Combined Effects of Biosolarization and Brassica Amendments on Survival of Biocontrol Agents and Inhibition of Fusarium oxysporum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Temperature and Associated Weather Parameters
2.3. Pathogen Stabilization
2.4. Pathogenicity Test
2.5. Morphological Characterization and Identification of the Foc
2.6. Inoculumpreparation
2.7. Experimental Design
2.7.1. Application of Biocontrol Agents and Pathogen Inoculum
2.7.2. Application of Brassica Amendment
2.7.3. Field Design
2.7.4. Experimentation Field Setup
2.8. Biological Assays
2.9. Statistical Analysis
3. Results
3.1. Reduction in Foc Population
3.2. Survival of T. harzianum Population
3.3. Survival of A. versicolor Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Solanki, P. Overall Scenario and Assessment of the Factors for Price Volatility of Cumin in Rajasthan. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2537–2545. [Google Scholar] [CrossRef]
- Agarwal, S. Volatile oil constituents and wilt resistance in cumin (Cuminum cyminum L.). Curr. Sci. 1996, 7, 1177–1178. [Google Scholar]
- Spice Board of India (n.d.) Statistics. Available online: http://indianspices.com/sites/default/files/Major-spic-wise-area-and-production-web-2015.pdf (accessed on 10 May 2022).
- Lodha, S.; Mawar, R. Cumin wilt management—A review. J. Spices Aromat. Crops 2014, 23, 145–155. [Google Scholar]
- Lodha, S.; Gupta, G.K.; Singh, S. Crop disease situation and some new records in Indian arid zone. Ann. Arid Zone 1986, 25, 311–320. [Google Scholar]
- Israel, S.; Lodha, S. Factors influencing population dynamics of Fusarium oxysporum f. sp. cumini in arid soils. Phytopathol. Mediterr. 2004, 43, 3–13. [Google Scholar]
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Tanović, B. Methods for management of soil-borne plant pathogens. Pestic. Fitomedicina 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soilborne Disease Management in Organic Vegetable Production. 2012. Available online: https://eorganic.org/node/7581 (accessed on 7 December 2019).
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soil-borne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef] [Green Version]
- Al-Kayssi, A.W.; Al-Karaghouli, A. A new approach for soil solarization by using paraffin-wax emulsion as a mulching material. Renew. Energy 2002, 26, 637–648. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- Lodha, S. Soil solarization, summer irrigation, and amendments for the control of Fusariumoxysporum f. sp. cumini and Macrophominaphaseolina in arid soils. Crop Prot. 1995, 14, 215–219. [Google Scholar]
- Gamliel, A.; Katan, J. Soil Solarization: Theory and Practice; APS Press: St. Paul, MN, USA, 2012. [Google Scholar]
- Okon-Levy, N.; Meller, Y.; Haile, Z.M.; Elad, Y.; Rav-David, E.; Jurkevitch, E.; Katan, J. Induced resistance to foliar diseases by soil solarization and Trichodermaharzianum. Plant Pathol. 2015, 64, 365–374. [Google Scholar] [CrossRef]
- Katan, J. Diseases caused by soil-borne pathogens: Biology, management and challenges. J. Plant Pathol. 2017, 99, 305–315. [Google Scholar]
- Israel, S.; Mawar, R.; Lodha, S. Soil solarization, amendments and bio-control agents for the control of Macrophominaphaseolina and Fusariumoxysporum f. sp. cumini in arid soils. Ann. Appl. Biol. 2005, 146, 481–491. [Google Scholar] [CrossRef]
- Keinath, A.P. Soil amendment with cabbage residue and crop rotation to reduce gummy stem blight and increased growth and yield of watermelon. Plant Dis. 1996, 80, 564–570. [Google Scholar] [CrossRef]
- Mayton, H.S.; Oliver, C.; Vaughan, S.F.; Loria, R. Correlation of fungicidal activity of Brassica species with allylisothiocyanates production in macerated leaf tissue. Phytopathology 1996, 86, 267–271. [Google Scholar] [CrossRef]
- Randall, T.E.; Fernandez-Bayo, J.D.; Harrold, D.R.; Achmon, Y.; Hestmark, K.V.; Gordon, T.R. Changes of Fusariumoxyporum f. sp. lactucae levels and soil microbial community during soil biosolarization using chitin as soil amendment. PLoS ONE 2020, 15, e0232662. [Google Scholar]
- Fernández-Bayo, J.D.; Randall, T.E.; Harrold, D.R.; Achmon, Y.; Hestmark, K.V.; Su, J.; Ruth, M.; Dahlquist-Willard, R.M.; Thomas, R.; Gordon, T.R.; et al. Effect of management of organic wastes on inactivation of Brassica nigra and Fusariumoxysporum f. sp. lactucae using soil biosolarization. Pest. Manag. Sci. 2018, 74, 1892–1902. [Google Scholar]
- Gameliel, A.; Stapleton, J.J. Characterization of antifungal volatile compounds evolved from solarized soil amended with cabbage residue. Phytopathology 1993, 83, 899–905. [Google Scholar] [CrossRef]
- Elad, Y.; Katan, J.; Chet, I. Physical, biological and chemical control integrated for soil-borne diseases in potatoes. Phytopathology 1980, 70, 418–422. [Google Scholar] [CrossRef]
- Tjamos, F.C.; Fravel, D.R. Deterimental effects of sub-lethal heating and Talaromycesflavus on microsclerotia of Verticilliumdahliae. Phytopathology 1995, 85, 388–392. [Google Scholar] [CrossRef]
- Israel, S.; Lodha, S. Biological control of Fusariumoxysporum f. sp. cumini with Aspergillusversicolor. Phytopathol. Mediterr. 2004, 44, 3–11. [Google Scholar]
- Ramirez-Villapudua, J.; Munnecke, D.E. Effects of solar heating and soil amendments of cruciferous residues on Fusariumoxysporum f. sp. conglutinans and other micro-organisms. Phytopathology 1988, 78, 289–295. [Google Scholar]
- Hami, A.; Rasool, R.S.; Khan, N.A.; Mansoor, S.; Mir, M.A.; Ahmed, N.; Masoodi, K.Z. Morpho-molecular identification and first report of Fusariumequiseti in causing chilli wilt from Kashmir (Northern Himalayas). Sci. Rep. 2021, 11, 3610. [Google Scholar] [CrossRef]
- Martin, J.P. Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Papavizas, G.C. Evaluation of various media and antimicrobial agents for isolation of Fusarium from soil. Phytopathology 1967, 57, 848–852. [Google Scholar]
- D’Addabbo, T.; Miccolis, V.; Basile, M.; Candido, V. Soil solarization and sustainable agriculture. In Sociology, Organic Farming, Climate Change, and Soil Science; Lichtfocus, E., Ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Mawar, R.; Lodha, S. Prior weakening of Macrophominaphaseolina and Fusarium propagules for enhancing the efficiency of Brassica amendments. Crop Prot. 2009, 28, 812–817. [Google Scholar] [CrossRef]
- Israel, S.; Mawar, R.; Lodha, S. Combining sub—Lethal heating and on-farm wastes: Effects on Fusariumoxysporum f. sp. cumini causing wilt of cumin. Phytoparasitica 2011, 39, 73–82. [Google Scholar]
- Israel, S. Effect of Amendments on Wilt of Cumin Caused by Fusariumoxysporum f. sp. cumini Prasad and Patel in Aridisols. Ph.D. Thesis, Jai Narain Vyas University, Jodhpur, India, 2002. [Google Scholar]
- Katan, J.; Greenberger, A.; Alon, H.; Grinstein, A. Solar heating by polyethylene mulching for the control of diseases caused by soil-borne pathogens. Phytopathology 1976, 66, 683–688. [Google Scholar] [CrossRef]
- Precht, H.; Christophersen, J.; Hensel, H.; Lartcher, W. Temperature and Life; Springer: Berlin, Germany, 1973; ISBN 978-3-642-88378-1. [Google Scholar]
- Nakamura, K.; Watanabe, S.; Ozaki, H.; Ikeura, Y.; Kotani, A. Soil temperature and moisture environments: Lot-management water requirements associated with soil solarization. Farml. Agric. 2011, 631, 2–10. [Google Scholar]
- Dai, Y.Y.; Kondo, M.; Ito, K.; Yoshiyama, K.; Zhang, P.F.; Zhang, F.P.; Senge, M. Study on irrigation water requirements for the control of Ralstoniasolanacearum via soil solarization in managing tomato cultivation. J. Irrig. Drain. Rural Eng. 2014, 294, 85–92. [Google Scholar]
- Lewis, J.A.; Papavizas, G.C. Effect of sulphur-containing volatile compounds and vapours from cabbage decomposition on Aphanomyceseuteiches. Phytopathology 1971, 61, 208–214. [Google Scholar] [CrossRef]
- Katan, J. Physical and cultural methods for the management of soil-borne pathogens. Crop Prot. 2000, 19, 725–731. [Google Scholar] [CrossRef]
- Simmons, C.W.; Higgins, B.; Staley, S.; Joh, L.D.; Simmons, B.A.; Singer, S.W.; Stapleton, J.J.; VanderGheynst, J.S. The role of organic matter amendment level on soil heating, organic acid accumulation, and development of bacterial communities in solarized soil. Appl. Soil Ecol. 2016, 106, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Diaz Hernandez, S.; Rodriguez Perez, A.; Dominguez Correa, P.; Gallo Liobet, L. Solar heating biofumigation and conventional chemical treatments for the control of corky root in tomato. Acta Hortic. 2005, 698, 311–314. [Google Scholar] [CrossRef]
- Guerrero, M.M.; Matinez, M.A.; Ros, C.; Martinez, M.C.; Barcelo, N.; Lacasa, A.; Guirao, P.; Bello, A.; Lopez, J.A. Biofumigationplus solarization efficacy for soil disinfestation in sweet pepper greenhouses in the southeast of Spain. Acta Hortic. 2005, 698, 293–298. [Google Scholar] [CrossRef]
- Jayaraj, J.; Radhakrishnan, N.V. Enhanced activity of introduced biocontrol agents in solarised soils and its implications on integrated control of tomato damping-off caused by Pythium spp. Plant Soil. 2012, 304, 189–197. [Google Scholar] [CrossRef]
- Charan, N.D.; Rajput, K.N.; Panchal, R.R.M. In vitro Evaluation of Biocontrol Agents against Fusariumoxysporum to Eliminate Wilting of Cumin. Biosc. Biotech. Res. Commun. 2021, 14, 88–92. [Google Scholar] [CrossRef]
- Sivan, A.; Chet, I. Integrated control of Fusarium crown rot of tomato with Trichodermaharzianum in combination with methyl bromide or soil solarization. Crop Prot. 1993, 12, 380–386. [Google Scholar] [CrossRef]
- Giannakou, I.O.; Anastasiadis, I.A.; Gowen, S.R.; Prophetou-Athanasiadou, D.A. Effects of a non-chemical nematicide combined with soil solarization for the control of root-knot nematodes. Crop Prot. 2011, 26, 1644–1654. [Google Scholar] [CrossRef]
- Cholil, A. Effect of heat treatment on the soil microflora of a potato field. Agrivita 1981, 4, 30–33. [Google Scholar]
- Freeman, S.; Ginzburg, C.; Katan, J. Heat shock protein synthesis in propagules of Fusariumoxysporum f. sp. niveum. Phytopathology 1989, 79, 1054–1058. [Google Scholar] [CrossRef]
- Kochhar, A.; Kaur, N.; Gupta, A.K. Immobilization of inulinase from Aspergillusversicolor for preparing fructose from inulin. J. Sci. Indus. Res. 1998, 57, 184–187. [Google Scholar]
- Singh, V.; Mawar, R.; Lodha, S. Combined effect of biocontrol agents and soil amendments on soil microbial populations, plant growth and incidence of charcoal rot on cowpea and wilt on cumin. Phytopathol. Mediterr. 2012, 51, 307–316. [Google Scholar]
- Kapulnik, Y.; Gamliel, A. Combioning Soil Solarization with beneficial microbial agents. In Soil Solarization: Theory and Practice; APS Press: St. Paul, MN, USA, 2012. [Google Scholar]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Loganathan, M.; Sible, G.V.; Maruthasalam, S.; Saravanakumar, D.; Raguchander, T.; Sivakumar, M.; Samiyappan, R. Trichoderma and chitin mixture based bioformulation for the management of head rot (Sclerotiniasclerotiorum (Lib.) de Bary)–root-knot (Meloidogyne incognita Kofoid and White; Chitwood) complex diseases of cabbage. Arch. Phytopathol. Plant Prot. 2010, 43, 1011–1024. [Google Scholar] [CrossRef]
- Rosskopf, E.; Di Gioia, F.; Hong, J.C.; Pisani, C.; Kokalis-Burelle, N. Organic amendments for pathogen and nematode control. Annu. Rev. Phytopathol. 2020, 58, 277–311. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, S.S.; Klarer, E.; Reed, A.J.; Leisso, R.; Poirier, B.; Honaas, L.; Rudell, D.R.; Mazzola, M. Temporal Dynamics of the Soil Metabolome and Microbiome During Simulated Anaerobic Soil Disinfestation. Front. Microbiol. 2019, 10, 2365. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.K.; Aujla, I.S.; De Bellis, L.; Luvisi, A. The Role of Soil Solarization in India: How an Unnoticed Practice Could Support Pest Control. Front. Plant Sci. 2017, 8, 1515. [Google Scholar] [CrossRef] [Green Version]
- Chellemi, D.O.; Gamliel, A.; Katan, J.; Subbarao, K.V. Development and deployment of system-based approaches for the management of soil-borne plant pathogens. Phytopathology 2016, 106, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, J.J. Soil solarization in various agriculture production systems. Crop Prot. 2000, 19, 837–841. [Google Scholar] [CrossRef]
- Scopa, A.; Dumontet, S. Soil solarization: Effect on soil microbiological parameters. J. Plant Nutr. 2007, 30, 537–547. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Zope, V.P.; Jadhav, H.P.; Sayyed, R.Z. Neem cake carrier prolongs shelf life of biocontrol fungus Trichoderma viridae. Indian J. Exp. Biol. 2019, 57, 372–375. [Google Scholar]
- Suriani, N.; Suprapta, D.; Novizar, N.; Parwanayoni, N.; Darmadi, A.; Dewi, D.; Sudatri, N.; Ahmad, F.; Sayyed, R.Z.; Syed, A.; et al. A Mixture of Piper Leaves Extracts and Rhizobacteria for Sustainable Plant Growth Promotion & Biocontrol of Blast Pathogen of Organic Bali Rice. Sustainability 2020, 12, 8490. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, A.; Dalela, M.; Sharma, S.; Sayyed, R.Z.; el Enshasy, H.A.; Elsayed, E.A. Linking organic metabolites as produced by Purpureocillium lilacinum 6029 cultured on Karanja deoiled cake medium for the sustainable management of root-knot nematodes. Sustainability 2020, 12, 8276. [Google Scholar] [CrossRef]
- Sukmawati, D.; Family, N.; Hidayat, I.; Sayyed, R.Z.; Elsayed, E.A.; Dailin, D.J.; Hanapi, S.Z.; Wadaan, M.A.; Enshasy, H.E. Biocontrol Activity of Aureubasidium pullulans and Candida orthopsilosis isolated from Tectona grandis L. Phylloplane against Aspergillus sp. In Post-Harvested Citrus Fruit. Sustainability 2021, 13, 7479. [Google Scholar] [CrossRef]
- Pourakbar, L.; Moghaddama, S.S.; Enshasy, H.E.; Sayyed, R.Z. Antifungal activity of the extract of a macroalgae, Gracilariopsis persica, against four plant pathogenic fungi in-vitro. Plants 2021, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Enshasy, H.E.; Ambehati, K.K.; Ashraf, F.; Ramchuran, S.; Sayyed, R.Z.; Amalin, D.; Dailin, D.; Hanapi, S.Z. Trichoderma: Biocontrol Agents for Promoting Plant Growth and Soil Health. In Agriculturally Important Fungi for Sustainable Agri-Culture: Vol 2: Functional Annotation for Crop Protection; Springer: Cham, Switzerland, 2020; pp. 239–259. [Google Scholar]
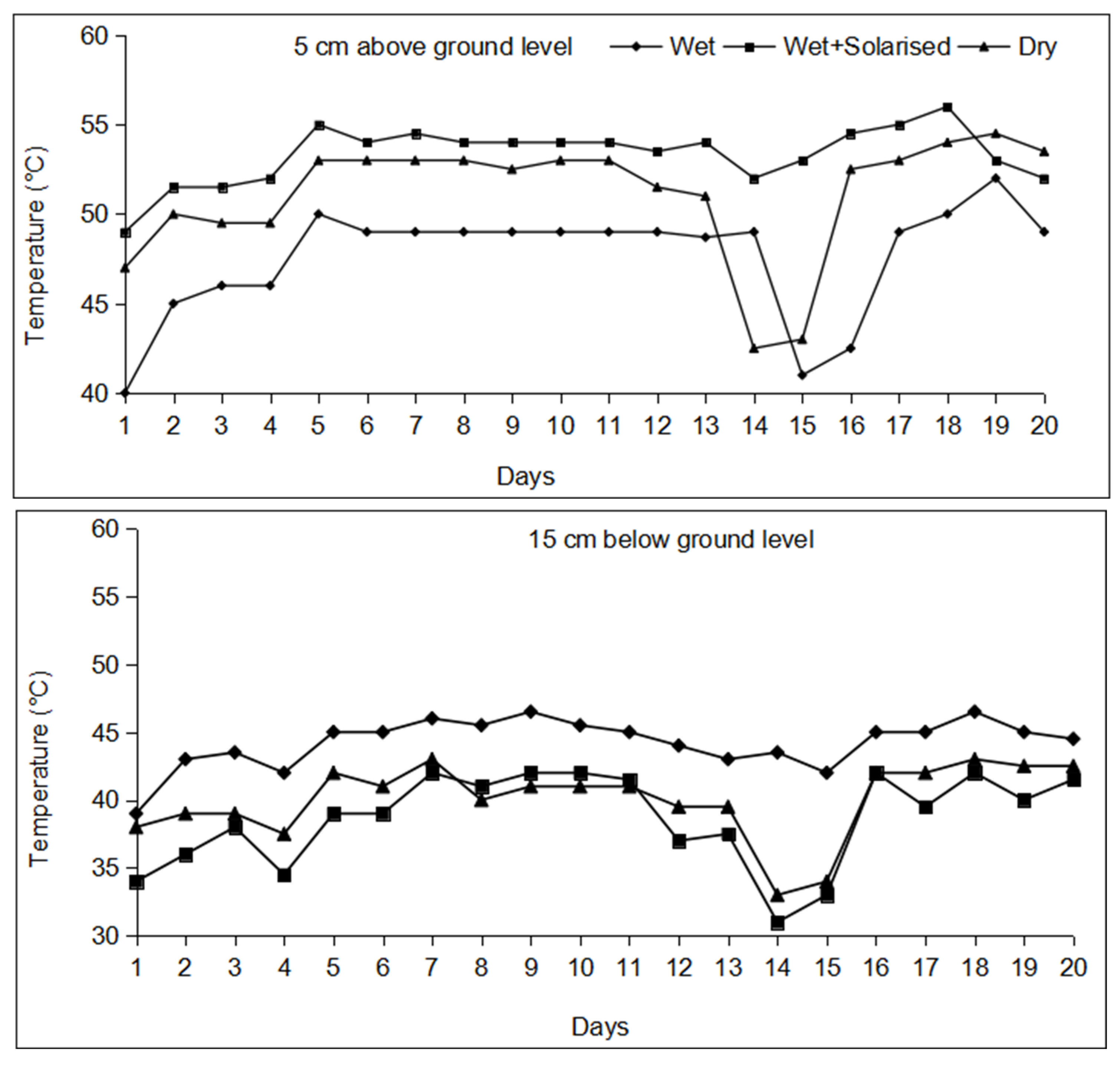

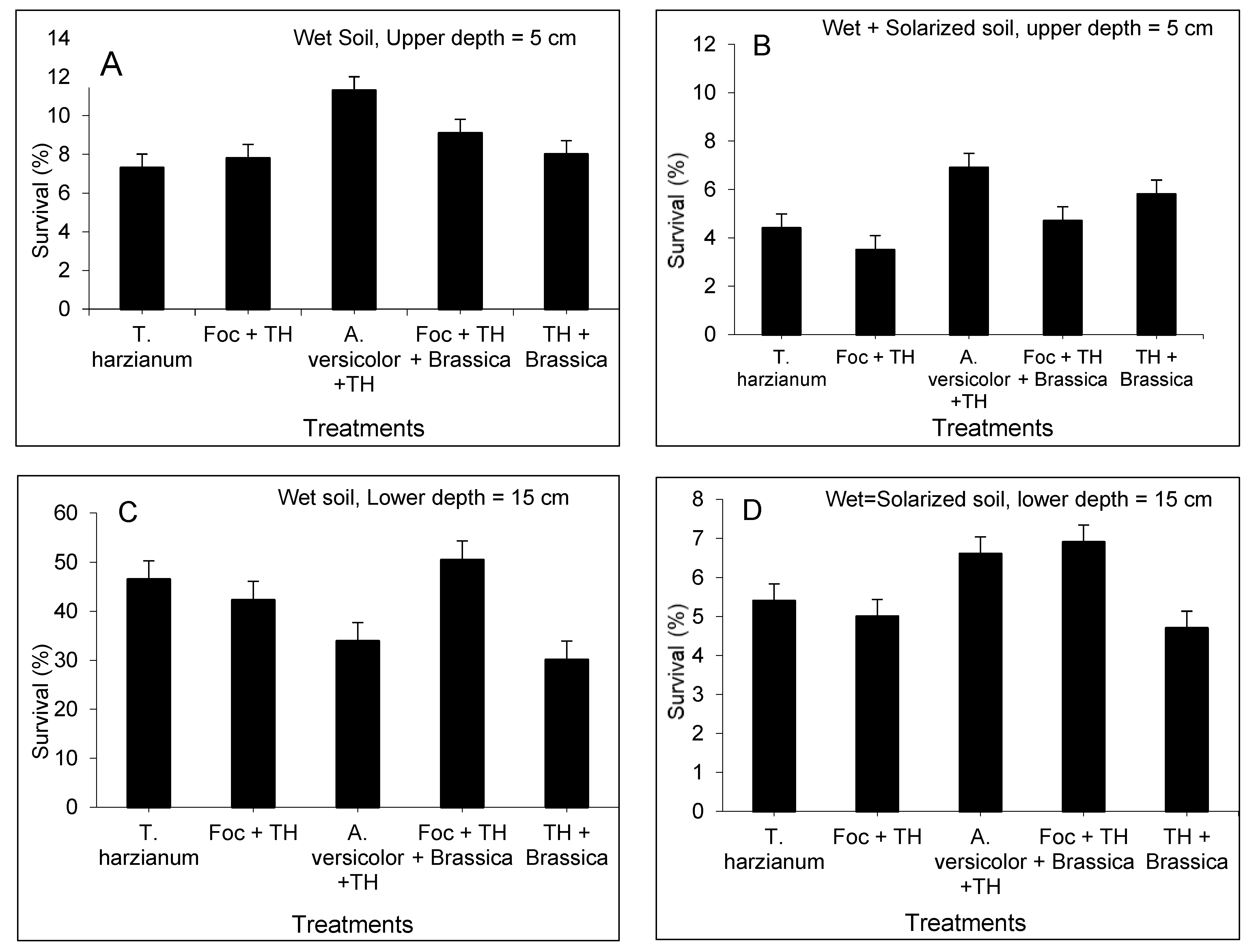

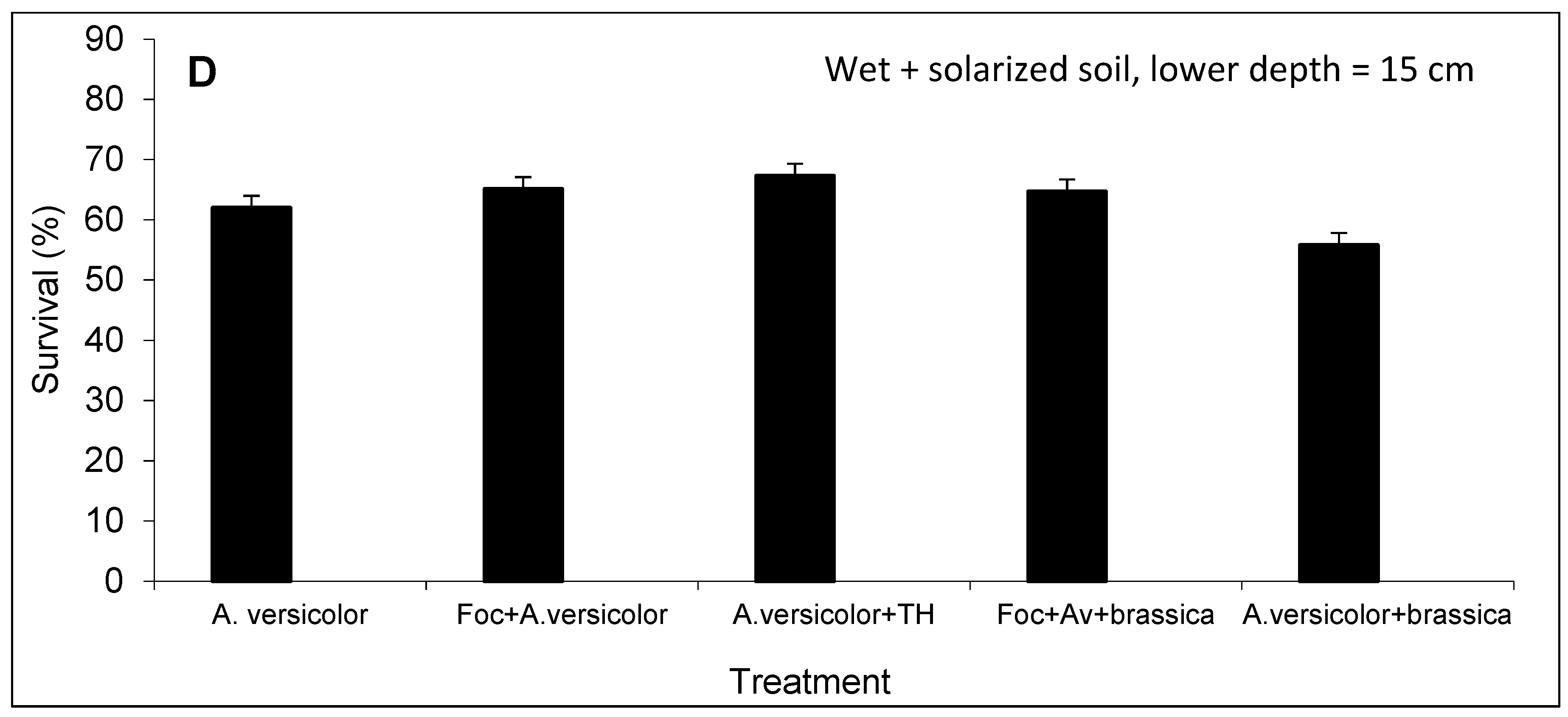
| Treatment * | Wet | Wet +Solarized | Dry soil | |||
|---|---|---|---|---|---|---|
| Soil Depth | ||||||
| 5cm | 15cm | 5cm | 15cm | 5cm | 15cm | |
| Foc | 36.7 (37.29) | 28.3 (32.14) | 92.8 (74.44) | 87.9 (69.6) | 3.9 (11.2) | 2.5 (9.0) |
| Foc + T. harzianum | 45.2 (42.13) | 36.8 (37.23) | 93.0 (77.30) | 91.0 (72.54) | - | - |
| Foc + A. versicolor | 46.3 (42.38) | 36.9 (37.35) | 93.7 (78.14) | 91.3 (72.85) | ||
| Foc + CR | 63.0 (52.53) | 43.3 (41.15) | 95.2 (77.08) | 92.4 (73.78) | - | - |
| Foc + T. harzianum + CR | 65.0 (53.61) | 45.2 (42.13) | 96.0 (78.17) | 93.2 (74.66) | - | - |
| Foc + A. versicolor + CR | 75.0 (60.0) | 45.8 (42.59) | 96.7 (79.22) | 94.0 (75.70) | - | - |
| Foc + A. versicolor + T. harzianum | 51.0 (45.46) | 38.5 (38.23) | 95.3 (77.21) | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawar, R.; Lodha, S.; Ranawat, M.; El Enshasy, H.A.; Rahman, R.A.; Gafur, A.; Reddy, M.S.; Ansari, M.J.; Obaid, S.A.; Sayyed, R.Z. Combined Effects of Biosolarization and Brassica Amendments on Survival of Biocontrol Agents and Inhibition of Fusarium oxysporum. Agronomy 2022, 12, 1752. https://doi.org/10.3390/agronomy12081752
Mawar R, Lodha S, Ranawat M, El Enshasy HA, Rahman RA, Gafur A, Reddy MS, Ansari MJ, Obaid SA, Sayyed RZ. Combined Effects of Biosolarization and Brassica Amendments on Survival of Biocontrol Agents and Inhibition of Fusarium oxysporum. Agronomy. 2022; 12(8):1752. https://doi.org/10.3390/agronomy12081752
Chicago/Turabian StyleMawar, Ritu, Satish Lodha, Madhavi Ranawat, Hesham Ali El Enshasy, Roshanida A. Rahman, Abdul Gafur, M. S. Reddy, Mohammad Javed Ansari, Sami Al Obaid, and R. Z. Sayyed. 2022. "Combined Effects of Biosolarization and Brassica Amendments on Survival of Biocontrol Agents and Inhibition of Fusarium oxysporum" Agronomy 12, no. 8: 1752. https://doi.org/10.3390/agronomy12081752
APA StyleMawar, R., Lodha, S., Ranawat, M., El Enshasy, H. A., Rahman, R. A., Gafur, A., Reddy, M. S., Ansari, M. J., Obaid, S. A., & Sayyed, R. Z. (2022). Combined Effects of Biosolarization and Brassica Amendments on Survival of Biocontrol Agents and Inhibition of Fusarium oxysporum. Agronomy, 12(8), 1752. https://doi.org/10.3390/agronomy12081752










