Genetic Parameters, Prediction of Gains and Intraspecific Hybrid Selection of Paspalum notatum Flügge for Forage Using REML/BLUP
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gasparetto, B.F.; Radunz, L.L.; Lopes, R.R.; Franke, L.B.; Martinelli, J.A. Fungi associated with Paspalum guenoarum seeds: Their impact on physiology and control. Cienc. Rural. 2021, 51, e20200497. [Google Scholar] [CrossRef]
- Gates, R.N.; Quarin, C.L.; Pedreira, C.G. Bahiagrass. In Warm-Season (C4) Grasses; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2004; Volume 45, pp. 651–680. [Google Scholar]
- Zilli, A.L.; Brugnoli, E.A.; Marcón, F.; Billa, M.B.; Rios, E.F.; Martínez, E.J.; Acuña, C.A. Heterosis and Expressivity of Apospory in Tetraploid Bahiagrass Hybrids. Crop Sci. 2015, 55, 1189–1201. [Google Scholar] [CrossRef]
- Blount, A.R.; Acuña, C.A. Bahiagrass. In Genetic Resources, Chromosome Engineering, and Crop Improvement Series: Forage Crops; Singh, R.J., Ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume 5, pp. 81–101. [Google Scholar]
- Wawu, F.U.K.; Sudita, I.D.N.; Rejeki, I.G.A.D.S. Physical quality of some types of grass on mixed planting with Arachis pintoi and organic fertilizing. J. Phys. Conf. Ser. 2021, 1869, 012049. [Google Scholar] [CrossRef]
- Steiner, M.G.; Dall’Agnol, M.; Nabinger, C.; Scheffer-Basso, S.M.; Weiler, R.L.; Simioni, C.; Schifino-Wittmann, M.T.; Motta, É.A.M.D. Forage potential of native ecotypes of Paspalum notatum and P. guenoarum. Acad. Bras. Ciênc. 2017, 89, 1753–1760. [Google Scholar] [CrossRef]
- Machado, J.M.; Dall’Agnol, M.; Motta, E.A.M.; Pereira, E.A.; Barbosa, M.R.; Neme, J.C.; Krycki, K.C. Productive potential of superior genotypes of Paspalum notatum Flügge in response to nitrogen fertilization. Rev. Bras. Saúde. Prod. Anim. 2019, 20, e03102019. [Google Scholar] [CrossRef]
- Fachinetto, J.; Dall’Agnol, M.; Schneider-Canny, R.; Janke-Porto, A.; de Souza, M.G. Genetic Similarity among Accessions of Paspalum notatum Flügge (Poaceae): A Potential to Parental Selection. Braz. Arch. Biol. Technol. 2021, 64, e21190007. [Google Scholar] [CrossRef]
- Motta, E.A.M.; da Graminho, L.A.; Dall’Agnol, M.; Pötter, L.; Nabinger, C.; Souza, C.H.L.; de Krycki, K.C.; dos Santos, T.N.; Weiler, R.L.; de Ávila, M.R. Response of Bahiagrass hybrids to nitrogen fertilization or mixture with legumes. Rev. Bras. De Zootec. 2021, 50, e20210015. [Google Scholar] [CrossRef]
- Burton, G.W. Breeding Pensacola bahiagrass, Paspalum notatum: Method of reproduction. Agron. J. 1955, 47, 311–314. [Google Scholar] [CrossRef]
- Forbes, I.; Burton, G.W. Induction of Tetraploidy and a Rapid Field Method of Detecting Induced Tetraploidy in Pensacola Bahiagrass 1. Crop Sci. 1961, 1, 383–384. [Google Scholar] [CrossRef]
- Quarin, C.L.; Espinoza, F.; Martinez, E.J.; Pessino, S.C.; Bovo, O.A. A rise of ploidy level induces the expression of apomixis in Paspalum notatum. Sex. Plant Reprod. 2001, 13, 243–249. [Google Scholar] [CrossRef]
- Weiler, R.L.; Krycki, K.C.; Guerra, D.; Simioni, C.; Dall’Agnol, M. Chromosome Doubling in Paspalum Notatum Var. Saure (Cultivar Pensacola). Crop Breed. Appl. Biotechnol. 2015, 15, 106–111. [Google Scholar] [CrossRef]
- Urbani, M.H.; Acuña, C.A.; Doval, D.W.; Sartor, M.E.; Galdeano, F.; Blount, A.R.; Quesenberry, K.H.; Mackowiak, C.L.; Quarin, C.L. Registration of ‘Boyero UNNE’ Bahiagrass. J. Plant Regist. 2017, 11, 26–32. [Google Scholar] [CrossRef]
- Jank, L.; Barrios, S.C.; do Valle, C.B.; Simeão, R.M.; Alves, G.F. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 2014, 65, 1132. [Google Scholar] [CrossRef]
- Nogler, G.A. Gametophytic apomixis. In Embryology of Angiosperms; Johri, B.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 475–518. ISBN 3-540-12739-9. [Google Scholar]
- Moser, L.E.; Burson, B.L.; Sollenberger, L.E. Warm-season (C4) grass overview. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004; pp. 1–14. [Google Scholar]
- Zilli, A.L.; Acuña, C.A.; Schulz, R.R.; Marcón, F.; Brugnoli, E.A.; Novo, S.F.; Quarin, C.L.; Martínez, E.J. Transference of natural diversity from the apomictic germplasm of Paspalum notatum to a sexual synthetic population. Ann. Appl. Biol. 2019, 175, 18–28. [Google Scholar] [CrossRef]
- Ortiz, J.P.A.; Quarin, C.L.; Pessino, S.C.; Acuña, C.; Martínez, E.J.; Espinoza, F.; Hojsgaard, D.H.; Sartor, M.E.; Cáceres, M.E.; Pupilli, F. Harnessing apomictic reproduction in grasses: What we have learned from Paspalum. Ann. Bot. 2013, 112, 767–787. [Google Scholar] [CrossRef]
- Burton, G.W. Artificial fog facilitates Paspalum emasculation. J. Am. Soc. Agron. 1948, 40, 281–282. [Google Scholar] [CrossRef]
- Quarin, C.L. Effect of pollen source and pollen ploidy on endosperm formation and seed set in pseudogamous apomictic Paspalum notatum. Sex. Plant Reprod. 1999, 11, 331–335. [Google Scholar] [CrossRef]
- Martínez, E.J.; Urbani, M.H.; Quarin, C.L.; Ortiz, J.P. Inheritance of apospory in bahiagrass, Paspalum notatum. Hereditas 2001, 135, 19–25. [Google Scholar] [CrossRef]
- Martínez, E.J.; Hopp, H.E.; Stein, J.; Ortiz, J.; Quarin, C.L. Genetic characterization of apospory in tetraploid Paspalum notatum based on the identification of linked molecular markers. Mol. Breed. 2003, 12, 319–327. [Google Scholar] [CrossRef]
- Pozzi, F.I.; Acuña, C.A.; Quarin, C.L.; Felitti, S.A. GG13: A candidate gene related to seed development and viability from apomictic Paspalum notatum. Euphytica 2021, 217, 148. [Google Scholar] [CrossRef]
- Marcón, F.; Brugnoli, E.A.; Nunes, J.A.R.; Gutierrez, V.A.; Martínez, E.J.; Acuña, C.A. Evaluating general combining ability for agro-morphological traits in tetraploid bahiagrass. Euphytica 2021, 217, 208. [Google Scholar] [CrossRef]
- Marcón, F.; Martínez, E.J.; Rodríguez, G.R.; Zilli, A.L.; Brugnoli, E.A.; Acuña, C.A. Genetic distance and the relationship with heterosis and reproductive behavior in tetraploid bahiagrass hybrids. Mol. Breed. 2019, 39, 89. [Google Scholar] [CrossRef]
- Azevedo, V.R.; de Wadt, L.H.O.; Pedrozo, C.A.; de Resende, M.D.V. Coeficiente de repetibilidade para produção de frutos e seleção de matrizes de Bertholletia excelsa (Bonpl.) em castanhais nativos do estado do Acre. Ciênc. Florest. 2020, 30, 135. [Google Scholar] [CrossRef]
- Ambrósio, M.; Viana, A.P.; Ribeiro, R.M.; Preisigke, S.C.; Cavalcante, N.R.; da Silva, F.A.; Torres, G.X.; de Sousa, C.M.B. Genotypic superiority of Psidium Guajava S1 families using mixed modeling for truncated and simultaneous selection. Sci. Agric. 2021, 78, e20190179. [Google Scholar] [CrossRef]
- Capistrano, M.D.C.; Andrade Neto, R.D.C.; Santos, V.B.D.; Lessa, L.S.; de Resende, M.D.V.; Mesquita, A.G.G.; Gurgel, F.D.L. Use of the REML/BLUP methodology for the selection of sweet orange genotypes. Pesq. Agropec. Bras. 2021, 56, e02032. [Google Scholar] [CrossRef]
- de Resende, M.D.V. Software Selegen-REML/BLUP: A useful tool for plant breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- de Resende, M.D.V. Genética Biométrica e Estatística no Melhoramento de Plantas Perenes; Embrapa Informação Tecnológica: Brasília, Brazil; Embrapa Florestas: Colombo, Brazil, 2002; p. 975. [Google Scholar]
- Carias, C.M.O.M.; Tomaz, M.A.; Ferrão, M.A.G.; da Fonseca, A.F.A.; Ferrão, R.G.; Gonçalves, L.S.A. Produtividade de grãos de cafeeiro conilon de diferentes grupos de maturação pelo procedimento REML/BLUP. Sem Ci Agric. 2014, 35, 707. [Google Scholar] [CrossRef][Green Version]
- Silva, F.H.D.L.; Viana, A.P.; Santos, E.A.; Freitas, J.C.D.O.; Rodrigues, D.L.; Amaral, A.T.D. Prediction of genetic gains by selection indexes and REML/BLUP methodology in a population of sour passion fruit under recurrent selection. Acta Sci. Agron. 2017, 39, 183–190. [Google Scholar] [CrossRef]
- Bernardo, R. Breeding for Quantitative Traits in Plants; Stemma Press: Woodbury, MN, USA, 2010. [Google Scholar]
- Robinson, G.K. That BLUP Is a Good Thing: The Estimation of Random Effects. Stat. Sci. 1991, 6, 15–32. [Google Scholar]
- Bernardo, R. Best linear unbiased predictor analysis. In The Genetics and Explotation of Heterosis in Crops; Coors, J.G., Pandey, S., Eds.; ASSA-CSSA-SSSA: Madison, WI, USA, 1999; pp. 269–276. [Google Scholar]
- Ferreira Coelho, I.; Peixoto, M.A.; Santana Pinto Coelho Evangelista, J.; Silva Alves, R.; Sales, S.; de Resende, M.D.V.; Naves Pinto, J.F.; Fialho dos Reis, E.; Bhering, L.L. Multiple-trait, random regression, and compound symmetry models for analyzing multi-environment trials in maize breeding. PLoS ONE 2020, 15, e0242705. [Google Scholar] [CrossRef]
- Zorić, M.; Gunjača, J.; Galić, V.; Jukić, G.; Varnica, I.; Šimić, D. Best Linear Unbiased Predictions of Environmental Effects on Grain Yield in Maize Variety Trials of Different Maturity Groups. Agronomy 2022, 12, 922. [Google Scholar] [CrossRef]
- Azevedo, C.V.G.; Val, B.H.P.; Araújo, L.C.A.D.; Juhász, A.C.P.; Mauro, A.O.D.; Unêda-Trevisoli, S.H. Genetic parameters of soybean populations obtained from crosses between grain and food genotypes. Acta Sci. Agron. 2020, 43. [Google Scholar] [CrossRef]
- De Albuquerque, J.R.T.; Lins, H.A.; dos Santos, M.G.; de Freitas, M.A.M.; de Oliveira, F.S.; de Souza, A.R.E.; da Silveira, L.M.; Henrique de Sousa Nunes, G.; Barros Júnior, A.P.; de Melo Jorge Vieira, P.F. Adaptability and stability of soybean (Glycine max L.) genotypes in semiarid conditions. Euphytica 2022, 218, 61. [Google Scholar] [CrossRef]
- Atagul, O.; Calle, A.; Demirel, G.; Lawton, J.M.; Bridges, W.C.; Gasic, K. Estimating Heat Requirement for Flowering in Peach Germplasm. Agronomy 2022, 12, 1002. [Google Scholar] [CrossRef]
- Matias, F.I.; Valle, C.B.D.; Gouveia, B.T.; Moro, G.V.; Barrios, S.C.L. Using additive indices and principal components to select sexual genitors and hybrids of Urochloa decumbens. Crop Breed. Appl. Biotechnol. 2020, 20, e28082022. [Google Scholar] [CrossRef]
- Moreno, J.A. Clima do Rio grande do Sul. Bol. Geográfico Do Rio Gd. Do Sul 1961, 11, 49–83. [Google Scholar]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2018; p. 356. [Google Scholar]
- Comissão de Química e Fertilidade do Solo RS/SC (CQFS RS/SC). Manual de Adubação E Calagem Para Estados do Rio Grande do Sul e Santa Catarina, 10th ed.; SBCS/NRS: Porto Alegre, Brazil, 2004. [Google Scholar]
- Quarin, C.L.; Urbani, M.H.; Blount, A.R.; Martinez, E.J.; Hack, C.M.; Burton, G.W.; Quesenberry, K.H. Registration of Q4188 and Q4205, Sexual Tetraploid Germplasm Lines of Bahiagrass. Crop Sci. 2003, 43, 745. [Google Scholar] [CrossRef]
- Fachinetto, J.M.; Schneider, R.; Hubber, K.G.C.; Dall’Agnol, M. Avaliação agronômica e análise da persistência em uma coleção de acessos de Paspalum notatum Flügge (Poaceae). Agraria 2012, 7, 189–195. [Google Scholar] [CrossRef]
- Machado, J.M.; Krycki, K.C.; Weiler, R.L.; Simioni, C.; Dall’Agnol, M. Reproduction mode and apospory expressivity of selected hybrids of Paspalum notatum Flügge. J. Plant Breed. Crop Sci. 2021, 13, 58–63. [Google Scholar] [CrossRef]
- Weiler, R.L.; Dall’Agnol, M.; Simioni, C.; Krycki, K.C.; Pereira, E.A.; Machado, J.M.; Motta, E.A.M. Intraspecific tetraploid hybrids of Paspalum notatum: Agronomic evaluation of segregating progeny. Sci Agric. 2018, 75, 36–42. [Google Scholar] [CrossRef]
- Resende, M.D.V. Matemática e Estatística na Análise de Experimentos e no Melhoramento Genético; Embrapa Florestas: Colombo, Brazil, 2007; p. 561. [Google Scholar]
- Cruz, C.D. Genes Software-extended and integrated with the R, Matlab and Selegen. Acta Sci. Agron. 2016, 38, 547–552. [Google Scholar] [CrossRef]
- Henriques, E.P.; Nunes, A.C.P.; de Moraes, M.L.T.; de Resende, M.D.V.; Sebbenn, A.M.; de Moraes, M.A. Seleção genética em teste de progênies de irmãos completos de Eucalyptus para a produção de carvão vegetal. Sci. For. 2018, 46, 119. [Google Scholar] [CrossRef]
- Krause, D.P.; Fachi, L.R.; Dalbosco, E.Z.; Campos, T.N.V.; Freitas, A.P.; Lima, K.S.; Krause, W. Estimativas de parâmetros genéticos e ganhos de seleção em progênies de maracujazeiro via metodologia REML/BLUP. Sci. Elec. Arch. 2021, 14, 42–48. [Google Scholar] [CrossRef]
- Lopes, R.R.; Franke, L.B.; de Souza, C.H.L.; Bertoncelli, P.; Graminho, L.A.; Pereira, É.A. Genetic parameters and predicted gains with selection of interspecific hybrids of Paspalum for seed production. Crop Breed. Appl. Biotechnol. 2018, 18, 284–291. [Google Scholar] [CrossRef]
- de Resende, M.D.V.; Duarte, J.B. Precisão e controle de qualidade em experimentos de avaliação de cultivares. Pesqui. Agropecu. Trop. 2007, 37, 182–194. [Google Scholar]
- Costa, M.M.; Di Mauro, A.O.; Unêda-Trevisoli, S.H.; Arriel, N.H.C.; Bárbaro, I.M.; Da Silveira, G.D.; Muniz, F.R.S. Analysis of direct and indirect selection and indices in soybean segregating populations. Crop Breed. Appl. Biotech. 2008, 8, 47–55. [Google Scholar] [CrossRef]
- Nogueira, T.A.P.C.; Nunes, A.C.P.; dos Santos, G.A.; Takahashi, E.K.; de Resende, M.D.V.; Corradi, I.S. Estimativa de parâmetros genéticos em progênies de irmãos completos de eucalipto e otimização de seleção. Sci. For. 2019, 47, 123. [Google Scholar] [CrossRef]
- Santos, W.D.; Araújo, E.G.; Souza, D.C.L.; da Silva, J.R.; Recco, C.R.S.B.; de Moraes, M.L.T.; de Aguiar, A.V. Divergência genética entre progênies de polinização aberta de Pinus caribaea var. hondurensis a partir de caracteres quantitativos. Pesq. Flor. Bras. 2016, 36, 127. [Google Scholar] [CrossRef]
- Resende, M.A.V.D.; Freitas, J.A.D.; Lanza, M.A.; Resende, M.D.V.D.; Azevedo, C.F. Divergência genética e índice de seleção via BLUP em acessos de algodoeiro para características tecnológicas da fibra. Pesqui. Agropecu. Trop. 2014, 44, 334–640. [Google Scholar] [CrossRef]
- Luz, J.M.Q.; Bitar, C.A.; Oliveira, R.C.; Nascimento, A.R.; Nogueira, A.P.O. Desempenho e divergência genética de genótipos de tomate para processamento industrial. Hortic. Bras. 2016, 34, 483–490. [Google Scholar] [CrossRef]
- Amaral Júnior, A.T.; Graça, A.J.P.; Vivas, M.; Viana, A.P.; Rodrigues, R. Prospecting of tomato hybrids for table and industry via mixed modeling and multivariate analysis. Hortic. Bras. 2017, 35, 20–25. [Google Scholar] [CrossRef]
- Peixoto, J.V.M.; Maciel, G.M.; Pereira, L.M.; da Silveira, A.J.; Clemente, A.A.; de Souza Miranda, M.; de Oliveira, C.S. Genetic divergence in round tomato germplasm: Optimization and hierarchical methods. Agraria 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Streck, E.A.; Aguar, G.A.; Magalhães Júnior, A.M.; Facchinello, H.K.; Oliveira, A.C. Phenotypic variability in genotypes of irrigated rice via multivariate analysis. Rev. Cienc. Agron. 2017, 48, 101–109. [Google Scholar] [CrossRef]
- Ferreira, D.S.; Canal, G.B.; Nascimento, M.; Nascimento, A.C.C.; Ferreira, J.M.S.; do Amaral, J.F.T.; Pereira, L.L.; Rodrigues, W.N.; Ribeiro, W.R.; Castanheira, D.T.; et al. Exploring the multivariate technique in the discrimination of Coffea arabica L. cultivars regarding the production and quality of grains under the effect of water management. Euphytica 2021, 217, 118. [Google Scholar] [CrossRef]
- Lei, G.; Song, C.; Wen, X.; Gao, G.; Qi, Y. Chemical Diversity and Potential Target Network of Woody Peony Flower Essential Oil from Eleven Representative Cultivars (Paeonia × suffruticosa Andr.). Molecules 2022, 27, 2829. [Google Scholar] [CrossRef]
- de Castro Sant’Anna, I.; Cruz, C.D.; Gouvêa, L.R.L.; Junior, E.J.S.; de Freitas, R.S.; de Souza Gonçalves, P. Genetic diversity associated with natural rubber quality in elite genotypes of the rubber tree. Sci. Rep. 2021, 11, 1081. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Z.; Yang, W.; Yang, E. Genetic Diversity Assessment of the International Maize and Wheat Improvement Center and Chinese Wheat Core Germplasms by Non-Denaturing Fluorescence in Situ Hybridization. Plants 2022, 11, 1403. [Google Scholar] [CrossRef]
- Mengistu, F.; Motoike, S.; Cruz, C.D. Molecular characterization and genetic diversity of the macaw palm ex situ germplasm collection revealed by microsatellite markers. Diversity 2016, 8, 20. [Google Scholar] [CrossRef]
- Kumar, V.; Lade, S.; Yadav, H.K. Evaluation of genetic diversity in Lepidium sativum L. germplasm based on multivariate analysis. Genet. Resour. Crop Evol. 2021, 68, 809–820. [Google Scholar] [CrossRef]
- Ihsan, M.; Nazir, N.; Ghafoor, A.; Khalil, A.A.K.; Zahoor, M.; Nisar, M.; Khames, A.; Ullah, R.; Shah, A.B. Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis. Agronomy 2021, 11, 1713. [Google Scholar] [CrossRef]
- Rasmusson, D.C.; Phillips, R.L. Plant breeding progress and genetic diversity from de novo variation and elevated epistasis. Crop Sci. 1997, 37, 303–310. [Google Scholar] [CrossRef]
- Brito, O.G.; Júnior, V.C.A.; Azevedo, A.M.; Donato, L.M.S.; Silva, A.J.M.; Oliveira, A.J.M. Genetic divergence between half-sibling progenies of kale using different multivariate approaches. Hortic. Bras. 2021, 39, 178–185. [Google Scholar] [CrossRef]

| ♂ (4x) | Origin | ♀ (4x) * | Hybrids |
|---|---|---|---|
| 30N | Santa Fé—Argentina | Q4188 | 121; 221; 321; 421; 521; 621; 721; 821; 921; 1021;1121 |
| 36N | Santa Fé—Argentina | C44X | 112; 212; 312; 412; 512; 612; 712; 812;912 |
| Q4205 | 132; 232; 332; 432; 532; 632;732 | ||
| 83N | Corrientes—Argentina | C44X | 115; 215; 315; 415; 515; 615 |
| Q4188 | 125; 225; 325; 425; 525; 625; 725; 825; 925 | ||
| 95N | Corrientes—Argentina | C44X | 116; 216; 316; 416 |
| Q4205 | 136; 236; 336; 436; 536; 636; 736; 836; 936; 1036; 1136; 1636 | ||
| Q4188 | 126; 226; 326; 426; 526; 626; 726; 826; 926; 1026; 1126 | ||
| V4 | Barra do Quaraí/RS—Brazil | Q4205 | 137; 237; 337; 437; 537 |
| Parameters | ATDM (kg DM Plant−1) | ALDM (kg DM Plant−1) | ASDM (kg DM Plant−1) | AIDM (kg DM Plant−1) | LSR | PH (cm) | TPD (Tillers Plant−1) |
|---|---|---|---|---|---|---|---|
| DEV genotype | 3518.7 | 3176.5 | 2786.2 | 2306.4 | 604.31 | 1418 | 2960.1 |
| DEV complete model | 2275.1 | 1803.9 | 1352.7 | 1042.5 | 199.27 | 351.43 | 1176.9 |
| LRT (χ2) | 1243.64 ** | 1372.53 ** | 1433.47 ** | 1263.93 ** | 405.04 ** | 1066.60 ** | 1783.22 ** |
| LI h2 (%) | 0.6939 | 0.6954 | 0.696 | 0.6942 | 0.5895 | 0.6902 | 0.6971 |
| LS h2 (%) | 1.2976 | 1.2995 | 1.3 | 1.2979 | 1.1544 | 1.2923 | 1.3019 |
| 13,917.2 | 4972.7 | 1535.6 | 361.29 | 1.8772 | 24.755 | 2596.6 | |
| 59.716 | 12.688 | 3.0655 | 1.4284 | 0.2758 | 0.2175 | 1.27 | |
| 13.977 | 4985.4 | 1538.7 | 362.72 | 2.1529 | 24.972 | 2597.9 | |
| 0.9957 | 0.9975 | 0.998 | 0.9961 | 0.8719 | 0.9913 | 0.9995 | |
| 0.9989 | 0.9994 | 0.9995 | 0.999 | 0.9646 | 0.9978 | 0.9999 | |
| 0.9995 | 0.9997 | 0.9998 | 0.9995 | 0.9821 | 0.9989 | 0.9999 | |
| CVg (%) | 64.656 | 65.597 | 78.706 | 73.344 | 51.781 | 27.866 | 49.857 |
| CVres (%) | 4.2352 | 3.3134 | 3.5165 | 4.6117 | 19.846 | 2.6118 | 1.1026 |
| CVr | 15.266 | 19.797 | 22.382 | 15.904 | 2.6091 | 10.669 | 45.217 |
| Grand mean | 182.46 | 107.501 | 49.789 | 25.916 | 2.646 | 17.855 | 102.21 |
| ATDM (kg DM Plant−1) | ALDM (kg DM Plant−1) | ASDM (kg DM Plant−1) | |||||||||||||
| Order | Hybrid | g | u + g | Gain | new | Hybrid | g | u + g | Gain | new | Hybrid | g | u + g | Gain | new |
| 1 | 336 | 399.51 | 581.97 | 399.51 | 581.97 | 336 | 239.64 | 347.14 | 239.64 | 347.14 | 437 | 130.86 | 180.65 | 130.86 | 180.65 |
| 2 | 332 | 332.58 | 515.04 | 366.05 | 548.51 | 332 | 226.72 | 334.22 | 233.18 | 340.68 | V4 | 117.15 | 166.94 | 124.01 | 173.79 |
| 3 | 437 | 319.52 | 501.98 | 350.54 | 533.00 | 132 | 171.29 | 278.80 | 212.55 | 320.05 | 336 | 111.68 | 161.47 | 119.90 | 169.69 |
| 4 | 132 | 262.96 | 445.42 | 328.64 | 511.10 | 437 | 155.89 | 263.40 | 198.39 | 305.89 | 221 | 96.12 | 145.90 | 113.95 | 163.74 |
| 5 | 30N | 206.79 | 389.25 | 304.27 | 486.73 | 30N | 108.63 | 216.13 | 180.43 | 287.94 | 332 | 79.61 | 129.40 | 107.08 | 156.87 |
| 6 | 221 | 200.32 | 382.79 | 286.95 | 469.41 | 236 | 103.66 | 211.16 | 167.64 | 275.14 | 515 | 70.68 | 120.47 | 101.02 | 150.80 |
| 7 | 236 | 188.31 | 370.77 | 272.86 | 455.32 | 221 | 102.77 | 210.27 | 158.37 | 265.87 | 236 | 66.33 | 116.12 | 96.06 | 145.85 |
| 8 | 137 | 168.76 | 351.22 | 259.84 | 442.31 | 95N | 99.84 | 207.34 | 151.05 | 258.56 | 116 | 60.28 | 110.07 | 91.59 | 141.38 |
| 9 | 95N | 168.08 | 350.54 | 249.65 | 432.11 | 725 | 99.00 | 206.50 | 145.27 | 252.77 | 30N | 49.69 | 99.48 | 86.93 | 136.72 |
| 10 | 515 | 162.79 | 345.25 | 240.96 | 423.42 | 137 | 91.42 | 198.92 | 139.89 | 247.39 | 137 | 48.64 | 98.43 | 83.10 | 132.89 |
| 11 | V4 | 148.26 | 330.72 | 232.54 | 415.00 | 926 | 82.56 | 190.06 | 134.67 | 242.18 | 132 | 43.18 | 92.97 | 79.47 | 129.26 |
| 12 | 48N | 143.36 | 325.82 | 225.10 | 407.56 | V4 | 68.46 | 175.96 | 129.16 | 236.66 | 70N | 42.64 | 92.43 | 76.40 | 126.19 |
| 13 | 216 | 121.01 | 303.47 | 217.10 | 399.56 | 636 | 67.07 | 174.57 | 124.38 | 231.88 | 337 | 42.48 | 92.27 | 73.79 | 123.58 |
| 14 | 926 | 98.23 | 280.69 | 208.61 | 391.07 | 225 | 62.94 | 170.44 | 119.99 | 227.49 | 316 | 40.70 | 90.49 | 71.43 | 121.22 |
| 15 | 337 | 95.81 | 278.27 | 201.09 | 383.55 | 721 | 59.34 | 166.84 | 115.95 | 223.45 | 48N | 40.26 | 90.05 | 69.35 | 119.14 |
| AIDM (kg DM Plant−1) | LSR | PH (cm) | |||||||||||||
| Order | Hybrid | g | u + g | Gain | new | Hybrid | g | u + g | Gain | new | Hybrid | g | u + g | Gain | new |
| 1 | 132 | 47.84 | 73.76 | 47.84 | 73.76 | Q4188 | 4.49 | 7.14 | 4.49 | 7.14 | 437 | 13.35 | 31.20 | 13.35 | 31.20 |
| 2 | 30N | 47.81 | 73.73 | 47.83 | 73.74 | 1026 | 4.09 | 6.73 | 4.29 | 6.94 | 525 | 11.74 | 29.60 | 12.54 | 30.40 |
| 3 | 336 | 47.62 | 73.53 | 47.76 | 73.67 | 525 | 4.07 | 6.71 | 4.22 | 6.86 | 332 | 8.93 | 26.78 | 11.34 | 29.19 |
| 4 | 48N | 46.40 | 72.32 | 47.42 | 73.33 | 225 | 3.39 | 6.03 | 4.01 | 6.65 | 115 | 8.15 | 26.01 | 10.54 | 28.40 |
| 5 | 515 | 39.64 | 65.55 | 45.86 | 71.78 | Q4205 | 3.07 | 5.72 | 3.82 | 6.47 | 636 | 8.13 | 25.98 | 10.06 | 27.91 |
| 6 | 212 | 35.92 | 61.83 | 44.20 | 70.12 | 1136 | 2.68 | 5.33 | 3.63 | 6.28 | 621 | 7.63 | 25.48 | 9.65 | 27.51 |
| 7 | 316 | 32.89 | 58.81 | 42.59 | 68.50 | 921 | 2.49 | 5.14 | 3.47 | 6.11 | 926 | 7.50 | 25.36 | 9.35 | 27.20 |
| 8 | 437 | 32.17 | 58.09 | 41.29 | 67.20 | 1636 | 2.31 | 4.96 | 3.32 | 5.97 | 336 | 6.77 | 24.63 | 9.03 | 26.88 |
| 9 | 95N | 32.10 | 58.02 | 40.27 | 66.18 | 532 | 1.63 | 4.28 | 3.14 | 5.78 | 1136 | 6.51 | 24.36 | 8.75 | 26.60 |
| 10 | 70N | 28.97 | 54.89 | 39.14 | 65.05 | 721 | 1.37 | 4.02 | 2.96 | 5.61 | 132 | 6.15 | 24.01 | 8.49 | 26.34 |
| 11 | 137 | 28.03 | 53.94 | 38.13 | 64.04 | 725 | 1.26 | 3.90 | 2.81 | 5.45 | 1036 | 6.13 | 23.98 | 8.27 | 26.13 |
| 12 | 116 | 27.51 | 53.43 | 37.24 | 63.16 | 536 | 1.14 | 3.79 | 2.67 | 5.31 | 936 | 6.01 | 23.86 | 8.08 | 25.94 |
| 13 | 332 | 25.67 | 51.58 | 36.35 | 62.27 | 825 | 1.10 | 3.74 | 2.55 | 5.19 | 836 | 5.95 | 23.80 | 7.92 | 25.77 |
| 14 | 415 | 24.26 | 50.17 | 35.49 | 61.40 | 912 | 0.98 | 3.62 | 2.43 | 5.08 | 1021 | 5.88 | 23.73 | 7.77 | 25.63 |
| 15 | 236 | 17.66 | 43.58 | 34.30 | 60.21 | 936 | 0.97 | 3.61 | 2.34 | 4.98 | 537 | 5.56 | 23.41 | 7.63 | 25.48 |
| TPD (Tillers Plant−1) | |||||||||||||||
| Order | Hybrid | g | u + g | Gain | new | ||||||||||
| 1 | 137 | 146 | 248 | 146 | 248 | ||||||||||
| 2 | 216 | 126 | 228 | 136 | 238 | ||||||||||
| 3 | 132 | 104 | 206 | 125 | 227 | ||||||||||
| 4 | 332 | 102 | 204 | 119 | 222 | ||||||||||
| 5 | 48N | 91 | 194 | 114 | 216 | ||||||||||
| 6 | 725 | 87 | 189 | 109 | 212 | ||||||||||
| 7 | 726 | 85 | 188 | 106 | 208 | ||||||||||
| 8 | 95N | 73 | 175 | 102 | 204 | ||||||||||
| 9 | 321 | 69 | 171 | 98 | 200 | ||||||||||
| 10 | 336 | 66 | 168 | 95 | 197 | ||||||||||
| 11 | 36N | 63 | 165 | 92 | 194 | ||||||||||
| 12 | 926 | 55 | 157 | 89 | 191 | ||||||||||
| 13 | 436 | 53 | 156 | 86 | 188 | ||||||||||
| 14 | V4 | 52 | 155 | 84 | 186 | ||||||||||
| 15 | 221 | 52 | 154 | 82 | 184 | ||||||||||
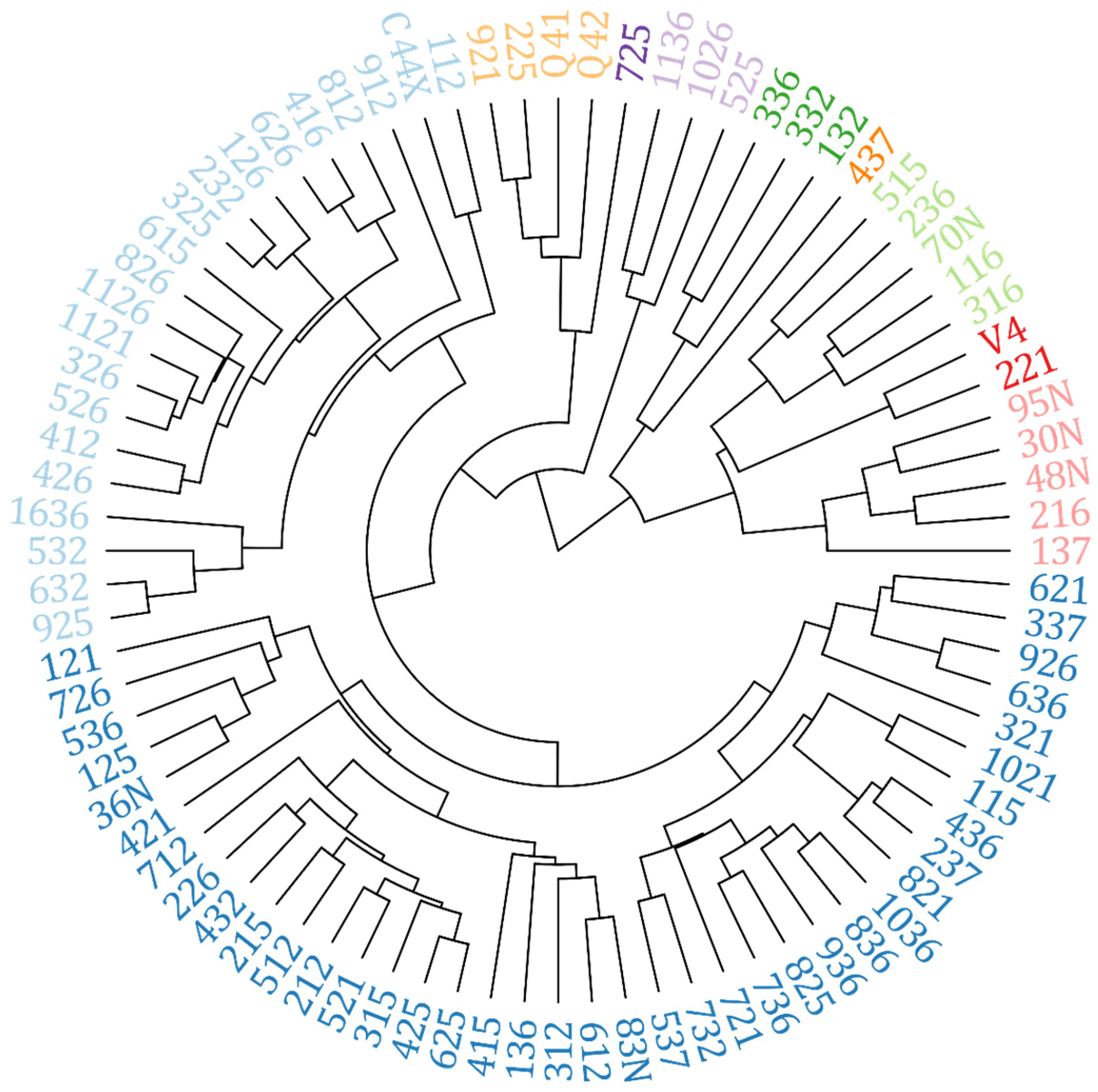
| Group | Hybrids |
|---|---|
| I | 232; 325; 126; 1121; 326; 526; 826; 1126; 626; 416; 426; 615; 412; 632; 925; 812; 521; 315; 625; 425; 212; 712; 112; 432; 512; 215; 226; 536; 612; 121; 125; 912; 83N; 312; 736; 825; 821; 237; 732; C44X; 421; 1036; 436; 537; 1021; 415; 136; 836; 36N; 726; 115; 936; 721; 1636 |
| II | 636; 926; 621; 337; 321; 316; 236; 515; 70N; 116; 95N; 30N; 48N; 221; V4 |
| III | 225; 921; Q4188; Q4205; 725 |
| IV | 1026; 1136; 525 |
| V | 332; 336; 132 |
| VI | 137; 216 |
| VII | 437 |
| VIII | 532 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, D.C.; Machado, J.M.; Motta, E.A.M.d.; Barbosa, M.R.; Simioni, C.; Weiler, R.L.; Mills, A.; Sampaio, R.; Brunes, A.P.; Dall’Agnol, M. Genetic Parameters, Prediction of Gains and Intraspecific Hybrid Selection of Paspalum notatum Flügge for Forage Using REML/BLUP. Agronomy 2022, 12, 1654. https://doi.org/10.3390/agronomy12071654
Silveira DC, Machado JM, Motta EAMd, Barbosa MR, Simioni C, Weiler RL, Mills A, Sampaio R, Brunes AP, Dall’Agnol M. Genetic Parameters, Prediction of Gains and Intraspecific Hybrid Selection of Paspalum notatum Flügge for Forage Using REML/BLUP. Agronomy. 2022; 12(7):1654. https://doi.org/10.3390/agronomy12071654
Chicago/Turabian StyleSilveira, Diógenes Cecchin, Juliana Medianeira Machado, Eder Alexandre Minski da Motta, Marlon Risso Barbosa, Carine Simioni, Roberto Luis Weiler, Annamaria Mills, Rodrigo Sampaio, André Pich Brunes, and Miguel Dall’Agnol. 2022. "Genetic Parameters, Prediction of Gains and Intraspecific Hybrid Selection of Paspalum notatum Flügge for Forage Using REML/BLUP" Agronomy 12, no. 7: 1654. https://doi.org/10.3390/agronomy12071654
APA StyleSilveira, D. C., Machado, J. M., Motta, E. A. M. d., Barbosa, M. R., Simioni, C., Weiler, R. L., Mills, A., Sampaio, R., Brunes, A. P., & Dall’Agnol, M. (2022). Genetic Parameters, Prediction of Gains and Intraspecific Hybrid Selection of Paspalum notatum Flügge for Forage Using REML/BLUP. Agronomy, 12(7), 1654. https://doi.org/10.3390/agronomy12071654






