Management of the Common Vole in the Czech Lands: Historical and Current Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Historical and Current Economic Importance of Voles in the Czech Republic
3.1. Economic Losses Caused by Voles in the Past
3.2. Current Economic Losses Caused by Voles
4. History and Present Status of M. arvalis Monitoring in Czech Lands
4.1. History of Vole Population Monitoring
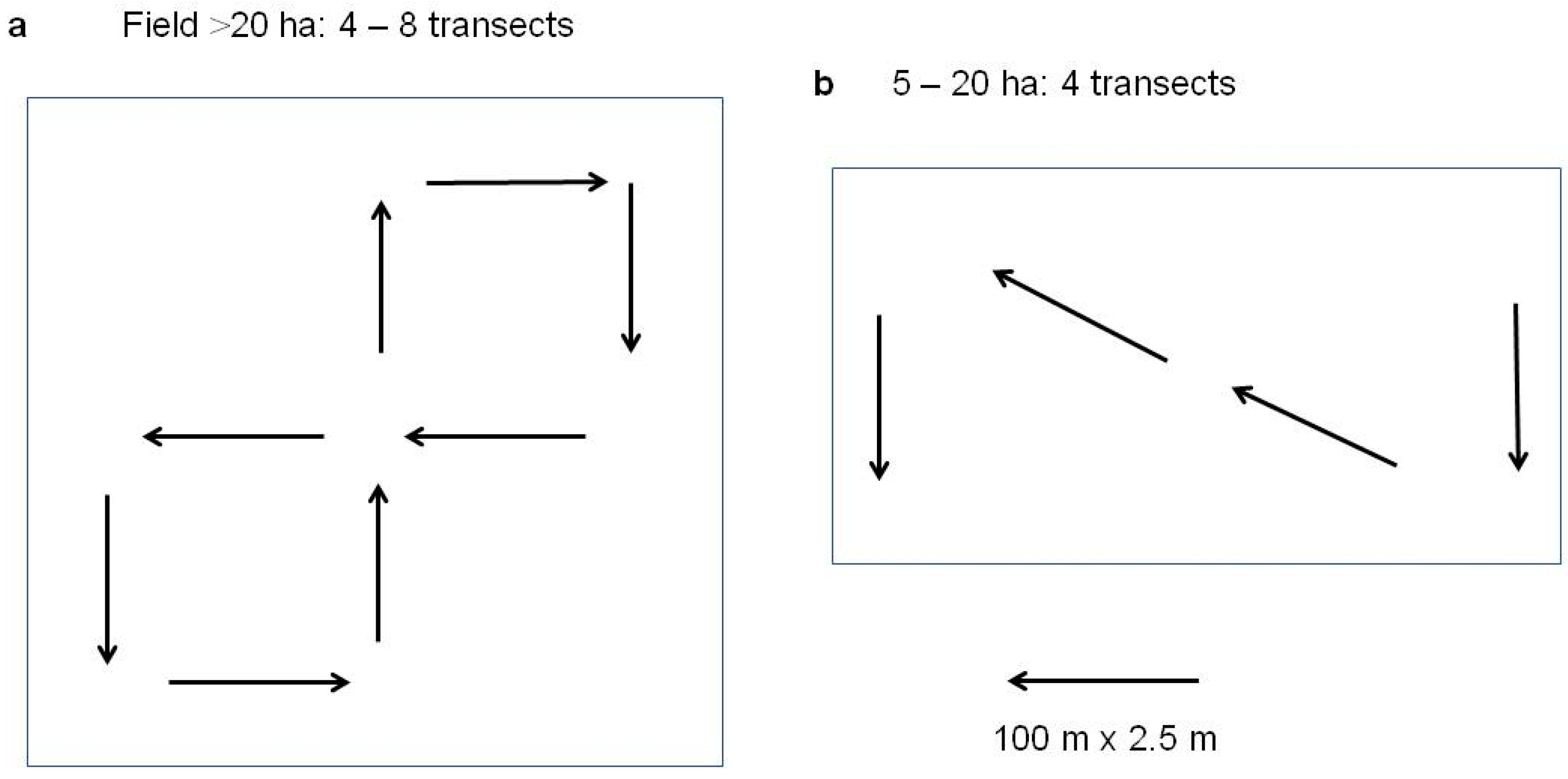
4.2. Present Status of Vole Monitoring
5. Methods of Controlling Common Voles
5.1. Agrotechnical Methods of Control and Landscape Management
5.2. Biological Control
5.2.1. Mammalian and Avian Predators
5.2.2. Microbial Methods
5.3. Chemical Control
5.3.1. Research and Preparation Used in the Early 1900s
5.3.2. Research and Preparation Used in the 1940s–1950s
Cartridge Generators of Smoke and Gas
Toxic Baits
Toxic Greasy and Sticky Materials (“Mushy Mass”)
Dusts and Ashes
Spray and Dust Insecticides
5.3.3. Preparations Used from the 1960s Onward
6. Stutox—A History of the Original Pelletised Bait in the Czech Republic
7. Ecological Aspects of Chemical Control
7.1. Surface vs. Subsurface (Burrow) Bait Applications in History and at Present
7.2. Chemical Vole Control and Primary or Secondary Poisoning of Nontarget Animals
8. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zapletal, M.; Obdržálková, D.; Pikula, J.; Pikula, J., Jr.; Beklová, M. Geographic distribution of the field vole (Microtus arvalis) in the Czech Republic. Plant Prot. Sci. 1999, 35, 139–146. [Google Scholar] [CrossRef][Green Version]
- Zapletal, M.; Obdržálková, D.; Pikula, J.; Zejda, J.; Pikula, J.; Beklová, M.; Heroldová, M. Common Vole Microtus arvalis (Pallas, 1778). Czech Republic, 1st ed.; Akademické nakladateství CERM, s.r.o.: Brno, Czech Republic, 2001; p. 128, (In Czech with English summary). [Google Scholar]
- Zejda, J.; Nesvadbová, J. Abundance and reproduction of the common vole, Microtus arvalis in crop rows and associated agricultural habitats. Folia Zool. 2000, 49, 261–268. [Google Scholar]
- Anděra, M.; Gaisler, J. Savci České Republiky: Popis, Rozšíření, Ekologie, Ochrana, 1st ed.; Academia Praha: Praha, Czech Republic, 2012; p. 285. (In Czech) [Google Scholar]
- Tkadlec, E.; Stenseth, N.C. A new geographical gradient in vole population dynamics. Proc. R Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Imholt, C.; Caminero-Saldaña, C.; Couval, G.; Giraudoux, P.; Herrero-Cófreces, S.; Horváth, G.; Luque-Larena, J.J.; Tkadlec, E.; Wymenga, E. Europe-wide outbreaks of common voles in 2019. J. Pest. Sci. 2020, 93, 703–709. [Google Scholar] [CrossRef]
- Giraudoux, P.; Villette, P.; Quéré, J.P.; Damange, J.P.; Delattre, P. Weather influences M. arvalis reproduction but not population dynamics in a 17-year time series. Sci. Rep. 2019, 9, 13942. [Google Scholar] [CrossRef] [PubMed]
- Bryja, J.; Nesvadbová, J.; Heroldová, M.; Jánová, E.; Losík, J.; Trebatická, L.; Tkadlec, E. Common vole (Microtus arvalis) population sex ratio: Biases and process variation. Can. J. Zool. 2005, 83, 1391–1399. [Google Scholar] [CrossRef]
- Heroldová, M.; Šipoš, J.; Suchomel, J.; Zejda, J. Influence of crop type on the common vole abundance in Central European agroecosystems. Agric. Ecosyst. Environ. 2021, 315, 107443. [Google Scholar] [CrossRef]
- Suchomel, J.; Heroldová, M. Extrémní přemnožení hraboše polního a škody v roce 2019. Úroda 2019, 9, 33–36. (In Czech) [Google Scholar]
- Frankova, M.; Kaftanova, B.; Aulicky, R.; Rodl, P.; Frynta, D.; Stejskal, V. Temporal production of coloured faeces in wild roof rats (Rattus rattus) following consumption of fluorescent non-toxic bait and a comparison with wild R. norvegicus and Mus musculus. J. Stored Prod. Res. 2019, 81, 7–10. [Google Scholar] [CrossRef]
- Maaz, D.; Herden, C.; Freise, J.; Wolf, R.; Stubbe, M.; Borkenhagen, P.; Ansorge, H.; Eccard, J.A.; Lang, J.; Jourdain, E.; et al. High genetic structuring of Tula hantavirus. Arch. Virol. 2016, 161, 1135–1149. [Google Scholar]
- Achazi, K.; Růžek, D.; Donoso-Mantke, O.; Schlegel, M.; Ali, H.S.; Wenk, M.; Schmidt-Chanasit, J.; Ohlmeyer, L.; Rühe, F.; Vor, T.; et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2011, 11, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Obigala, A.; Jeske, K.; Augustin, M.; Król, N.; Fischer, S.; Mertens-Scholz, K.; Imholt, C.; Suchomel, J.; Heroldova, M.; Tomaso, H.; et al. Highly prevalent bartonellae and other vector-borne pathogens in small mammal species from the Czech Republic and Germany. Parasites Vectors 2019, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Fraňková, M.; Stejskal, V.; Aulický, R. Comparison of risks of voles and other rodents on sugar beet and other crops. Listy Cukrov. A Řepařské 2021, 137, 308–314, (In Czech with English abstract). [Google Scholar]
- Treml, F.; Pejčoch, M.; Holešovská, Z. Small mammals—Natural reservoir of pathogenic leptospires. Vet. Med. 2002, 47, 309–314. [Google Scholar] [CrossRef]
- Pejčoch, M.; Kriz, B. Hantaviruses in the Czech Republic. Emerg. Infect. Dis. 2003, 9, 756–757. [Google Scholar] [CrossRef] [PubMed]
- Heroldová, M.; Pejcoch, M.; Bryja, J.; Jánová, E.; Suchomel, J.; Tkadlec, E. Tula virus in populations of small terrestrial mammals in a rural landscape. Vector Borne Zoonotic Dis. 2010, 10, 599–603. [Google Scholar] [CrossRef]
- Obiegala, A.; Woll, D.; Karnath, C.; Silaghi, C.; Schex, S.; Eßbauer, S.; Pfeffer, M. Prevalence and genotype allocation of pathogenic Leptospira species in small mammals from various habitat types in Germany. PLoS Negl. Trop. Dis. 2016, 10, e0004501. [Google Scholar] [CrossRef]
- Jeske, K.; Tomaso, H.; Imholt, C.; Schulz, J.; Beerli, O.; Suchomel, J.; Heroldova, M.; Jacob, J.; Staubach, C.; Ulrich, R.G. Detection of Francisella tularensis in three vole species in Central Europe. Transbound Emerg. Dis. 2019, 66, 1029–1032. [Google Scholar] [CrossRef]
- Rodríguez-Pastor, R.; Escudero, R.; Lambin, X.; Vidal, M.D.; Gil, H.; Jado, I.; Rodríguez-Vargas, M.; Luque-Larena, J.J.; Mougeot, F. Zoonotic pathogens in fluctuating common vole (Microtus arvalis) populations: Occurrence and dynamics. Parasitology 2019, 146, 389–398. [Google Scholar] [CrossRef]
- Tkadlec, E.; Václavík, T.; Široký, P. Rodent host abundance and climate variability as predictors of tickborne disease risk 1 year in advance. Emerg. Infect. Dis. 2019, 25, 1738–1741. [Google Scholar] [CrossRef]
- Mendenhall, V.M.; Pank, L.F. Secondary poisoning of owls by anticoagulant rodenticides. Wildl. Soc. Bull. 1980, 8, 311–315. [Google Scholar]
- Merson, M.H.; Byers, R.E. Residues of the rodenticide brodifacoum in voles and raptors after orchard treatment. J. Wildl. Manag. 1984, 48, 212–216. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Balát, F.; Folk, Č.; Grulich, I.; Havlín, J.; Holišová, V.; Hudec, K.; Pelikán, J.; Rosický, B.; Sýkora, J.; et al. Common vole (Microtus arvalis); CAV Edition: Prague, Czech Republic, 1959; p. 359, (In Czech with summary in German). [Google Scholar]
- Swanepoel, L.H.; Swanepoel, C.M.; Brown, P.R.; Eiseb, S.J.; Goodman, S.M.; Keit, M.; Kirsten, F.; Leir, H.; Mahlaba, T.A.M.; Makundil, H.; et al. Correction: A systematic review of rodent pest research in Afro-Malagasy small-holder farming systems: Are we asking the right questions? PLoS ONE 2017, 12, e0176621. [Google Scholar] [CrossRef] [PubMed]
- Farský, O. Příspěvek k řešení otázky hubení hraboše polního. (Arvicola arvalis). Sborník Výzkumných Ust. Zemědělských 1925, 8, 1–69. (In Czech) [Google Scholar]
- Rambousek, F. Hubení hrabošů v zimě. Ochr. Rostl. 1927, VII, 101–105. (In Czech) [Google Scholar]
- Straňák, F. Ochrana kulturních rostlin na jaře. Venkov 1916, XI, 4. (In Czech) [Google Scholar]
- Uher, F. Ničení hrabošů polních. Venkov 1911, VI, 4. (In Czech) [Google Scholar]
- Špatný, F. O polních myších. Živa 1858, 4, 59–62. (In Czech) [Google Scholar]
- Blattný, C. Škody způsobené myšmi a hraboši a jak jim čelit. Věstník České Akad. Zemědělské 1942, 18, 558–569. (In Czech) [Google Scholar]
- Durdik, F. Hubení hrabošů. Venkov 1920, XV, 12. (In Czech) [Google Scholar]
- Anonymous. Přehled dosud platných zákonů a nařízení v ochraně rostlin. Ochr. Rostl. 1947, XIX–XX, 10–11, 156–157. (In Czech) [Google Scholar]
- Jacob, J.; Tkadlec, E. Rodent Outbreaks in Europe: Dynamics and Damage. In Rodent Outbreaks—Ecology and Impacts, 1st ed.; Singleton, G.R., Belmain, S., Brown, P.R., Hardy, B., Eds.; International Rice Research Institute: Los Baños, Philippines, 2010; pp. 207–223. [Google Scholar]
- Suchomel, J.; Šipoš, J.; Čepelka, L.; Heroldová, M. The impact of Microtus arvalis and Lepus europaeus on apple trees by trunk bark gnawing. Plant. Prot. Sci. 2019, 55, 142–147. [Google Scholar] [CrossRef]
- Modlinger, R.; Čepelka, L.; Homolka, M. Současné možnosti ochrany kultur před ohryzem způsobeným hlodavci. Lesn. Práce 2015, 10, 44–45. (In Czech) [Google Scholar]
- Suchomel, J.; Šipoš, J.; Ouředníčková, J.; Skalský, M.; Heroldová, M. Bark gnawing by rodents in orchards during the growing season—Can we detect relation with forest damages? Agronomy 2022, 12, 251. Available online: https://www.mdpi.com/2073-4395/12/2/251 (accessed on 19 April 2022). [CrossRef]
- Suchomel, J.; Purchart, L.; Čepelka, L.; Heroldová, M. Factors influencing vole bark damage intensity in managed mountain-forest plantations of Central Europe. Eur. J. For. Res. 2016, 135, 331–342. [Google Scholar] [CrossRef]
- Suchomel, J.; Heroldová, M.; Šipoš, J. Contribution to the knowledge of the damage caused by common vole on sugar beet. Listy Cukrov. A Řepařské 2020, 136, 11–414, (In Czech with English abstract). [Google Scholar]
- Heroldová, M.; Michalko, R.; Suchomel, J.; Zejda, J. Influence of no-tillage versus tillage system on common vole (Microtus arvalis) population density. Pest. Manag. Sci. 2018, 74, 1346–1350. [Google Scholar] [CrossRef]
- Czech Statistical Office 2020. Available online: https://www.czso.cz/csu/czso/home (accessed on 11 April 2022). (In Czech).
- Heroldová, M.; Šipoš, J.; Suchomel, J.; Zejda, J. Interactions between common vole and winter rape. Pest. Manag. Sci. 2021, 77, 599–603. [Google Scholar] [CrossRef]
- Suchomel, J.; Šipoš, J.; Dokulilová, M.; Heroldová, M. Spill over of the common voles from rape fields to adjacent crops. Biologia 2021, 76, 1747–1752. [Google Scholar] [CrossRef]
- Prášil, F. Hraboš polní a jeho ničení. Venkov 1907, II, 190–191. (In Czech) [Google Scholar]
- Prášil, F. Hraboš polní a jeho ničení. Venkov 1907, II, 198–199. (In Czech) [Google Scholar]
- Anonymous. Vzrůstající nouze. Venkov 1910, V, 3. (In Czech) [Google Scholar]
- Bradáč, B. Řeč posl. Boh. Bradáče. Venkov 1911, VI, 3. (In Czech) [Google Scholar]
- Teichmann, K. O hraboši polním a zápasu hospodáře s ním. Venkov 1911, VI, 1–2. (In Czech) [Google Scholar]
- Horák, J. Píše Josef Horák, rolník ve Žluticích p. Vysoké Veselí. Venkov 1911, VI, 2. (In Czech) [Google Scholar]
- Kašpárek, T.; Senft, E. O prostředcích k hubení hrabošů. Časových Sp. 1911, 2, 444. (In Czech) [Google Scholar]
- Humpál, J. Ničte polního hraboše. Venkov 1911, VI, 3. (In Czech) [Google Scholar]
- Mráz, J. K boji s hraboši. Venkov 1912, VII, 2. (In Czech) [Google Scholar]
- Straňák, F. Srovnávací pokusy s některými prostředky a přístroji určenými k hubení hrabošů. Venkov 1915, X, 9–10. (In Czech) [Google Scholar]
- Straňák, F. Ochrana rostlin na podzim. Venkov 1916, XI, 1. (In Czech) [Google Scholar]
- Řepa, F. Myši, myš. Venkov 1916, XI, 2–3. (In Czech) [Google Scholar]
- Batlík, J. K hubení myší. Venkov 1917, XII, 3–4. (In Czech) [Google Scholar]
- Jičínský, F. Záplavy hrabošů v ozimech. Venkov 1919, XIV, 10–11. (In Czech) [Google Scholar]
- Farský, O. Hraboši. Ochrana rostlin 1923, III, 55–56. (In Czech) [Google Scholar]
- Anonymous. Proti myším záplavám. Venkov 1923, XVIII, 9–10. (In Czech) [Google Scholar]
- Anonymous. Sklizeň v našem státě ke dni 1. září 1925. Venkov 1925, XX, 7. (In Czech) [Google Scholar]
- Baudyš, E. Časové otázky z ochrany rostlin. Ceskoslov. Zemědělec Hospodářská Příloha Venk. 1927, IX, 428. (In Czech) [Google Scholar]
- Procházka, F.B. Hubení hrabošů. Ceskoslov. Zemědělec 1928, X, 571. (In Czech) [Google Scholar]
- Anonymous. Osení lepší než loni. Lid. Nov. 1930, 38, 9. (In Czech) [Google Scholar]
- Vojtěch, L. Příspěvek k hubení hrabošů. Ceskoslov. Zemědělec 1930, XII, 219. (In Czech) [Google Scholar]
- Sedláček, J. Zkušenosti s letošním jarním a loňským podzimním setím a ošetřováním obilí s ohledem k abnormálnímu počasí a výskytu škůdců. Ceskoslov. Zemědělec Příloha Venk. 1937, XIX, 1. (In Czech) [Google Scholar]
- Anonymous. Předejděme hrozícímu množení se myší. Venkov 1940, XXXV, 12. (In Czech) [Google Scholar]
- Blattný, C.; Novák, S.; Kac, A.; Ryžkov, N. Zpráva o škodlivých činitelích obilovin a řepy zelenin, pícnin a obchodních plodin, ovocných plodin a okrasných rostlin v Čechách ve vegetačním období 1938–1939. Ochr. Rostl. 1940, XVI, 5–6. (In Czech) [Google Scholar]
- Klúz, Z. Nestřílejte po dravcích. Venkov 1943, XXXVIII, 3. (In Czech) [Google Scholar]
- Farský, O. K otázce hubení hrabošů. Ochr. Rostl. 1925, V, 8–9. (In Czech) [Google Scholar]
- Daněk, F. Ochrana Proti Hraboši Polnímu v Polních Kulturách. Oborová Norma ON 46 6021; Ministerstvo zemědělství, lesního a vodního hospodářství: Praha, Czech Republic, 1963. (In Czech) [Google Scholar]
- Zacha, V. Prognóza a Signalizace v Ochraně Rostlin, 1st ed.; SZN: Praha, Czech Republic, 1966; p. 182. (In Czech) [Google Scholar]
- Tkadlec, E.; Zbořil, J.; Losík, J.; Gregor, P.; Lisická, L. Winter climate and plant productivity predict abundances of small herbivores in central Europe. Clim. Res. 2006, 32, 99–108. [Google Scholar] [CrossRef]
- Lisická, L.; Losík, J.; Zejda, J.; Heroldová, M.; Nesvadbová, J.; Tkadlec, E. Measurement error in burrow index to monitor relative population size in the common vole. Folia zool. 2007, 56, 169–176. [Google Scholar]
- Available online: http://eagri.cz/public/app/srs_pub/fytoportal/fy-public/?k=0#mon|modul:mapy|mapy:mapa (accessed on 30 March 2022).
- Regulation (EU) No 1306/2013 of the European Parliament and of the Council of 17 December 2013 on the Financing, Management and Monitoring of the Common Agricultural Policy and Repealing Council Regulations (EEC) No 352/78, (EC) No 165/94, (EC) No 2799/98, (EC) No 814/2000, (EC) No 1290/2005 and (EC) No 485/2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32013R1306 (accessed on 15 February 2022).
- Schlötelburg, A.; Plekat, A.; Bellingrath-Kimura, S.; Jacob, J. Self-service traps inspected by avian and terrestrial predators as a management option for rodents. Pest. Manag. Sci. 2020, 76, 103–110. [Google Scholar] [CrossRef]
- Jacob, J. Short-term effects of farming practices on populations of common voles. Agric. Ecosyst. Environ. 2003, 95, 321–325. [Google Scholar] [CrossRef]
- Rodrígez-Pastor, R.; Lugue-Larena, J.; Lambin, X.; Mougeot, F. “Living on the edge”: The role of field margins for common vole (Microtus arvalis) populations in recently colonised Mediterranean farmland. Agric. Ecosyst. Environ. 2016, 231, 206–217. [Google Scholar] [CrossRef]
- Tönisalu, G.; Väli, Ü. Edge effect in rodent populations at the border between agricultural landscapes and forests. Eur. J. Wildl. Res. 2022, 68, 34. [Google Scholar] [CrossRef]
- Bonnet, T.; Crespinc, L.; Pinot, A.; Bruneteaue, L.; Bretagnolle, V.; Gauffrea, B. How the common vole copes with modern farming: Insights from a capture–mark–recapture experiment. Agric. Ecosyst. Environ. 2013, 177, 21–27. [Google Scholar] [CrossRef]
- Jacob, J.; Hempel, N. Effects of farming practices on spatial behaviour of common voles. J. Ethol. 2003, 21, 45–50. [Google Scholar] [CrossRef]
- Tattersall, F.H.; Macdonald, D.W.; Hart, B.J.; Johnson, P.; Manley, W.J.; Feber, R.E. Is habitat linearity important for small mammal communities on farmland? J. Appl. Ecol. 2002, 39, 643–652. [Google Scholar] [CrossRef]
- Delattre, P.; Giradoux, P.; Baudry, J.; Musard, P.; Toussaint, M.; Trucheteff, D.; Stahl, P.; Poule, M.L.; Artois, M.; Damange, J.P.; et al. Land use patterns and types of common vole (Microtus arvalis) population kinetics. Agric. Ecosyst. Environ. 1992, 39, 53–169. [Google Scholar] [CrossRef]
- Labuschagne, L.; Swanepoel, L.H.; Taylor, P.J.; Belmain, S.R.; Keith, M. Are avian predators effective biological control agents for rodent pest management in agricultural systems? Biol. Control. 2016, 101, 94–102. [Google Scholar] [CrossRef]
- Paz, A.; Jareño, D.; Arroyo, L.; Viñuela, J.; Arroyo, B.; Mougeot, F.; Luque-Larena, J.J.; Fargallo, J.A. Avian predators as a biological control system of common vole (Microtus arvalis) populations in north-western Spain: Experimental set-up and preliminary results. Pest. Manag. Sci. 2013, 69, 444–450. [Google Scholar] [CrossRef]
- Garcia, K.; Olimpi, E.M.; Karp, D.S.; Gonthier, D.J. The good, the bad, and the risky: Can birds be incorporated as biological control agents into Integrated Pest Management programs? J. Integr. Pest. Manag. 2020, 11, 11. [Google Scholar] [CrossRef]
- Balát, F.; Folk, Č.; Havlín, J.; Hudec, K. Myšilovní ptáci hubící hraboše polního v Československu. In Common Vole (Microtus arvalis), 1st ed.; Kratochvíl, J., Balát, F., Eds.; CAV Edition: Prague, Czech Republic, 1959; pp. 250–274. (In Czech) [Google Scholar]
- Grulich, I. Myšilovní savci, hubící hraboše polního v Československu. In Common Vole (Microtus arvalis), 1st ed.; Kratochvíl, J., Balát, F., Eds.; CAV Edition: Prague, Czech Republic, 1959; pp. 275–279. (In Czech) [Google Scholar]
- Zejda, J.; Zapletal, M.; Pikula, J.; Obdržálková, D.; Heroldová, M.; Hubálek, Z. Hlodavci v Zemědělské a Lesnické Praxi, 1st ed.; Agrospoj s.r.o.: Praha, Czech Republic, 2002; p. 360. (In Czech) [Google Scholar]
- Machar, I.; Harmacek, J.; Vrublova, K.; Filippovova, J.; Brus, J. Biocontrol of common vole populations by avian predators versus rodenticide application. Pol. J. Ecol. 2017, 65, 434–444. [Google Scholar] [CrossRef]
- Grulich, I. Boj proti hraboši polnímu. In Common Vole (Microtus arvalis), 1st ed.; Kratochvíl, J., Balát, F., Eds.; CAV Edition: Prague, Czech Republic, 1959; pp. 285–316. (In Czech) [Google Scholar]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry Pest Control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Ramey, C.A.; Schafer, E.W., Jr. The evolution of APHIS two gas cartridges. Proc. Vertebr. Pest. Conf. 1996, 17, 219–224. [Google Scholar]
- Lemay, A.; Hall, T. The Use of Carbon Monoxide in Wildlife Damage Management. In Human Health and Ecological Risk Assessment for the Use of Wildlife Damage Management Methods by USDA-APHIS-Wildlife Services; Hall, T., Algeo, T., Green, M., Lemay, A., Wang-Cahill, F., Warren, J., Wimberly, R., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2017; pp. 1–41. [Google Scholar]
- Rödl, P.; Fraňková, M.; Aulický, R.; Stejskal, V. Možnosti regulace hraboše polního a dalších škodlivých hlodavců v cukrové řepě. Listy Cukrov. A Reparske 2020, 136, 9–12, (In Czech with English abstract). [Google Scholar]
- Kác, A. Hraboši v Zemědělství-Voles in Agriculture, 1st ed.; ZN-Praha: Praha, Czech Republic, 1948. (In Czech) [Google Scholar]
- Farský, O. Zpráva o chorobách a škůdcích lesa za rok 1920. Lesn. Práce 1924, 3, 1–2. (In Czech) [Google Scholar]
- Farský, O. Hubení myší strichninovým ovsem. Leták Fytopath. Sekce mor. Zem. Výzk. Úst. Zem. 1926, 5, 7–9. (In Czech) [Google Scholar]
- Traut, I.I.; Semenov, N.M. Opyty po bor´be s suslikami otravlennymi primankami. Zašč. Rast. 1927, 4, 14–15. [Google Scholar]
- Traut, I.I.; Semenov, N.M. Ješcë ob issledovanii voprosov primenenija otravlennych primanok v bor´be s suslikami. Tr. Nauč. Issl. Lab. Otravljajuščich veščestv 1928, 4, 8–10. [Google Scholar]
- Tkadlec, E. Response of voles to the concentration of crimidine in rodenticidal baits. Crop. Prot. 1994, 13, 474–478. [Google Scholar] [CrossRef]
- Brown, P.R.; Chambers, L.K.; Singleton, G.R. Pre-sowing control of house mice (Mus domesticus) using zinc phosphide: Efficacy and potential non-target effects. Wildl. Res. 2002, 29, 27–37. [Google Scholar] [CrossRef]
- Mutze, G.J.; Sinclair, R. Efficacy of zinc phosphide, strychnine and chlorpyrifos as rodenticides for the control of house mice in South Australian cereal crops. Wildl. Res. 2004, 31, 249–257. [Google Scholar] [CrossRef]
- Ambrož, A. O hubení hrabošů. Venkov 1911, VI, 2. (In Czech) [Google Scholar]
- Tyburetz, F.W. Prostředky pro hubení hlodavců I. SCILLA. Ochr. Rostl. 1942, XVIII, 78–86. (In Czech) [Google Scholar]
- Martelli, G. Contributo alla conoscenza della vita e dei costumi delle Arvicole in Puglia. Bolletino Del Lab. Di Zool. Gen. E Agrar. Portici 1919, 13, 193–316. [Google Scholar]
- Sachtleben, H. Rodentia-Nagetiere. In Handb. D. Pflanzenkrankheiten, 1st ed.; Appel, O., Reh, L., Eds.; Paul Parey Edition: Berlin, Germany, 1932; pp. 858–926. (In Germany) [Google Scholar]
- Kuzjakin, A.P.; Rezinko, D.S.; Makarov, N.I. Metody zaščity lesnach nasaždenij ot suslikov I drugich gryzunov. Rukopis 1952, 10, 14–19. [Google Scholar]
- Vaškov, V.J. Rukovodstvo po Desinfekcii, Desinsekcii i Deratizacii, 1st ed.; Medgiz: Moskva, SSSR, 1952; p. 654. [Google Scholar]
- Dvořák, K. Ochrana rolníkova proti myším. Letáky Čes. Odb. Zem. Rady Mor. 1930, 37, 5–6. (In Czech) [Google Scholar]
- Markovič, R. Boj proti mysiam. Oráč 1950, 49, 15–17. (In Slovak) [Google Scholar]
- Raška, K. Desinfekce, Desinsekce, Deratizace, 1st ed.; Státní zdravotnické nakladatelství: Praha, Czech Republic, 1953; p. 286. (In Czech) [Google Scholar]
- Zbirovský, M.; Myška, J. Insekticidy, Fungicidy, Rodenticidy, 1st ed.; Československá akademie věd: Praha, Czech Republic, 1957. (In Czech) [Google Scholar]
- Anonymous. Popis Deratizačních, Insekticidních, Ektoparasitárních i Antibiotických Přípravků. In Veterinární zprávy 1; Spolana a.s.: Prague, Czech Republic, 1955. (In Czech) [Google Scholar]
- Turček, F. Hlodavci v pôdohospodárstve. Oráč 1950, 49, 6–9. (In Slovak) [Google Scholar]
- Sviridenko, P.A. Myševidnyje gryzuny I zaščita ot nich urožaja zapasov produktov I drevesnych kuľtur. Izd. AN USSR 1953, 15, 30–31. [Google Scholar]
- Falkenstein, B.J.; Vinogradov, B.C. Myševidnyje gryzuny, vredjaščije pitomnikam I lesonasaždenijam I mery boŕby s nimi. AN SSSR 1952, 11, 24–26. [Google Scholar]
- Schindler, U. Eine neue wirksame Methode zur Bekämpfung der Erdmaus (Micr. Agrestis). Allg. Forstz. 1955, 10, 384–387. (In German) [Google Scholar]
- Schindler, U. Erdmaus-Bekämpfungsversuche im Gradationsjahr 1955. Z. Für Pflanzenkrankh. Pflanzenpathol. Und Pflanzenschutz 1956, 63, 694–704. (In German) [Google Scholar]
- Buckle, A.P.; Eason, C.T. Control Methods: Chemicals. In Rodent Pests and Their Control, 2nd ed.; Buckle, A.P., Smith, R.H., Eds.; CABI: Wallingford, UK, 2015. [Google Scholar]
- Jacob, J.; Buckle, A. Use of Anticoagulant Rodenticides in Different Applications around the World. In Anticoagulant Rodenticides and Wildlife; Springer: Cham, Switzerland, 2018; pp. 11–43. [Google Scholar]
- Kupper, J.; Grobosch, T.; Kistler, R.; Sydler, T.; Naegeli, H. Bromadiolone poisoning in foxes. Schweiz Arch. Tierheilkd 2006, 148, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Padilla, J.; López-Idiáquez, D.; López-Perea, J.J.; Mateo, R.; Paz, A.; Viñuela, J. A negative association between bromadiolone exposure and nestling body condition in common kestrels: Management implications for vole outbreaks. Pest. Manag. Sci. 2017, 73, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Jokić, G.; Vukša, P.; Vukša, M. Comparative efficacy of conventional and new rodenticides against Microtus arvalis (Pallas, 1778) in wheat and alfalfa crops. Crop. Prot. 2010, 29, 487–491. [Google Scholar] [CrossRef]
- Jokić, G.; Vukša, M.; Elezović, I.; Đedović, S.; Kataranovski, D. Application of grain baits to control common vole Microtus arvalis (Pallas, 1778) in alfalfa crops. Serbia Arch. Biol. Sci. 2012, 64, 629–637. [Google Scholar] [CrossRef]
- Jokić, G.; Blažić, T. Control of common vole (Microtus arvalis) in alfalfa crops using reduced content of anticoagulants. Agronomy 2022, 12, 53. [Google Scholar] [CrossRef]
- Mazánek, L.; Žerníčková, O.; Kenša, M. Large-Scale Intoxication of Black-Headed Gull (Chroicocephalus ridibundus) Chomutovske Lake in the Spring 2010. In Proceedings of the 12th Conference on Disinfection and Vector Control—2016 Přívorovy dny, Poděbrady, Czech Republic, 9–11 May 2016. (In Czech). [Google Scholar]
- Fraňková, M.; Stejskal, V.; Aulický, R. Suppression of food intake by house mouse (Mus musculus) following ingestion of brodifacoum-based rodenticide bait. Crop. Prot. 2017, 100, 134–137. [Google Scholar] [CrossRef]
- Frankova, M.; Stejskal, V.; Aulicky, R. Efficacy of rodenticide baits with decreased concentrations of brodifacoum: Validation of the impact of the new EU anticoagulant regulation. Sci. Rep. 2019, 9, 16779. [Google Scholar] [CrossRef] [PubMed]
- AO 20 45 01; Shaped Bait to Control the Numbers of Harmful Rodents. Institute for Inventions and Discoveries: Prague, Czech Republic, 1978. (In Czech)
- Grulich, I. Bait for Combating Exo-Anthropic and Hemi-Synanthropic Species of Mammals Using the Method of Surface Sowing. Patent Application PV 392-72, 21 January 1972. (In Czech). [Google Scholar]
- Rychnovský, B.; Janalík, B. Rozpad granulí Stutox® vlivem některých povětrnostních činitelů a následné změny v obsahu účinné látky. Agrochémia 1981, 21, 26–29. (In Czech) [Google Scholar]
- Novotný, I.; Rychnovský, B.; Janalík, B.; Wohlgemuth, E. Inovace nástrahového přípravku Stutox®. Vertebr. Zprávy 1984, 15, 75–89. (In Czech) [Google Scholar]
- Rychnovský, B.; Novotný, I.; Janalík, B. Zkušenosti a poznatky z používání nástrahy Stutox® proti hraboši polnímu. Agrochemia 1989, 29, 138–142. (In Czech) [Google Scholar]
- Hood, G.A. Zinc phosphide–a new look at an old rodenticide for field rodents. In Proceedings of the 5th Vertebrate Pest Conference, Fresno, CA, USA, 7–9 March 1972; 16. University of Nebraska: Lincoln, OR, USA, 1972; pp. 85–92. [Google Scholar]
- Johnson, G.D.; Fagerstone, K.A. Primary and Secondary Hazards of Zinc Phosphide to Nontarget Wildlife—A Review of the Literature; DWRC Research Report No. 11-55-005. U.S.; Department of Agriculture, Animal and Plant. Health Inspection Service: Washington, DC, USA, 1994; p. 26. [Google Scholar]
- Tkadlec, E.; Rychnovský, B. Residues of Zn3P2 in the common vole (Microtus arvalis) and secondary poisoning hazards to predators. Folia Zool. 1990, 39, 147–156. [Google Scholar]
- Zapletal, M.; Obdržálková, D. Hraboš polní stále aktuální nejen pro zemědělce. Agromanuál 2017, 12, 56–57. (In Czech) [Google Scholar]
- Beránek, J. Přemnožení hraboše polního v souvislostech. Fórum Ochr. Přírody 2020, 3, 29–32. (In Czech) [Google Scholar]
- Tkadlec, E.; Rychnovský, B. Optimum concentration of zinc phosphide in rodenticidal baits against the common vole Microtus arvalis. Folia Zool. 1990, 39, 227–236. [Google Scholar]
- EFSA Conclusion on the peer review of the pesticide risk assessment of the active substance zinc phosphide. EFSA J. 2010, 8, 1671. Available online: https://efsa.onlinelibrary.wiley.com/doi/pdf/10.2903/j.efsa.2010.1671 (accessed on 13 January 2022). [CrossRef][Green Version]
- Berny, P.; Esther, A.; Jacob, J.; Prescott, C. Risk Mitigation Measures for Anticoagulant Rodenticides as Biocidal Products; Report; European Union: Luxembourg, 2014; p. 104. [Google Scholar]
- MoA. National Action Plan for the Safe Use of Pesticides in the Czech Republic 2018–2022; Ministry of Agriculture: Prague, Czech Republic, 2018; p. 42. [Google Scholar]
- MoE. National Strategy for Dealing with Illegal Killings and Poisoning of Wild Animals in the Czech Republic 2020–2030; Ministry of the Environment: Prague, Czech Republic, 2019; p. 33. [Google Scholar]
- Vašák, S. Nejlacinější hubení hraboše. Hospodářský List. 1901, XXVI, 173. (In Czech) [Google Scholar]
- Beklova, M.; Krizkova, S.; Supalkova, V.; Mikelova, R.; Adam, V.; Pikula, J.; Kizek, R. Determination of bromadiolone in pheasants and foxes by differential pulse voltammetry. J. Environ. Anal. Chem. 2007, 87, 459–469. [Google Scholar] [CrossRef]
- Vidal, D.; Alzaga, V.; Luque-Larena, J.J.; Mateo, R.; Arroyo, L.; Viñuela, J. Possible interaction between a rodenticide treatment and a pathogen in common vole (Microtus arvalis) during a population peak. Sci. Total. Environ. 2009, 408, 267–271. [Google Scholar] [CrossRef]
- Olea, P.; Sánchez-Barbudo, I.; Viñuela, J.; Barja, I.; Mateo-Tomás, P.; Piñeiro, A.; Mateo, R.; Purroy, F. Lack of scientific evidence and precautionary principle in massive release of rodenticides threatens biodiversity: Old lessons need new reflections. Environ. Conserv. 2009, 36, 1–4. [Google Scholar] [CrossRef]
- Fraňková, M.; Aulický, R.; Radostná, T.; Stejskal, V. Rodenticides for common voles: An overview of formulations and the effect of bait composition on their acceptance by voles. DDD J. 2020, 29, 146–150, (In Czech with English abstract). [Google Scholar]
| Year | Toxic Mushy Mass and Pills (kg) | Strychnine-Prepared Grain (kg) |
|---|---|---|
| 1920 | 2100 | 18 |
| 1921 | 3430 | 302 |
| 1922 | 0 | 17 |
| 1923 | 5000 | 6127 |
| 1924 | 2500 | 2707 |
| Total | 13,030 | 9171 |
| Year | Reference |
|---|---|
| 1893 | Prášil [45] |
| 1901–1902 | Prášil [45,46] |
| 1910–1912 | Anonym [47]; Bradáč [48]; Teichmann [49]; Horák [50]; Uher [30]; Kašpárek and Senft [51]; Humpál [52]; Mráz [53] |
| 1915–1917 | Straňák [54]; Straňák [55]; Řepa [56]; Batlík [57] |
| 1919–1925 | Jičínský [58]; Farský [59]; Anonym [60]; Farský [27]; Anonym [61] |
| 1927–1930 | Baudyš [62]; F.B.P. [63]; Anonym [64]; L.V. [65] |
| 1936–1940 | Sedláček [66]; Anonym [67]; Blattný et al. [68] |
| 1943 | Klúz [69] |
| Season | Index of Reopened Burrow Entrances | |||
|---|---|---|---|---|
| Low | Medium | High | Extremely High | |
| Spring (March) | 0–5 | 6–25 | 26–50 | 51 and more |
| Summer (July) | 100–290 | 300–990 | 1000–5000 | 5100 and more |
| Autumn (September) | 500–990 | 1000–2900 | 3000–10,000 | 10,500 and more |
| Winter (January) | 100–290 | 300–590 | 600–1000 | 1050 and more |
| Season of the Year | Index of Reopened Burrow Entrances | |||
|---|---|---|---|---|
| Low | Medium | High | Extremely High | |
| Spring (March) | 1–40 | 41–100 | 101–200 | 201 and more |
| Summer (July) | 100–290 | 300–650 | 660–5000 | 5100 and more |
| Autumn (September) | 500–990 | 1000–2000 | 2100–10,000 | 10,500 and more |
| Winter (January) | 100–290 | 300–450 | 460–1000 | 1050 and more |
| Season | Index of Active Burrow Entrances | ||
|---|---|---|---|
| Low | Medium | High | |
| Spring | 10–40 | 50–200 | 210 and more |
| Summer | 10–200 | 210–600 | 610 and more |
| Autumn | 10–400 | 410–2000 | 2010 and more |
| Active Ingredient | Chemical Category | Mode of Action | Formulation | Product |
|---|---|---|---|---|
| camphechlor | organochlorine | acute | dust, liquid | Melipax Spritzmittel |
| endosulphan | organochlorine | acute | liquid | Thiodan 35 EC |
| disulfoton + thiometon and O,O-methylethyl-S-(2 ethylthioethyldithiophosphate) | organophosphate + organothiophosphate | acute | liquid | Developmental preparation |
| demeton | organophosphate | acute | liquid | Systox |
| thiometon | organothiophosphate | acute | liquid | Intration |
| dimefox | organophosphate | acute | liquid, grain | Terrasytam |
| disulfoton | organophosphate | acute | grain | Di Syston |
| zinc phosphide | anorganic | acute | grain, pellets | Niva zrna, M-Köder, Arrex E, Grazinn pellets, developmental preparation |
| calcium phosphide | anorganic | acute | pellets | Polytanol |
| aluminium phosphide | anorganic | acute | pellets | Delicia Gastoxin |
| crimidine | convulsant heterocyclic compound | acute | pellets | Castrix Pellets |
| scilliroside | botanical glycoside | acute | grain | Rascil |
| carbofuran | carbamate | acute | liquid | Furadan 75 WP |
| alpha-naphthyl thiourea (ANTU) | organic synthetic compound | acute | Dirax | |
| endrin | organochlorine | acute | liquid | Endrin 20 |
| chlorophacinone | anticoagulant | chronic | grain, pellets | Developmental preparation, Delicia pellets |
| chlorophacinone + scilliroside | anticoagulant + botanical glycoside | chronic | grain | Developmental preparation |
| warfarin | anticoagulant | chronic | pellets | Neratox |
| difenacoum | anticoagulant | chronic | pellets | Ratac |
| Preparation | Active Substance | End of Sale |
|---|---|---|
| Arvalin Forte | zinc phosphide | 30 April 2025 |
| Delicia Gastoxin | aluminium phosphide | 31 August 2023 |
| Polytanol | calcium phosphide | 2 March 2021 (end of sale) and 2 September 2022 (end of use) |
| Ratron GL | zinc phosphide | 30 April 2025 |
| Ratron GW | zinc phosphide | 30 April 2025 |
| Stutox II | zinc phosphide | 30 April 2025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aulicky, R.; Tkadlec, E.; Suchomel, J.; Frankova, M.; Heroldová, M.; Stejskal, V. Management of the Common Vole in the Czech Lands: Historical and Current Perspectives. Agronomy 2022, 12, 1629. https://doi.org/10.3390/agronomy12071629
Aulicky R, Tkadlec E, Suchomel J, Frankova M, Heroldová M, Stejskal V. Management of the Common Vole in the Czech Lands: Historical and Current Perspectives. Agronomy. 2022; 12(7):1629. https://doi.org/10.3390/agronomy12071629
Chicago/Turabian StyleAulicky, Radek, Emil Tkadlec, Josef Suchomel, Marcela Frankova, Marta Heroldová, and Vaclav Stejskal. 2022. "Management of the Common Vole in the Czech Lands: Historical and Current Perspectives" Agronomy 12, no. 7: 1629. https://doi.org/10.3390/agronomy12071629
APA StyleAulicky, R., Tkadlec, E., Suchomel, J., Frankova, M., Heroldová, M., & Stejskal, V. (2022). Management of the Common Vole in the Czech Lands: Historical and Current Perspectives. Agronomy, 12(7), 1629. https://doi.org/10.3390/agronomy12071629







