Abstract
The intensification of highly specialized viticulture has led to a dramatic decrease of soil fertility that can be restored by increasing soil organic matter using organic fertilizers. The aim of the present experiment was to evaluate the effect of different organic amendments on vine vegetative growth and nutritional status, soil N availability and microbial biomass, as well as on yield and grape quality. The experiment was carried out in 2020 and 2021, on cv. Sangiovese (Vitis vinifera L.) vines grafted on 110 Richter (V. berlandieri × V. rupestris) planted in February 2019. Plants were fertilized yearly in spring with (1) mineral fertilization (MIN), (2) municipal organic waste compost (MOW), and (3) sewage sludge compost (SS). The application of SS increased nitrate availability in both years, while the supply of organic matter (no matter the source) enhanced soil microbial biomass content. Plant nutritional status was in the optimal range for all treatments, with an increase of N in SS and K in MOW. Fruit yield in 2020 was not influenced by treatments, while in 2021 it was enhanced by MIN and MOW, which also induced a higher berry quality. Plant vegetative growth was stimulated by the application of SS. In conclusion, from these preliminary results we observed a higher N availability as a consequence of SS supply that resulted in a higher plant biomass, but reduced yield and berry quality, supporting the theory that for vineyards, N should be carefully managed to reach an equilibrium between vegetative and reproductive activity.
1. Introduction
The rise of the world population and urbanization is leading to increasing demand for food and production of urban organic waste. In the meantime, soils suffer from fertility degradation mainly due to the loss of soil organic carbon (SOC). Composting would recycle urban organic biowaste [1] into a valuable nutrient-rich end-product that could be used to sustainably improve soil fertility management [2,3]. During composting, organic wastes are biologically stabilized under controlled aerobic conditions to form a stable, humus-like final product [4], whose nutrient composition, organic carbon (C) content, and microbial activity could vary in relation to its sources [2,5].
As for all other fruit crops, viticulture is subjected to highly specialized and intensive production systems that generally exploit soil to its maximum productivity. This issue, associated with the inadequate use of organic fertilizer, is leading to significant decreases of soil organic matter in many Italian regions [6] with a loss of soil fertility that negatively impacts vineyard yield and grape quality [7].
As for woody plants [8,9], the effects of compost on grapevine growth, yield, berry composition [10,11], and on changes induced on soil chemical, biological, and physical properties [12,13] have been widely examined. Whereas inorganic fertilizers are promptly available for plants, organic amendments decompose gradually, mineralizing nutrients over time, and consequently supplying plants with elements during the whole growing season [14]. One of the main issues of compost supply is the difficulty of synchronizing nitrogen (N) release and plants’ needs during the vegetative season. This process depends on several factors, such as the compost C/N ratio, the concentration of organic and inorganic N in the amendments, the environmental condition, and the soil type. Among all, the C/N ratio is the most predictive index of the N mineralization [15,16]. According to the literature [15,16,17], organic amendments with a C/N ratio lower than 30 release mineral N, while substrates with a higher C/N ratio promote soil N immobilization [15].
Among nutrients, N plays a key role in plant nutrition. This is particularly true for grapevines since a correct N availability is fundamental for the balanced growth of shoots and the development of clusters [18]. Fertilization of non-bearing trees aims at building plant structure and preparing them to yield. In this phase, N is usually the most important nutrient [19,20]; however, young vineyards are sensitive to N excess that could lead to high vigor, dense canopy, large dark-green leaves, and an extended vegetative growth period [21]. Consequently, N surplus should be prevented to avoid excessive vegetative and unbalanced growth.
In the prestigious wine-growing districts of Italy and France, economic success depends more on fruit quality rather than on yield. Therefore, in these areas, the economic output of wine producers is mainly determined by the optimal balance between grape quality and yield. Sugars, acidity, polyphenols, anthocyanins, and N content in grapes [22,23] provide information on berry composition, and consequently, wine quality. It is well-known that grape composition, including aromas, is influenced by agronomic practices [24,25]. Therefore, the correct fertilization strategy plays a key role for achieving the desired vine performances [18,20]. Consequently, it is essential to accurately choose the correct organic matter source, which should be mature, correctly stabilized, and with a medium- to long-term N availability. Thus, the big challenge for a safe and sustainable use of organic matter is the choice of products with elevated quality standards able to satisfy soil and plant needs without exceeding the N availability.
Municipal organic waste (MOW) compost is probably the most used type of compost in Italy, and it derives from the combination of aerobic and anaerobic digestion of municipal solid and food industry-related wastes. It is usually characterized by an uncompacted structure and a C/N ratio lower than 20. An alternative to MOW could be sewage sludge (SS) compost, that derives from the combination of organic material from municipal and industrial waste combined with sludges deriving from water depuration. It usually has a higher C/N ratio than MOW compost and a dense structure due to its high moisture content.
The aim of the present experiment was to evaluate the effects of three fertilization strategies, admitted by the Integrated Crop Management (ICM) Guidelines of the Emilia-Romagna Region [26], including the use of mineral fertilizer and compost, on soil fertility (evaluated through soil N availability and microbial biomass activity), plant vegetative growth and nutritional status, yield, and grape quality. The results presented in this paper are from the first two years of cultivation of a long-term experiment that has the ambitious target to increase SOC, ameliorate wine quality, and alleviate the emission of greenhouse gases responsible for climate change.
2. Materials and Methods
2.1. Vineyard Description and Treatments
The trial was conducted at the Experimental Station of the University of Bologna, Italy (44°33′ N; 11°24′ E), on a clay-loam Cambisol soil [27]. The main soil properties are described in Table 1. The climate in the area is temperate with an average annual precipitation of 605 mm (in the period 2000–2022) and an average temperature of 14.4°C [28]. The average temperature was 14.3 °C both in 2020 and 2021, while the total precipitation was 512 mm in 2020 and 458 mm in 2021 [28].

Table 1.
Main soil characteristics of the vineyard at the beginning of the experiment.
The experiment was conducted from 2020 to 2021, on cv. Sangiovese (Vitis vinifera L.) vines (clone Tea 10D) grafted on 110 Richter (V. berladieri × V. rupestris) rootstock planted in February 2019. Vines were trained to vertical shoot-positioned spur-pruned cordons and spaced at 2.75 m between rows and 1 m within the row. The cordon was 0.80 m high and six two-bud spurs per vine were left with winter pruning. Shoots were trimmed twice during summer (June and August) only in 2021 at 1.2 m above the cordon. In 2020, the first year of cultivation summer shoot trimming was not performed due to reduced vegetative growth.
Since plantation, the following treatments were compared in a randomized block design with four replicates of five plants each:
- (1)
- Mineral fertilization (MIN): N supply was split into spring (half May) and summer (half June) and fertilization was performed with N, phosphorous (P), and potassium (K) fertilizer, and urea, respectively. Fertilizers were distributed manually on the soil at a total rate (spring + summer) of 40 and 60 kg N ha−1, in 2020 and 2021, respectively. The increased rate from 2020 to 2021 is a consequence of the increase of plants’ needs due to plant growth and is in line with the ICM Guidelines of the Emilia-Romagna region [26].
- (2)
- MOW compost (MOW): applied at a rate of 12.9 and 19.1 t DW ha−1 in 2020 and 2021, respectively.
- (3)
- SS compost (SS): applied at a rate of 18.6 and 11.1 t DW ha−1 in 2020 and 2021, respectively.
The rate of compost supplied was defined to provide the same quantity of available N, equal to 120 kg N ha−1 (according to the ICM Guidelines of the Emilia-Romagna region [26]), and it was counted considering the amended N concentration (Table 2), the mineralization coefficient (K2 = 0.3 and 0.4 for MOW and SS, respectively), and the state of hydration of the matrix (Table 2). The quantity of N applied with amendments is higher than mineral fertilizations since it considers the different N availability between mineral fertilization (prompt release) and compost (slow release).

Table 2.
Main chemical and physical characteristics of the amendments used in the experiment in 2020 and 2021.
The ICM Guidelines of the Emilia-Romagna region define the maximum quantity of nutrients that should be applied in vineyards each year according to soil analysis, age of the vineyard, and area characteristics [26]. In detail, since we were not in a nitrate-vulnerable zone, mineral fertilization admitted the following N, P, and K rates (in kg ha−1): 40 N (first year) and 60 N (second year), 15 P (first year) and 25 P (second year), and 20 K (first year) and 40 K (second year). Moreover, since the level of soil organic matter is considered low (Table 1), the quantity of N that could be supplied with organic material is maximum, 120 kg N ha−1 [26].
Before compost application, a 0.25 m-depth furrow was open under the vines in a 1.5 m-wide strip. Immediately after compost application, performed on 20 April 2020 and 17 June 2021, it was closed using a disk harrow.
The vineyard was regularly watered during the entire vegetative season with a drip irrigation system to return the daily evapotranspiration rate measured thanks to a class A evaporimeter positioned next to the vineyard. The soil was tilled superficially (0.25 m) in a 1.5 m-wide strip on the vine row, while alleys were covered with a mixture of Festuca rubra 36%, F. ovina 25%, Lolium perenne 35%, and Trifolium repens 4%, mowed 3 times a year. Soil tillage in the under-row was performed twice per year by an offset-type cultivator to control the growth of the spontaneous weed, and therefore no chemical herbicides were applied. Phytosanitary management was conducted according to the ICM Guidelines of the Emilia-Romagna region [26].
2.2. Soil Sampling and Analysis
To assess the effect of treatments on soil nitrate (NO3−)-N and ammonium (NH4+)-N, periodic sampling was performed from December 2019 to December 2021 at a depth of 0.10–0.40 m on the row, 50 cm from the plants. Two cores from each plot were sampled and mixed together. Nitrate-N and NH4+-N were extracted from 10 g of soil by a solution of 100 mL of KCl (2 M). Samples were shaken at 100 rpm for 1 h, and after soil sedimentation, a clear solution was collected and stored at −20 °C until colorimetric analysis [29], performed with an auto analyzer (Auto Analyzer AA3; Bran + Luebbe, Norderstadt, Germany).
Soil microbial biomass C was periodically measured using the substrate-induced respiration method [30] on soil samples collected at the depth of 0.05–0.15 m. Then, 50 g of fresh soil was sieved (diameter of 2 mm), placed in a 250 mL glass jar, and equilibrated at room temperature for at least 24 h. The samples were then mixed with 200 mg of glucose and incubated at 25 °C for 3 h. Carbon dioxide (CO2) evolution was measured by an infrared gas analyzer (EGM-4; PP system; Hitchin, UK), and CO2 concentration was converted into microbial C according to the following formula [30]:
Cmic = 40.04 × CO2 + 0.37
2.3. Leaf Sampling and Analysis
In July of 2020 and 2021, a representative sample of young and fully expanded leaves was collected from the apical part of shoots of the five vines of each plot. Chlorophyll content was measured with a SPAD-502 chlorophyll meter (Minolta Camera Co., Osaka, Japan). Leaves were then washed, oven-dried, weighed, milled, and analyzed for N, P, K, calcium (Ca), magnesium (Mg), sulfur (S), boron (B), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) concentrations. Briefly, N concentration was measured by the Kjeldahl method [31] by mineralizing 0.3 g of leaves with 10 mL of H2SO4 (95%), at 420 °C, for 180 min of distillation with 32% (v/v) NaOH and titration with 0.1 N H2SO4. Metal concentration was determined by a plasma spectrometer (ICP-OES; Ametek Spectro, Arcos, Kleve, Germany) on samples previously mineralized by US EPA Methods 3052 [32] by treating 0.3 g of leaves in an Ethos TC microwave lab station (Milestone, Bergamo, Italy) with 8 mL of HNO3 (65%) and 2 mL of H2O2 (30%), at 180 °C for 20 min. At leaf abscission (in 2020 and 2021), all plants from each plot were enclosed into a plastic net and abscised leaves were periodically collected to measure fresh and dry weight. A sample of leaves was then analyzed for macro- and micro-nutrients, as described above.
2.4. Berry Sampling and Analysis
At harvest in 2020 and 2021, all the clusters from the experimental plots were collected and weighed. Around 100 g of berries were crushed, and then sieved for total soluble solids and titratable acidity analysis. Total soluble solids (TSS) concentration was measured by a refractometer (Atago PR32), and titratable acidity (expressed as g L−1 of tartaric acid equivalents) was determined using a Micro TT 2022 automatic titrator (Crison, Barcelona, Spain) by titration with 0.1 N NaOH. A 30-berry sample was collected by cutting through the pedicel with scissors and frozen at −80 °C for anthocyanins and total polyphenols’ determination [33].
2.5. Vegetative Growth Determination
The effect of treatments on vine vegetative growth was determined thanks to the measurement of shoot weight removed with summer and winter pruning. In detail, summer pruning was performed in June and August 2021 by trimming shoots at 1.2 m above the cordon, and the total fresh weight of removed shoots was recorded. A representative sample was then dried to constant weight at 65 °C to determine the dry weight. In winter of 2020 and 2021, plants were pruned to leave six two-bud spurs per vine, and pruning dry weight was determined as described above.
2.6. Statistical Analysis
Data were statistically analyzed using the software SAS (SAS Institute Inc., Cary, NC, USA), in a factorial experimental design with amendment supply (3 levels: MIN, MOW, and SS) and sampling year (2 levels: 2020 and 2021) as main factors. When the analysis of variance (ANOVA) showed an effect of treatment that was statistically significant (p ≤ 0.05), means were separated by the Student–Newman–Keuls (SNK) test. Data of nitrate and ammonium soil concentration as well as microbial biomass C were analyzed according to the repeated measure model using proc mixed and compound symmetry as the covariance structure. The analysis evidenced a positive interaction between treatments and data (p ≤ 0.001, for all variables) and the same correlation for each set of sampling data. Consequently, a separate ANOVA was used to describe the effect of treatments at each sampling time.
Data were also analyzed, using the R statistical software (candisc package), according to the canonical discriminant analysis (CDA). CDA is a dimension-reduction technique related to principal component analysis and canonical correlation that determines the best way to separate or discriminate two or more groups of treatments, using quantitative measurements of different variables for each treatment [34].
3. Results
3.1. Soil Analysis
The application of SS increased the soil NO3−-N concentration in comparison to the other treatments both in 2020 (Figure 1A) and 2021 (Figure 1B). In detail, NO3−-N availability was enhanced by SS application from May 2020 until January 2021, with the only exception being August 2020, when no significant differences among treatments were observed (Figure 1A). In 2021, the trend was almost the same, with an increased availability as a consequence of amendment supply that was maintained until the end of the year (Figure 1B). In December 2019, February 2020, and February, March, April, and June 2021, no differences between treatments were observed (Figure 1A,B).
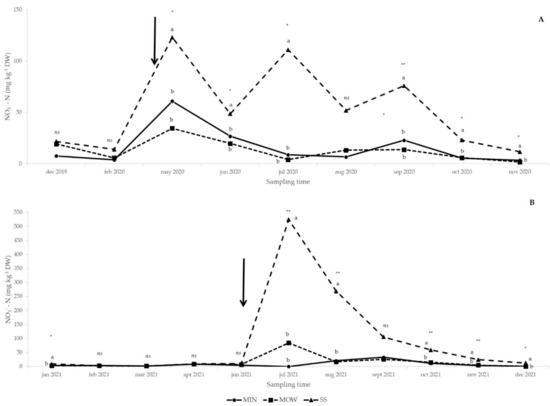
Figure 1.
Effect of fertilization treatments on soil nitrate-N (NO3−-N) concentration from December 2019 to December 2020 (A) and from January 2021 to December 2021 (B). Arrows: amendment applications (20 April 2020 and 17 June 2021). MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost. ns, *, **: effect not significant or significant at p ≤ 0.05 and p ≤ 0.01, respectively. Means followed by the same letter are not statistically different (p ≤ 0.05).
Ammonium-N availability was higher in SS than MIN in May 2020 and July 2021, and MOW showed intermediate values not different from SS and MIN (Figure 2A,B). In August 2021, the application of SS significantly increased the soil NH4+-N concentration in comparison to the other treatments, while from September 2021 MIN induced higher ammonium availability than the other treatments (Figure 2B). No significative differences were observed on other sampling dates (Figure 2A,B).
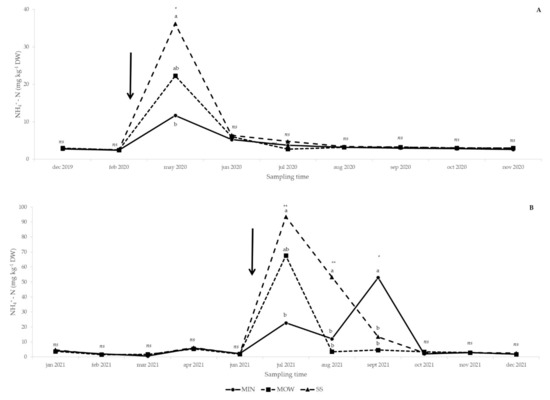
Figure 2.
Effect of fertilization treatments on soil ammonium-N (NH4+-N) concentration from December 2019 to December 2020 (A) and from January 2021 to December 2021 (B). Arrows: amendment applications (20 April 2020 and 17 June 2021). MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost. ns, *, **: effect not significant or significant at p ≤ 0.05 and p ≤ 0.01, respectively. Means followed by the same letter are not statistically different (p ≤ 0.05).
In July 2020 and October 2021, soil microbial C increased as a consequence of municipal organic waste addition in comparison to mineral fertilization and sewage sludge supply (Figure 3). In August and November 2020, both the organic amendments, no matter their source, increased microbial C in comparison to mineral fertilization, while in July 2021 the supply of SS induced a higher microbial C content than the other treatments (Figure 3). On all the other sampling dates, no significant differences between treatments were observed (Figure 3).
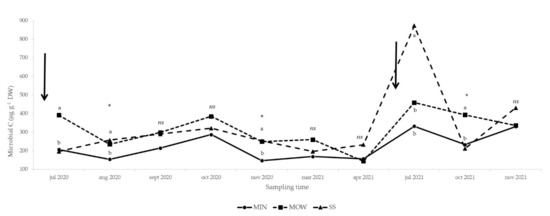
Figure 3.
Effect of fertilization treatments on soil microbial biomass C concentration from July 2020 to November 2021. Arrows: amendment applications (20 April 2020 and 17 June 2021). MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost. ns, *: effect not significant or significant at p ≤ 0.05, respectively. Means followed by the same letter are not statistically different (p ≤ 0.05).
3.2. Leaf Analysis
The interaction between fertilization treatment and year was not significantly different for leaf chlorophyll and macronutrient concentrations; consequently, in Table 3, only the effect of main factors are reported. Leaf N concentration was higher in SS than MOW, while MIN showed intermediate values, not different from the other treatments (Table 3). The highest leaf K concentration was measured in MOW, followed by SS and MIN (Table 3). No significant differences were observed for leaf chlorophyll and other nutrients (Table 3). The values of chlorophyll, N, K, Ca, and Mg were higher in 2020 than 2021, while the opposite was observed for P and S leaf concentrations (Table 3).

Table 3.
Effect of fertilization treatment and year on leaf chlorophyll (chl) measured as SPAD unit and macronutrient concentration (g 100 g−1 DW) in summer.
Fertilization treatment and year did not interact with leaves micronutrient concentrations that were not significantly influenced by the treatments. The average values were (in mg kg−1 DW): 74, 63, 71, 42, and 50 for B, Cu, Fe, Mn, and Zn, respectively.
The canonical discriminant analysis performed on leaf data showed that all the gradient of variability of this dataset was represented by the first canonical variable (Can1); consequently, the distribution of objects was represented only for this axis (Figure 4). The box plot separated the leaves sampled in autumn from those sampled in summer. Taking into consideration the structure of the data, we observed that N, K, P, S, B, and Zn were higher in summer than in autumn, and the opposite was observed for other nutrients (Figure 4).
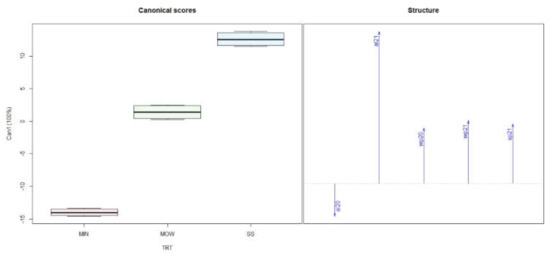
Figure 4.
Discriminant canonical analysis of nutrient concentration sampled in summer and autumn of 2020 and 2021. Since no significant difference between years was observed, data from both years were pooled together. Bar charts (left) of discriminant functions and arrows on the right side represent the direction of each parameter analyzed.
3.3. Yield and Berry Composition
Vine yield was not significantly influenced by the treatments in the first year of production (Figure 5). In 2021, MOW and MIN showed similar yields, higher than SS (Figure 5).
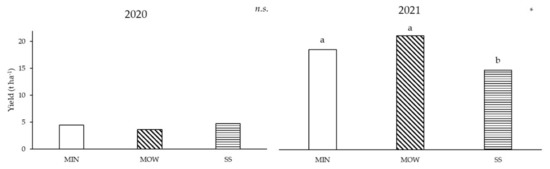
Figure 5.
Effect of fertilization treatment on yield in 2020 and 2021. MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost. ns, *: effect not significant or significant at p ≤ 0.05, respectively. Means followed by the same letter are not statistically different (p ≤ 0.05).
The discriminant canonical analysis of berry composition at harvest in 2020 and 2021 evidenced two main clusters: SS on the left side of the plot, and MOW and MIN on the right side of the plot, with a more evident separation among treatments in 2020 than in 2021 (Figure 6). In 2020, a clear separation in plot space was also evident among anthocyanins, acidity, and TSS that were positioned in the bottom-right side of the plot and flavonoids that were on the upper-right side (Figure 6). In 2021, the situation was different, with flavanols and TSS positioned on the bottom-right side of the plot, while anthocyanins and acidity were on the upper-right side (Figure 6).
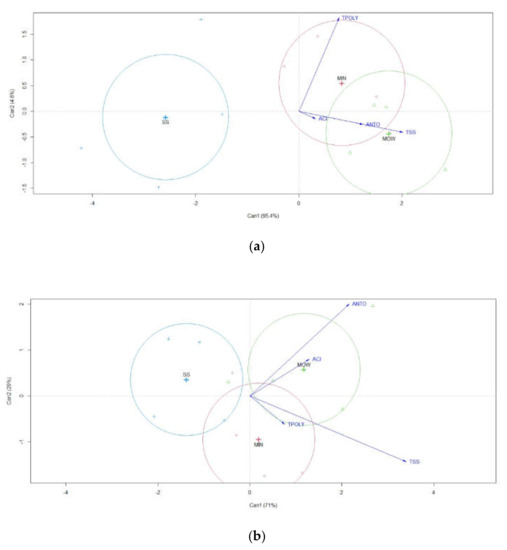
Figure 6.
Discriminant canonical analysis of berry composition in 2020 (a) and 2021 (b). MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost; TSS: total soluble solid; ACI: acidity; TPOLY: total polyphenols; ANTO: anthocyanins.
3.4. Vegetative Growth
The canonical discriminant analysis performed on vegetative parameters evidenced that all the gradients of variability were represented by the first canonical variable (Can1); consequently, the distribution of objects was represented only for this axis (Figure 7). The box plot separated the different treatments, with the lowest values for MIN, intermediate for MOW, and higher for SS (Figure 7). When analyzing the structure of the data, we observed that all parameters but abscised leaves in 2020 (al20) were higher as a consequence of amendment application, with a more evident effect for SS than MOW (Figure 7).
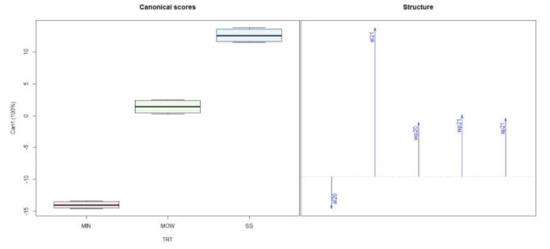
Figure 7.
Discriminant canonical analysis of biomass parameter in 2020 and 2021. MIN: mineral; MOW: municipal organic waste compost; SS: sewage sludge compost; al = abscised leaves; wp = winter pruning; sp = summer pruning.
In detail, winter pruning in 2020 accounted for (in g DW plant−1) 1165, 1261, and 1477 for MIN, MOW, and SS, respectively, while the values in 2021 were (in g DW plant−1): 1346 for mineral, 1435 for municipal organic waste compost, and 1346 for sewage sludge compost. The total dry biomass of summer pruning (2021) was (in g DW plant−1): 583 for MIN, 644 for MOW, and 1011 for SS.
4. Discussion
In the present manuscript, we refer to the non-bearing period of the vineyard, in which vines build their structures and reach a lower yield level than in the following years. In the first years after vine plantation, root development is limited, and nutrient uptake efficiency is low. Consequently, on the one hand, N is required to build up the plant skeleton as fast as possible, and on the other hand, excess N is easily achieved with an increase of the risk of pollution. For this reason, the ICM Guidelines of the Emilia-Romagna Region recommend only small amounts of N per hectare (40 and 60 kg ha−1 in the first and second years of cultivation, respectively), eventually localized near the root system by fertigation. The use of organic fertilizer has the advantage of increasing the supply of soil OM, and consequently the distribution rate is usually set with the aim to achieve this goal. The results on soil microbial activity (Figure 3) could hint at an OM increase since the values were higher as a consequence of organic amendments in comparison to mineral fertilization. It is well-known [35,36] that systems receiving high OM inputs have greater microbial activity compared to systems that receive only mineral fertilizers. The values of soil microbial C observed in the present experiment ranged between 150 and 800 μg C g−1 DW soil (Figure 3) and were slightly higher than those (40 and 500 μg C g−1 DW) reported by Sparling [37] in the description of the substrate-induced methods. In addition, the values recorded in this trial were also higher than those recorded (30 and 250 μg C g−1 DW) in a similar experiment conducted in a commercial peach orchard [38]. These differences could be due to different sources of the organic material, different soil properties, and the meteorological conditions. According to Leifel and co-authors [39], temperature and moisture are the main factors influencing biomass activity in soil. In detail, when temperatures are too high, and soil tends to dry, soil microbial activity slows down, and this behavior was evident in the first year (2020; Figure 3) when the values of microbial C decreased in August and increased in September and October. A different trend was observed in 2021 (Figure 3), when in July, we registered a peak of microbial C related to the application of organic amendments performed 20 days before soil sampling. In this case, microbial biomass was probably more affected by the supply of organic matter than by meteorological conditions.
Municipal organic waste and SS evidenced a different behavior, with a sharp increase after SS supply followed by a rapid decrease, while MOW induced a more gradual reduction, evidence of a slower mineralization rate. According to Insam and Domsch [40], microbial activity rose as a consequence of the increase of the soil organic C concentration. Sewage sludge showed a higher concentration of organic C than MOW (Table 2); consequently, we could assume that the soil treated with SS was richer in organic C concentration, thus enhancing microbial biomass.
In comparison to mineral fertilization, the application of N from organic sources presents the additional advantage of improving soil chemical and biological properties. However, the release of nutrients from organic matter must be controlled to avoid possible negative environmental impacts if the availability of N in soil is not synchronized with plant uptake. When organic matter mineralizes, ammonium is released and rapidly converted into nitrate, behavior clearly evidenced by the data of the present experiment, where the NH4+-N concentration significantly increased immediately after amendment application and then rapidly decreased (Figure 2). Nitrate soil concentration followed almost the same trend (Figure 1), but its concentration remained above 10 ppm during the entire vegetative season. In 2021, the peaks of NH4+-N and NO3−-N were higher than in 2020, probably because amendments were applied when the temperature was above 20 °C, thus stimulating a faster mineralization. The release of mineral N was higher as a consequence of SS than MOW supply, probably due to the higher moisture content of SS than MOW that has enhanced mineralization [41]. The big issue regarding the high NO3−-N concentration induced by SS in some periods is that the nitrate ion produced by the fast oxidation of ammonium is not withheld by the soil adsorption capacity, and consequently it could be lost by water percolation promoting ground water pollution [42,43]. Moreover, the excess of soil available N in SS plots induced an increase in vegetative growth, as evidenced by the higher biomass production recorded in summer and winter and the weight of abscised leaves in 2021 (Figure 7). As was also previously demonstrated [44,45], the excess of N stimulates plant vigor, leading to competition with grape due to an extended vegetative growth period [46]. Consequently, the higher N availability induced by SS impaired yield and grape quality that, in the second year, were higher as a consequence of MOW and MIN application than SS (Figure 5).
If N becomes a limiting factor, the C/N ratio will increase, and tree metabolism moves toward a higher synthesis of secondary metabolism compounds such as polyphenolic compounds or terpenoids [47]. If N is highly available, then vegetative growth will increase [48], and a decrease in plant secondary metabolite synthesis is expected. This is supported by experimental evidence on fruit trees, where the synthesis of ascorbic acid increased in response to low N availability [49]. Moreover, as a consequence of increased N availability, a decrease of the concentration of phenolic antioxidants [49] and a contemporary increase of proteins [48] were observed. Similarly, excess soil N promotes the vine vigor, and the following increase of bunch shading may reduce anthocyanins and tannins concentrations [50,51,52,53], confirming our results. In addition, the increased vine vigor can reduce grape quality [54] due to the detrimental effect on the expression of the genes involved in the flavonoid pathway [55,56].
Nutrient leaf concentrations were only slightly influenced by fertilization treatments (Table 3) and were in line with the average standard for this variety [57], showing that all the fertilization strategies were able to induce an optimal nutritional status. The nutritive elements contained in compost are released slowly [58], and consequently contribute to the enhancement of plant performances, as previously evidenced [57,59]. Even if not influenced by the treatments, it is interesting to focus on seasonal changes of nutrient leaf concentrations. The importance of stored N to support shoot growth in the following spring is widely recognized [58]. However, other nutrients could be equally important for the next season’s growth, although the different mobility of each nutrient determines the accumulation and mobilization from the perennial structure. Usually, K and Ca are present, in storage organs, in similar concentration ranges to N, while P and Mg are present in smaller amounts [60,61]. In detail, we observed, beside N, a remobilization from abscised leaves of K, P, and S (Table S1). Similar results were previously obtained for K, P, and S in grapevine [60]. On the other hand, our results showed that Ca and Mg accumulated in senescent leaves sampled in autumn (Table S1), evidencing a substantial autoregulation of vines to remove of the excess nutrient and potentially harmful for cells during dormancy [62,63,64,65,66]. This behavior was reported for Ca and Mg, whose poor redistribution into the perennial structure during senescence as a consequence of low mobility was previously studied [67].
The concentrations of Cu, Fe, and Mn in abscised leaves were (in mg kg−1 ss): 158, 155, and 77, respectively, evidencing a clear accumulation at the end of the season. These nutrients are less studied than macronutrients, so little information is available for Cu, Fe, and Mn behavior. An investigation on several species, including V. vinifera [68], evidenced an increase of Fe, Mn, and Cu content in leaves from spring to autumn. Nevertheless, in the same research, an increase of B and Zn concentrations during the vegetative season was also observed, in contrast to our results (74 vs. 52 mg B kg−1, in summer and autumn respectively, and 50 vs. 34 mg Zn kg−1, in summer and autumn, respectively), but adding evidence to the ability of vines to maintain the concentration of nutrients during dormancy. This different behavior could also be due to the different environmental condition, soil nutrient availability, rootstock uptake efficiency, variety accumulation ability, etc.; however, further research should be carried out to shed light on nutrient behavior in grape plants to optimize nutrient supply.
5. Conclusions
Grapevine is one of the most economically valuable crops worldwide and, since viticulture is evolving towards sustainable production systems, the use of compost could be a win–win solution able to improve soil fertility while recycling wastes. The results obtained in this study demonstrated that the application of compost in a vineyard can have beneficial effects on soil fertility, but that if mineralization is too fast, it could lead to an excess of N in soil. If, on the one hand, the increased N availability stimulated plant growth, being a positive feature for building vine structure, on the other it reduced the yield and grape quality. These preliminary results evidenced that the chemical composition of compost (moisture, nutrient concentration, etc.) must be accurately defined to achieve a balanced canopy/grape growth and a satisfactory berry quality. Our study confirms that MOW compost is the amendment that better combines the improvement of soil biological properties with gradual and constant N availability (comparable to mineral fertilizers), inducing the optimal balance between vegetative and reproductive activity. A possible strategy that would overcome the risk of excess nitrate in soil could be the split supply in spring to sustain vegetative growth and yield and in autumn to improve soil properties and enhance the restoration of reserves.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12071604/s1, Table S1: Effect of fertilization treatment and year on macronutrient concentration in autumn.
Author Contributions
Conceptualization, M.T., I.F., C.M. and E.B.; methodology, M.T., M.Q. and E.B.; validation, E.B., M.T., C.M. and I.F.; formal analysis, G.P. and E.B.; investigation, G.P., M.G., G.N.L., G.A., C.P., M.M. and M.Q.; data curation, E.B. and G.P.; writing—original draft preparation, E.B.; writing—review and editing, E.B., M.T. and M.M.; visualization, G.A. and I.F.; supervision, M.T. and I.F.; project administration, C.C., C.M. and M.T.; funding acquisition, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
HERAMBIENTE & PARTNERS: Herambiente SpA; AIMAG; Sogliano ambiente SpA; Salerno Pietro.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cerda, A.; Artola, A.; Font, X.; Barrena, R.; Gea, T.; Sánchez, A. Composting of food wastes: Status and challenges. Bioresour. Technol. 2018, 248, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Olanrewaju, O.S.; Babalola, O.O.; Odeyemi, O. Waste management through composting: Challenges and potentials. Sustainability 2020, 12, 4456. [Google Scholar] [CrossRef]
- Tondello, A.; Fasolo, A.; Marcato, S.; Treu, L.; Bonato, T.; Zanardi, W.; Concheri, G.; Squartini, S.; Baldan, B. Characterization of bacterial communities isolated from municipal waste compost and screening of their plant-interactive phenotypes. Sci. Total Environ. 2022, 806, 150592. [Google Scholar] [CrossRef] [PubMed]
- López-González, J.A.; Suárez-Estrella, F.; Vargas-García, M.C.; López, M.J.; Jurado, M.M.; Moreno, J. Dynamics of bacterial microbiota during lignocellulosic waste composting: Studies upon its structure, functionality and biodiversity. Bioresour. Technol. 2015, 175, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.A.; Dazzi, C. The Soils of Italy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- White, R.E.; Balachandra, L.; Edis, R.; Chen, D. The soil component of terroir. OENO One 2007, 41, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Carey, P.L.; Benge, J.R.; Haynes, R.J. Comparison of soil quality and nutrient budgets between organic and conventional kiwifruit orchards. Agric. Ecosyst. Environ. 2009, 132, 7–15. [Google Scholar] [CrossRef]
- Baldi, E.; Marcolini, G.; Quartieri, M.; Sorrenti, G.; Muzzi, E.; Toselli, M. Organic fertilization in nectarine (Prunus persica var. nucipersica) orchard combines nutrient management and pollution impact. Nutr. Cycl. Agroecosyst. 2016, 105, 39–50. [Google Scholar] [CrossRef]
- Morlat, R.; Chaussod, R. Long-term additions of organic amendments in a Loire Valley vineyard. I. Effects on properties of a calcareous sandy soil. Am. J. Enol. Vitic. 2008, 59, 353–363. [Google Scholar]
- Morlat, R.; Symoneaux, R. Long-term additions of organic amendments in a Loire Valley vineyard on a calcareous sandy soil. III. Effects on fruit composition and chemical and sensory characteristics of Cabernet franc wine. Am. J. Enol. Vitic. 2008, 59, 375–386. [Google Scholar]
- Nendel, C.; Reuter, S. Soil biology and nitrogen dynamics of vineyard soils as affected by a mature biowaste compost application. Compost Sci. Util. 2007, 15, 70–77. [Google Scholar] [CrossRef]
- Gaiotti, F.; Marcuzzo, P.; Belfiore, N.; Lovat, L.; Fornasier, F.; Tomasi, D. Influence of compost addition on soil properties, root growth and vine performances of Vitis vinifera cv Cabernet sauvignon. Sci. Hort. 2017, 225, 88–95. [Google Scholar] [CrossRef]
- Ambus, P.; Kure, L.K.; Jensen, E.S. Gross N transformation after application of household compost and domestic sewage sludge on agricultural soils. Agronomie 2002, 22, 723–730. [Google Scholar] [CrossRef]
- Mohanty, M.; Reddy, K.S.; Probert, M.E.; Dalal, R.C.; Rao, A.S.; Menzies, N.W. Modelling N mineralization from green manure and farmyard manure from a laboratory incubation study. Ecol. Model. 2011, 222, 719–726. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen mineralization dynamics of different valuable organic amendments commonly used in agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Gioacchini, P.; Montecchio, D.; Gnudi, E.; Terzi, V.; Stanca, A.M.; Ciavatta, C.; Marzadori, C. Fate of N in soil amended with 15N-labelled residues of winter cereals combined with an organic N fertiliser. Soil Res. 2016, 54, 182–190. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Austr. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Vega, A.; Bouguyon, E.; Krouk, G.; Gojon, A.; Coruzzi, G.; Gutiérrez, R.A. Nitrate transport, sensing, and responses in plants. Mol. Plant 2016, 9, 837–856. [Google Scholar] [CrossRef] [Green Version]
- Verdenal, T.; Dienes-Nagy, Á.; Spangenberg, J.E.; Zufferey, V.; Spring, J.L.; Viret, O.; Carbonne, J.M.; van Leeuwen, C. Understanding and managing nitrogen nutrition in grapevine: A review. OENO One 2021, 55, 1–43. [Google Scholar] [CrossRef]
- Alem, H.; Rigou, P.; Schneider, R.; Ojeda, H.; Torregrosa, L. Impact of agronomic practices on grape aroma composition: A review. J. Sci. Food Agric. 2019, 99, 975–985. [Google Scholar] [CrossRef]
- Cordovil, C.D.S.; Coutinho, J.; Goss, M.; Cabral, F. Potentially mineralizable nitrogen from organic materials applied to a sandy soil: Fitting the one-pool exponential model. Soil Use Manag. 2005, 21, 65–72. [Google Scholar] [CrossRef]
- Reeve, J.R.; Carpenter-Boggs, L.; Reganold, J.P.; York, A.L.; McGourty, G.; McCloskey, L.P. Soil and wine grape quality in biodynamically and organically managed vineyards. Am. J. Enol. Vitic. 2005, 56, 367–376. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Möller, K. Soil fertility status and nutrient input–output flows of specialized organic cropping systems: A review. Nutr. Cycl. Agroecosyst. 2018, 112, 147–164. [Google Scholar] [CrossRef]
- Emilia-Romagna PDO and PGI Products: Created Here, Enjoyed Worldwide. Available online: https://www.regione.emilia-romagna.it/en/agriculture-and-food (accessed on 18 March 2022).
- WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports 106. FAO, Rome. Available online: http://www.fao.org/soils-portal/soil-survey/soil-classification/world-reference-base/en/ (accessed on 18 March 2022).
- Weather Forecast. Data and Observations. Available online: https://www.arpae.it/it/temi-ambientali/meteo/dati-e-osservazioni (accessed on 18 March 2022).
- Bran+Luebbe Auto Analyzer III Applications and Operation Manual, November 1998, Germany. Method No. G-109-94 Rev. 1 (multitest MT7/MT8) for NO2/NO3-N analysis and Method No. G-102-93 Rev. 1 (Multitest MT7/MT8) for NH4-N Analysis.
- Anderson, J.; Domsch, K. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Schumann, G.E.; Stanley, M.A.; Knudsen, D. Automated total nitrogen analysis of soil and plant samples. Soil Sci. Soc. Am. J. 1973, 37, 480–481. [Google Scholar] [CrossRef]
- Kingston, H.M. Microwave Assisted Acid Digestion of Siliceous and Organically-Based Matrices, Method 3052 1988 U.S. Environmental Protection Agency IAG DWI-393254-01-0, Quarterly Report, 1 January–31 March. Available online: https://www.scienceopen.com/document?vid=c835d7c2-a13d-4634-812e-58fa2a6853cd (accessed on 18 March 2022).
- Di Stefano, R.; Mattivi, F.; Carburazzi, M.; Giustini, E.; Bonifazi, L. Evoluzione della composizione fenolica dell’uva Sagrantino durante la maturazione. Riv. Vitic. Enol. 2008, 1, 39–61. [Google Scholar]
- Cruz-Castillo, J.G.; Ganeshanandam, S.; MacKay, B.R.; Lawes, G.S.; Lawoko, C.R.O.; Woolley, D.J. Applications of canonical discriminant analysis in horticultural research. HortScience 1994, 29, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Laudicina, V.A.; Badalucco, L.; Palazzolo, E. Effects of compost input and tillage intensity on soil microbial biomass and activity under Mediterranean conditions. Biol. Fert. Soil. 2011, 47, 63–70. [Google Scholar] [CrossRef]
- Nemet, F.; Perić, K.; Lončarić, Z. Microbiological activities in the composting process: A review. Columella J. Agric. Environ. Sci. 2021, 8, 41–53. [Google Scholar] [CrossRef]
- Sparling, G. The substrate induced respiration method. In Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; pp. 397–404. [Google Scholar]
- Baldi, E.; Toselli, M.; Marcolini, G.; Quartieri, M.; Cirillo, E.; Innocenti, A.; Marangoni, B. Compost can successfully replace mineral fertilizers in the nutrient management of commercial peach orchard. Soil Use Manag. 2010, 26, 346–353. [Google Scholar] [CrossRef]
- Leifeld, J.; Siebert, S.; Kogel-Knabner, I. Changes in the chemical composition of soil organic matter after application of compost. Europ. J. Soil Sci. 2002, 53, 299–309. [Google Scholar] [CrossRef]
- Insam, H.; Domsch, K.H. Relationship between soil organic carbon and microbial biomass on chrono sequences of reclamation sites. Microb. Ecol. 1988, 15, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Murwira, H.K.; Kirchmann, H.; Swift, M.J. The effect of moisture on the decomposition rate of cattle manure. Plant Soil 1990, 122, 197–199. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching in temperate agroecosystems: Sources, factors and mitigating strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Forster, S.S.D.; Cripps, A.C.; Smith-Carington, A. Nitrate leaching to groundwater. Philosophical Transactions of the Royal Society of London. Biol. Sci. 1982, 296, 477–489. [Google Scholar]
- Holzapfel, B.P.; Treeby, M.T. Effects of timing and rate of N supply on leaf nitrogen status, grape yield and juice composition from Shiraz grapevines grafted to one of three different rootstocks. Austr. J. Grape Wine Res. 2007, 13, 14–22. [Google Scholar] [CrossRef]
- Gatti, M.; Squeri, C.; Garavani, A.; Vercesi, A.; Dosso, P.; Diti, I.; Poni, S. Effects of variable rate nitrogen application on cv. Barbera performance: Vegetative growth and leaf nutritional status. Am. J. Enol. Vitic. 2018, 69, 196–209. [Google Scholar] [CrossRef]
- Thomidis, T.; Zioziou, E.; Koundouras, S.; Karagiannidis, C.; Navrozidis, I.; Nikolaou, N. Effects of nitrogen and irrigation on the quality of grapes and the susceptibility to Botrytis bunch rot. Sci. Hort. 2016, 212, 60–68. [Google Scholar] [CrossRef]
- Winter, C.K.; Davis, S.F. Organic food. J. Food Sci. 2006, 71, 117–124. [Google Scholar] [CrossRef]
- Brandt, K.; Mølgaard, J.P. Organic agriculture: Does it enhance or reduce the nutritional value of plant foods? J. Sci. Food Agri. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Mitchell, A.E.; Chassy, A.W. Antioxidants and the nutritional quality of organic agriculture The Mitchell Lab–Phytochemicals & Health–Beyond Antioxidants. Ann. Meet. Am. Advancem. Sci. Chic. 2009, 12–16. [Google Scholar]
- Duchene, E.; Schneider, C.; Gaudillere, J.P. Effects of nitrogen nutrition timing on fruit set of grapevine, cv. Grenache. Vitis-Geilwelerhof 2011, 40, 45–46. [Google Scholar]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Portu, J.; Moreno-Simunovic, Y.; Martínez-Gil, A.M. Foliar nitrogen application in Cabernet Sauvignon vines: Effects on wine flavonoid and amino acid content. Food Res. Intern. 2017, 96, 46–53. [Google Scholar] [CrossRef]
- Hilbert, G.; Soyer, J.-P.; Giraudon, J.; Milin, S.; Gaudillère, J.P. Effects of nitrogen supply on must quality and anthocyanin accumulation in berries of cv. Merlot. Vitis 2003, 42, 69–76. [Google Scholar]
- Schreiner, R.P.; Osborne, J.; Skinkis, P.A. Nitrogen requirements of Pinot noir based on growth parameters, must composition, and fermentation behavior. Am. J. Enol. Vitic. 2018, 69, 45–58. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of crop level and crop load on growth, yield, must and wine composition, and quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 125–131. [Google Scholar]
- Dai, Z.W.; Ollat, N.; Gomès, E.; Decroocq, S.; Tandonnet, J.P.; Bordenave, L.; Pieri, P.; Hilbert, G.; Kappel, C.; van Leeuwen, C.; et al. Ecophysiological, genetic, and molecular causes of variation in grape berry weight and composition: A review. Am. J. Enol. Vitic. 2011, 62, 413–425. [Google Scholar] [CrossRef] [Green Version]
- Soubeyrand, E.; Colombié, S.; Beauvoit, B.; Dai, Z.; Cluzet, S.; Hilbert, G.; Christel, R.; Christel, R.; Maneta-Peyret, L.; Dieuaide-Noubhani, M.; et al. Constraint-based modeling highlights cell energy, redox status and α-ketoglutarate availability as metabolic drivers for anthocyanin accumulation in grape cells under nitrogen limitation. Front. Plant Sci. 2018, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, J.I.; Rombolà, A.D. Nutrition of grapes in relation to cultivation techniques. Inf. Agrar. 2012, 68, 56–58. [Google Scholar]
- Sikora, J.L. Effect of compost-fertilizer blends on crop growth. In The Science of Composting; Bertoldi, M., Sequi, P., Lammers, B., Papi, T., Eds.; Blackie Academic & Professional Publications: Glasgow, UK, 1995; pp. 423–430. [Google Scholar]
- Pinamonti, F. Compost mulch effects on soil fertility, nutritional status and performance of grapevine. Nutr. Cycl. Agroecosyst. 1998, 51, 239–248. [Google Scholar] [CrossRef]
- Holzapfel, B.P.; Smith, J.P.; Field, S.K. Seasonal vine nutrient dynamics and distribution of Shiraz grapevines. OENO One 2019, 53, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, R.P.; Scagel, C.F.; Baham, J. Nutrient uptake and distribution in a mature ‘Pinot Noir’ vineyard. HortScience 2006, 41, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Pradubsuk, S.; Davenport, J.R. Seasonal Uptake and Partitioning of Macronutrients in Mature ‘Concord’ Grape. J. Am. Soc. Hort. Sci. 2012, 135, 474–483. [Google Scholar] [CrossRef] [Green Version]
- Briat, J.F.; Gojon, A.; Plassard, C.; Rouached, H.; Lemaire, G. Reappraisal of the central role of soil nutrient availability in nutrient management in light of recent advances in plant nutrition at crop and molecular levels. Europ. J. Agron. 2020, 116, 126069. [Google Scholar] [CrossRef]
- Forde, B.G. The role of long-distance signalling in plant responses to nitrate and other nutrients. J. Exp. Bot. 2002, 53, 39–43. [Google Scholar]
- Touraine, B.; Clarkson, D.T.; Muller, B. Regulation of nitrate uptake at the whole plant level. In A Whole Plant Perspective on Carbon-Nitrogen Interactions; SPB Academic Publishing: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Imsande, J.; Touraine, B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994, 105, 3. [Google Scholar] [CrossRef] [Green Version]
- Conradie, W.J. Seasonal uptake of nutrients by Chenin blanc in sand culture: I. Phosphorus, potassium, calcium and magnesium. S. Afr. J. Enol. Vitic. 1981, 2, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Bäßler, R.; Zou, C.; Römheld, V. Is iron phloem mobile during senescence in trees? A reinvestigation of Rissmüller’s finding of 1874. Plant Physiol. Biochem. 2011, 49, 489–493. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).