Biomarker Discovery for Detecting the Seed Ageing Degree and Priming Effect of Tobacco
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Accelerated Seed Ageing Treatment
2.3. Seed Priming
2.4. Seed Germination
2.5. Identification of NtPIMT1 and NtOGG1 in Tobacco
2.6. Expression Analysis
2.7. Data Analysis
3. Results
3.1. Identification of NtPIMT and NtOGG1 in Tobacco
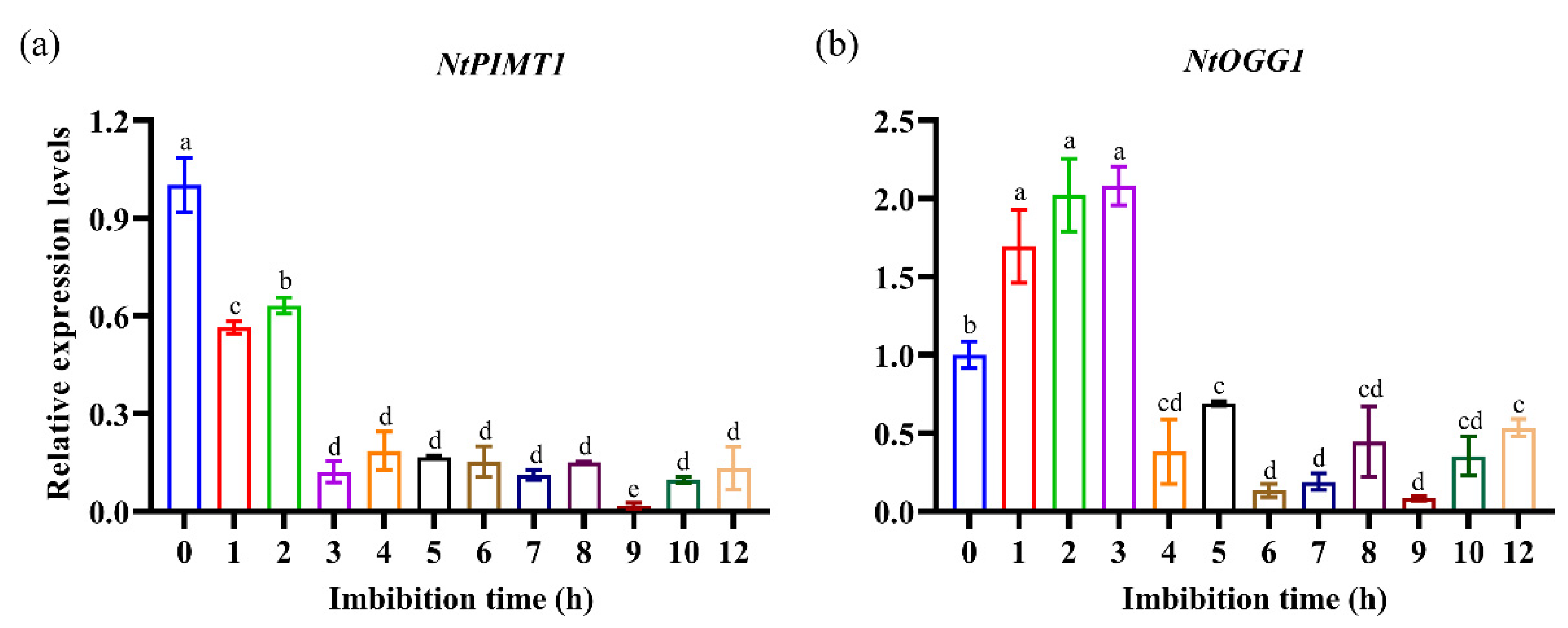
3.2. Expression Patterns of NtPIMT1 and NtOGG1 during Early Seed Germination
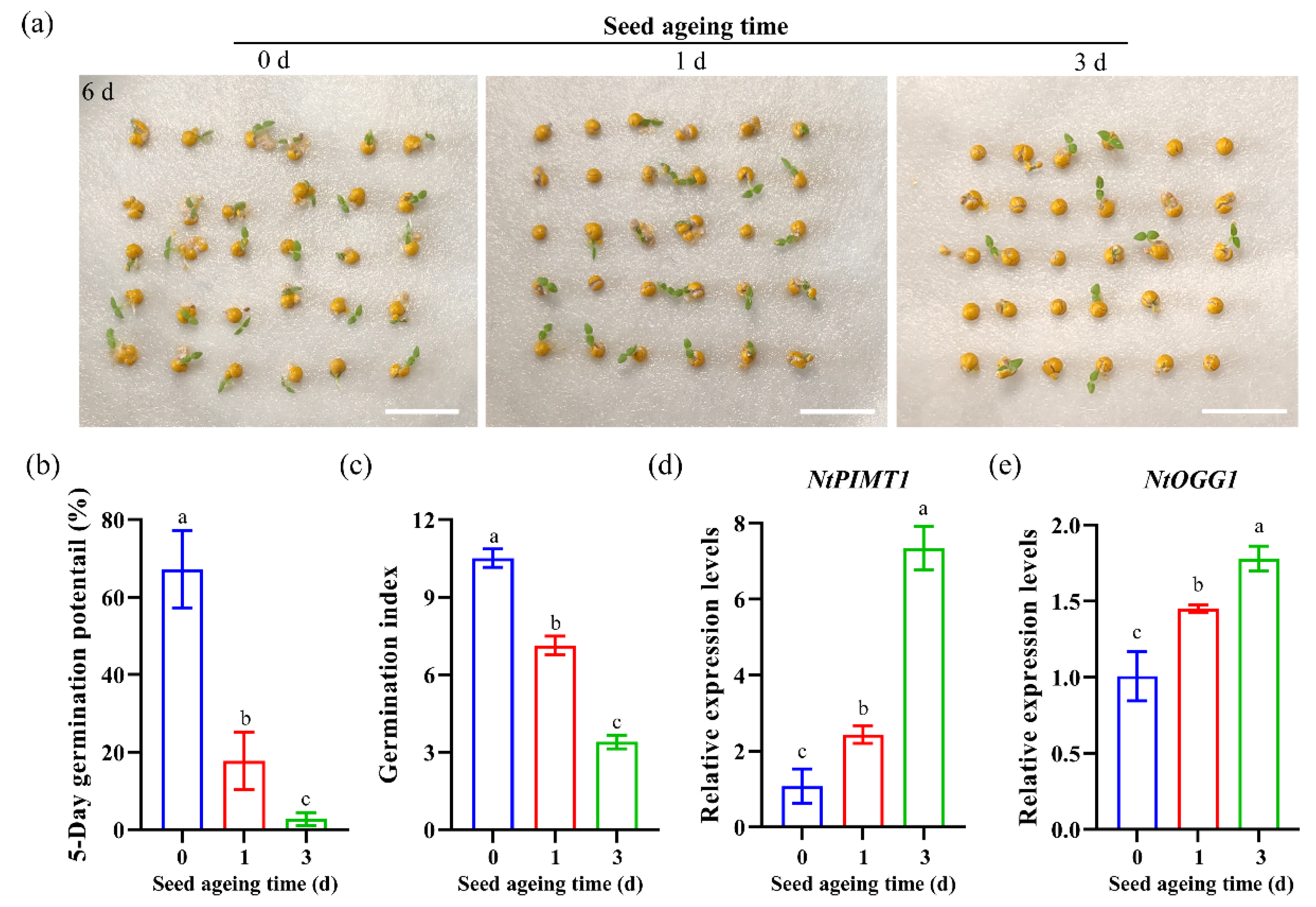
3.3. Expression Patterns of NtPIMT1 and NtOGG1 in Non-Pelleted Seeds after Artificial Ageing Treatments
3.4. Expression Patterns of NtPIMT1 and NtOGG1 in Pelleted Seeds after Artificial Ageing Treatments
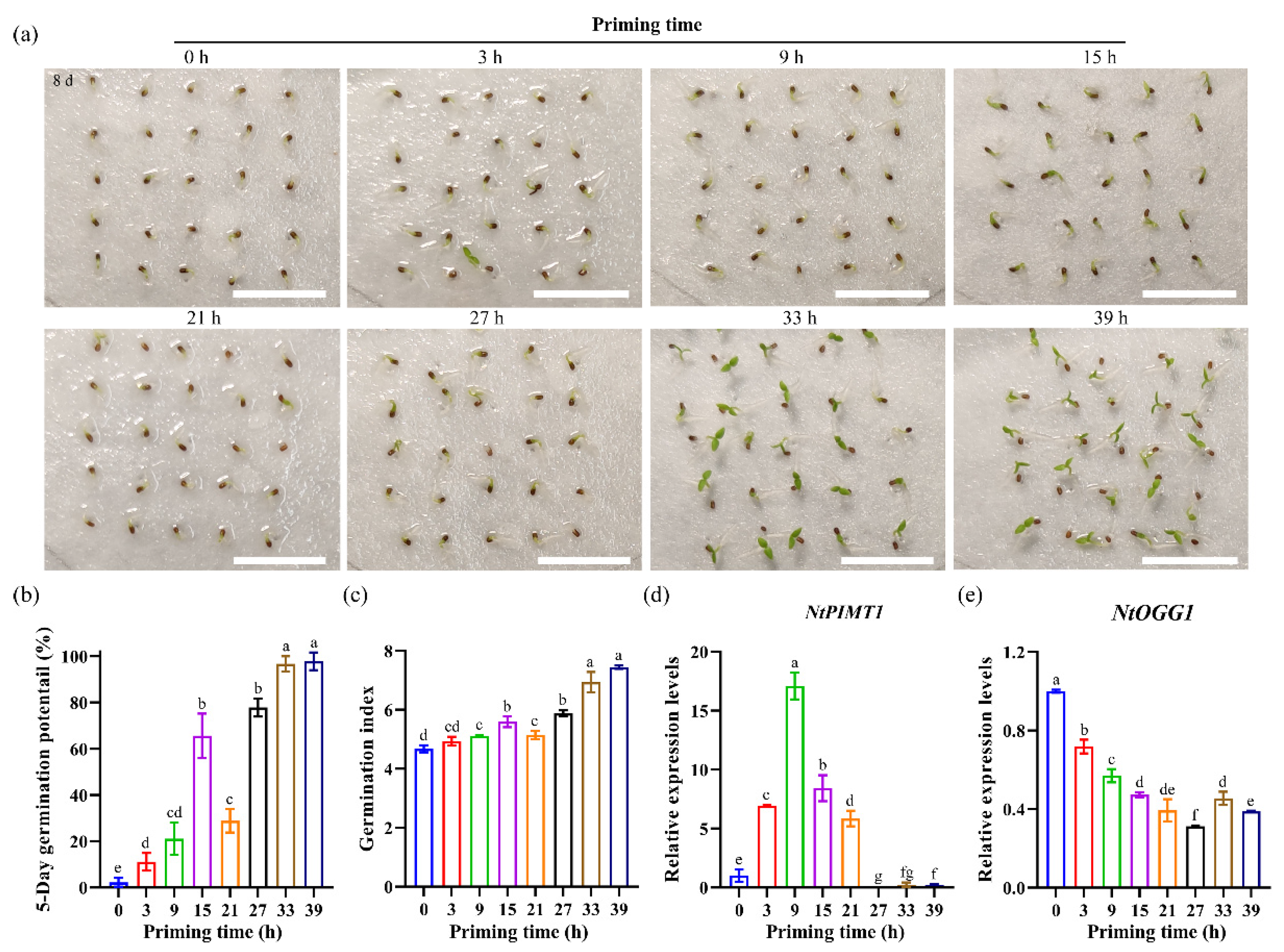
3.5. Expression Patterns of NtPIMT1 and NtOGG1 in Primed Seeds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.X.; Fu, H.; Gao, J.D.; Zhang, Y.X.; Huang, W.J.; Chen, Z.J.; Zhang, Q.; Yan, S.J.; Liu, J. Identification of metabolomic biomarkers of seed vigor and aging in hybrid rice. Rice 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, Y.; Zheng, Y.; Wang, Z. Advances in the understanding of reactive oxygen species-dependent regulation on seed dormancy, germination, and deterioration in crops. Front. Plant Sci. 2022, 13, 826809. [Google Scholar] [CrossRef] [PubMed]
- Mudgett, M.B.; Lowenson, J.D.; Clarke, S. Protein repair L-isoaspartyl methyltransferase in plants. Pphylogenetic distribution and the accumulation of substrate proteins in aged barley seeds. Plant Physiol. 1997, 115, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Oge, L.; Bourdais, G.; Bove, J.; Collet, B.; Godin, B.; Granier, F.; Boutin, J.P.; Job, D.; Jullien, M.; Grappin, P. Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis. Plant Cell 2008, 20, 3022–3037. [Google Scholar] [CrossRef]
- Verma, P.; Kaur, H.; Petla, B.P.; Rao, V.; Saxena, S.C.; Majee, M. PROTEIN L-ISOASPARTYL METHYLTRANSFERASE2 is differentially expressed in chickpea and enhances seed vigor and longevity by reducing abnormal isoaspartyl accumulation predominantly in seed nuclear proteins. Plant Physiol. 2013, 161, 1141–1157. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, H.; Diao, L.; Zhu, Y.; Xie, H.; Cai, Q.; Wu, F.; Wang, Z.; Zhang, J.; Xie, H. Protein repair L-isoaspartyl methyltransferase 1 (PIMT1) in rice improves seed longevity by preserving embryo vigor and viability. Plant Mol. Biol. 2015, 89, 475–492. [Google Scholar] [CrossRef]
- Córdoba-Cañero, D.; Roldán-Arjona, T.; Ariza, R.R. Arabidopsis ZDP DNA 3′-phosphatase and ARP endonuclease function in 8-oxoG repair initiated by FPG and OGG1 DNA glycosylases. Plant J. 2014, 79, 824–834. [Google Scholar] [CrossRef]
- Osborne, D.J.; Dell’Aquila, A.; Elder, R.H. DNA repair in plant cells. An essential event of early embryo germination in seeds. Folia Biologica 1984, 30, 155–169. [Google Scholar]
- Waterworth, W.M.; Drury, G.E.; Bray, C.M.; West, C.E. Repairing breaks in the plant genome: The importance of keeping it together. New Phytol. 2011, 192, 805–822. [Google Scholar] [CrossRef]
- Girard, P.M.; Guibourt, N.; Boiteux, S. The Ogg1 protein of Saccharomyces cerevisiae: A 7,8-dihydro-8-oxoguanine DNA glycosylase/AP lyase whose lysine 241 is a critical residue for catalytic activity. Nucleic Acids Res. 1997, 25, 3204–3211. [Google Scholar] [CrossRef]
- Sandigursky, M.; Yacoub, A.; Kelley, M.R.; Xu, Y.; Franklin, W.A.; Deutsch, W.A. The yeast 8-oxoguanine DNA glycosylase (Ogg1) contains a DNA deoxyribophosphodiesterase (dRpase) activity. Nucleic Acids Res. 1997, 25, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chu, P.; Zhou, Y.; Li, Y.; Liu, J.; Ding, Y.; Tsang, E.W.; Jiang, L.; Wu, K.; Huang, S. Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. J. Exp. Bot. 2012, 63, 4107–4121. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Balestrazzi, A.; Confalonieri, M.; Faé, M.; Carbonera, D. New insights on the barrel medic MtOGG1 and MtFPG functions in relation to oxidative stress response in planta and during seed imbibition. Plant Physiol. Biochem. 2011, 49, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed longevity: Survival and maintenance of high germination ability of dry seeds. Comptes Rendus Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Garza-Caligaris, L.E.; Avendaño-Vázquez, A.O.; Alvarado-López, S.; Zúñiga-Sánchez, E.; Orozco-Segovia, A.; Pérez-Ruíz, R.V.; Gamboa-deBuen, A. At3g08030 transcript: A molecular marker of seed ageing. Ann. Bot. 2012, 110, 1253–1260. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef]
- Boucelha, L.; Abrous-Belbachir, O.; Djebbar, R. Is protein carbonylation a biomarker of seed priming and ageing? Funct. Plant Biol. 2021, 48, 611–623. [Google Scholar] [CrossRef]
- Butler, L.H.; Hay, F.R.; Ellis, R.H.; Smith, R.D.; Murray, T.B. Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Ann. Bot. 2009, 103, 1261–1270. [Google Scholar] [CrossRef]
- Heydecker, W.; Higgins, J.; Gulliver, R.L. Accelerated germination by osmotic seed treatment. Nature 1973, 246, 42–44. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Konzen, L.H.; Rodrigues, D.B.; Mazon, A.S. Parameters of the accelerated aging test for the determination of tobacco (Nicotiana tabacum L.) seed vigor. J. Agric. Stud. 2020, 8, 247–263. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, J.F.; Bao, Y.M.; Wang, F.H.; Zhang, H.S. Quantitative trait loci analysis for rice seed vigor during the germination stage. J. Zhejiang Univ. Sci. B 2010, 11, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Gallardo, K.; Job, C.; Groot, S.P.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef]
- Cheah, K.S.E.; Osborne, D.J. DNA lesions occur with loss of viability in embryos of aging rye seed. Nature 1978, 272, 593–599. [Google Scholar] [CrossRef]
- Osborne, D.J. Biochemical control-systems operating in the early hours of germination. Can. J. Bot. 1983, 61, 3568–3577. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, L.; Zeng, P.; He, Y.; Zhou, R.; Zhang, H.; Wang, Z. Identification of genes involved in rice seed priming in the early imbibition stage. Plant Biol. 2017, 19, 61–69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cheng, J.; He, Y.; Yang, B.; Cheng, Y.; Yang, C.; Zhang, H.; Wang, Z. Influence of isopropylmalate synthase OsIPMS1 on seed vigour associated with amino acid and energy metabolism in rice. Plant Biotech. J. 2019, 17, 322–337. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Zheng, Y.; Zhou, D.; Zhao, J.; Wang, C.; Wang, Z.; Zhang, L. Biomarker Discovery for Detecting the Seed Ageing Degree and Priming Effect of Tobacco. Agronomy 2022, 12, 1897. https://doi.org/10.3390/agronomy12081897
Niu Y, Zheng Y, Zhou D, Zhao J, Wang C, Wang Z, Zhang L. Biomarker Discovery for Detecting the Seed Ageing Degree and Priming Effect of Tobacco. Agronomy. 2022; 12(8):1897. https://doi.org/10.3390/agronomy12081897
Chicago/Turabian StyleNiu, Yongzhi, Yunye Zheng, Dongjie Zhou, Jia Zhao, Chengjing Wang, Zhoufei Wang, and Limeng Zhang. 2022. "Biomarker Discovery for Detecting the Seed Ageing Degree and Priming Effect of Tobacco" Agronomy 12, no. 8: 1897. https://doi.org/10.3390/agronomy12081897
APA StyleNiu, Y., Zheng, Y., Zhou, D., Zhao, J., Wang, C., Wang, Z., & Zhang, L. (2022). Biomarker Discovery for Detecting the Seed Ageing Degree and Priming Effect of Tobacco. Agronomy, 12(8), 1897. https://doi.org/10.3390/agronomy12081897






