ± 0.00 a | 50.0 ± 0.00 a | 65.8 ± 0.00 b |
| NO3-N (mg·kg−1) | 22.5 ± 2.50 a | 27.5 ± 2.5 a | 105± 5.00 b | 15 ± 0.00 a | 35 ± 0.00 a.b | 55 ± 0.00 b |
| NH4-N (mg·kg−1) | 2.17 ± 0.17 a | 2.15 ± 0.15 a | 5.65 ± 0.05 b | 3.5 ± 0.00 a.b | 4.9 ± 0.00 a | 2.6 ± 0.00 b |
| P (mg·kg−1) | 1374 ± 0.00 | 1374 ± 0.00 | 1374 ± 0.00 | 1511 ± 0.00 | 1511 ± 0.00 | 1511 ± 0.00 |
| K (mg·kg−1) | 2240 ± 150 | 2080 ± 226 | 1880 ± 113 | 2400 ± 0.00 | 2520 ± 0.00 | 2160 ± 0.00 |
| Heavy Metals | End of 1st Cycle | End of 2nd Cycle | European Norms * | ||||
|---|---|---|---|---|---|---|---|
| C0 | C1 | C2 | C0 | C1 | C2 | ||
| mg·kg−1 | |||||||
| Cd | 0.7 ± 0.00 | 0.8 ± 0.00 | 0.7 ± 0.09 | 0.7 ± 0.00 | 0.8 ± 0.09 | 0.6 ± 0.05 | 4 |
| Cr | 143.3 ± 4.71 | 136.7 ± 4.71 | 146.7 ± 4.71 | 140 ± 0.00 | 136 ± 4.71 | 146.7 ± 4.71 | 300 |
| Hg | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 2 |
| Ni | 88.0 ± 2.16 | 85.7 ± 1.89 | 90 ± 2.94 | 85.3 ± 3.68 | 81.0 ± 2.16 | 85.7 ± 3.30 | 110 |
| Pb | 14.3 ± 0.94 | 15.0 ± 0.82 | 14.0 ± 0.82 | 18.3 ± 2.05 | 15.3 ± 0.47 | 14.7 ± 1.25 | 450 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragonezi, C.; Nunes, N.; Oliveira, M.C.O.; de Freitas, J.G.R.; Ganança, J.F.T.; de Carvalho, M.Â.A.P. Sewage Sludge Fertilization—A Case Study of Sweet Potato Yield and Heavy Metal Accumulation. Agronomy 2022, 12, 1902. https://doi.org/10.3390/agronomy12081902
Ragonezi C, Nunes N, Oliveira MCO, de Freitas JGR, Ganança JFT, de Carvalho MÂAP. Sewage Sludge Fertilization—A Case Study of Sweet Potato Yield and Heavy Metal Accumulation. Agronomy. 2022; 12(8):1902. https://doi.org/10.3390/agronomy12081902
Chicago/Turabian StyleRagonezi, Carla, Nuno Nunes, Maria Cristina O. Oliveira, José G. R. de Freitas, José Filipe T. Ganança, and Miguel Â. A. Pinheiro de Carvalho. 2022. "Sewage Sludge Fertilization—A Case Study of Sweet Potato Yield and Heavy Metal Accumulation" Agronomy 12, no. 8: 1902. https://doi.org/10.3390/agronomy12081902
APA StyleRagonezi, C., Nunes, N., Oliveira, M. C. O., de Freitas, J. G. R., Ganança, J. F. T., & de Carvalho, M. Â. A. P. (2022). Sewage Sludge Fertilization—A Case Study of Sweet Potato Yield and Heavy Metal Accumulation. Agronomy, 12(8), 1902. https://doi.org/10.3390/agronomy12081902








