Row Spacing and the Use of Plant-Available Water in Sugarcane Cultivation in Water-Abundant Louisiana
Abstract
1. Introduction
2. Materials and Methods
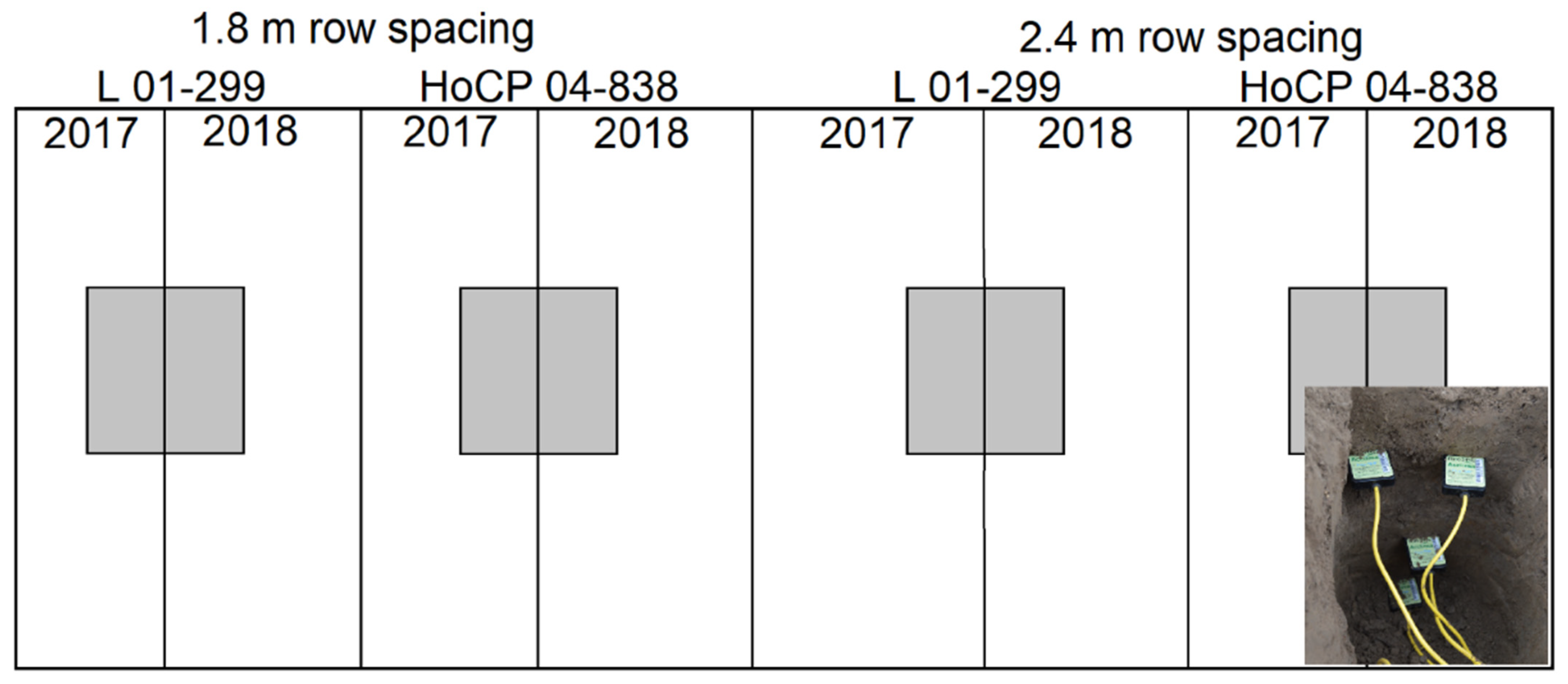
2.1. Site Description
2.2. Volumetric Water Content and the Determination of Plant-Available Water (PAW)
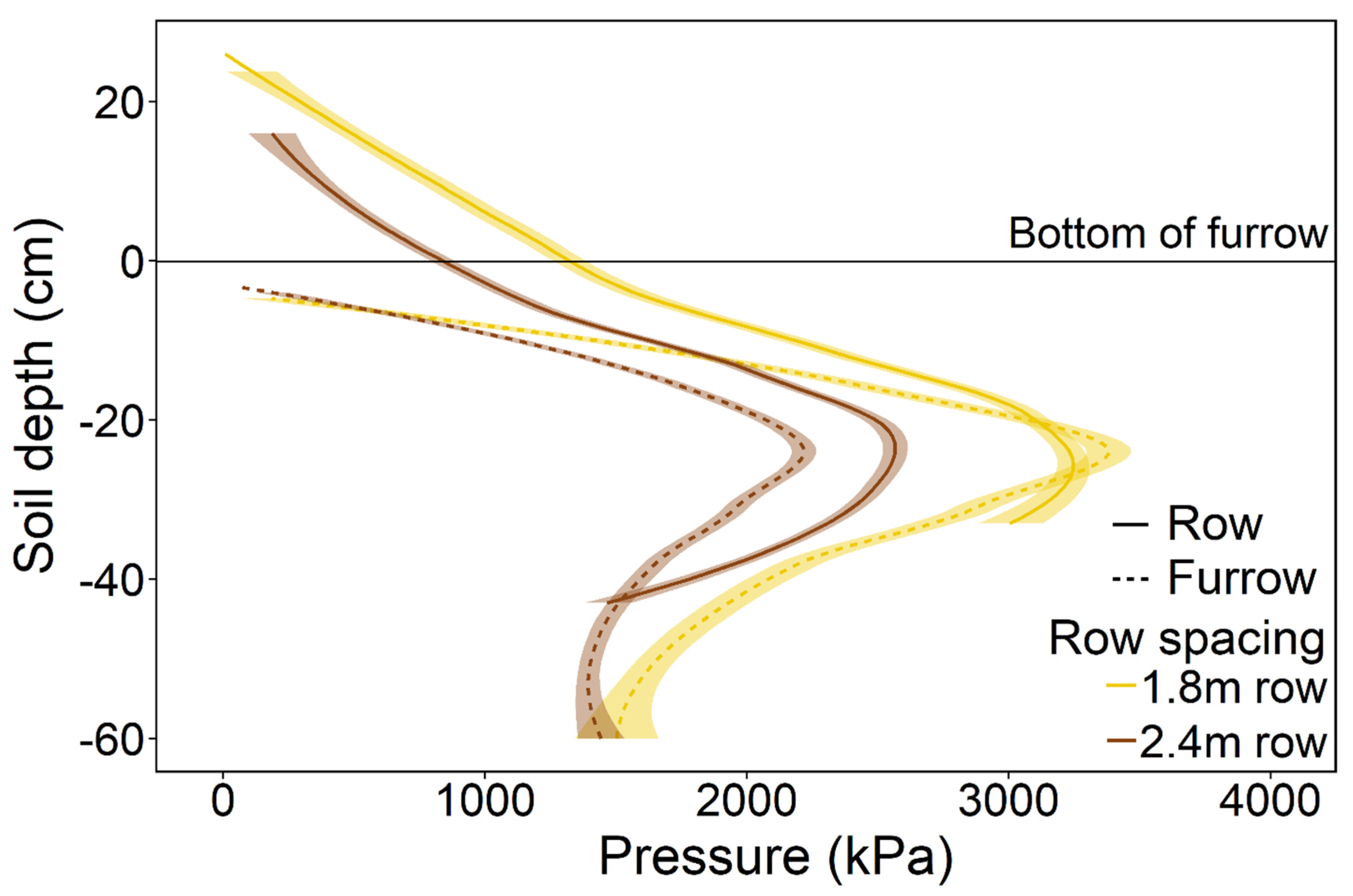
2.3. Soil Compaction
2.4. Statistical Analysis
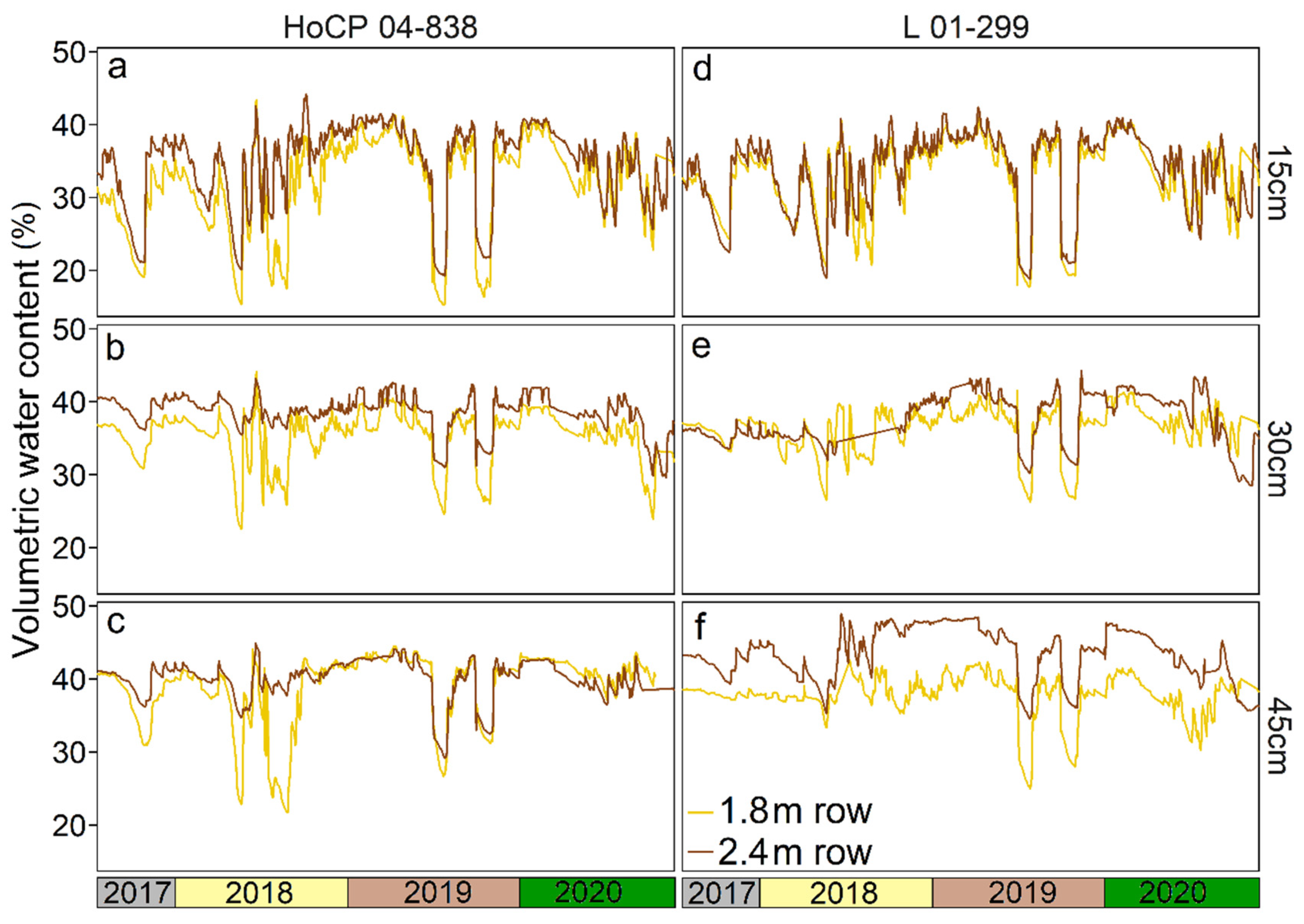
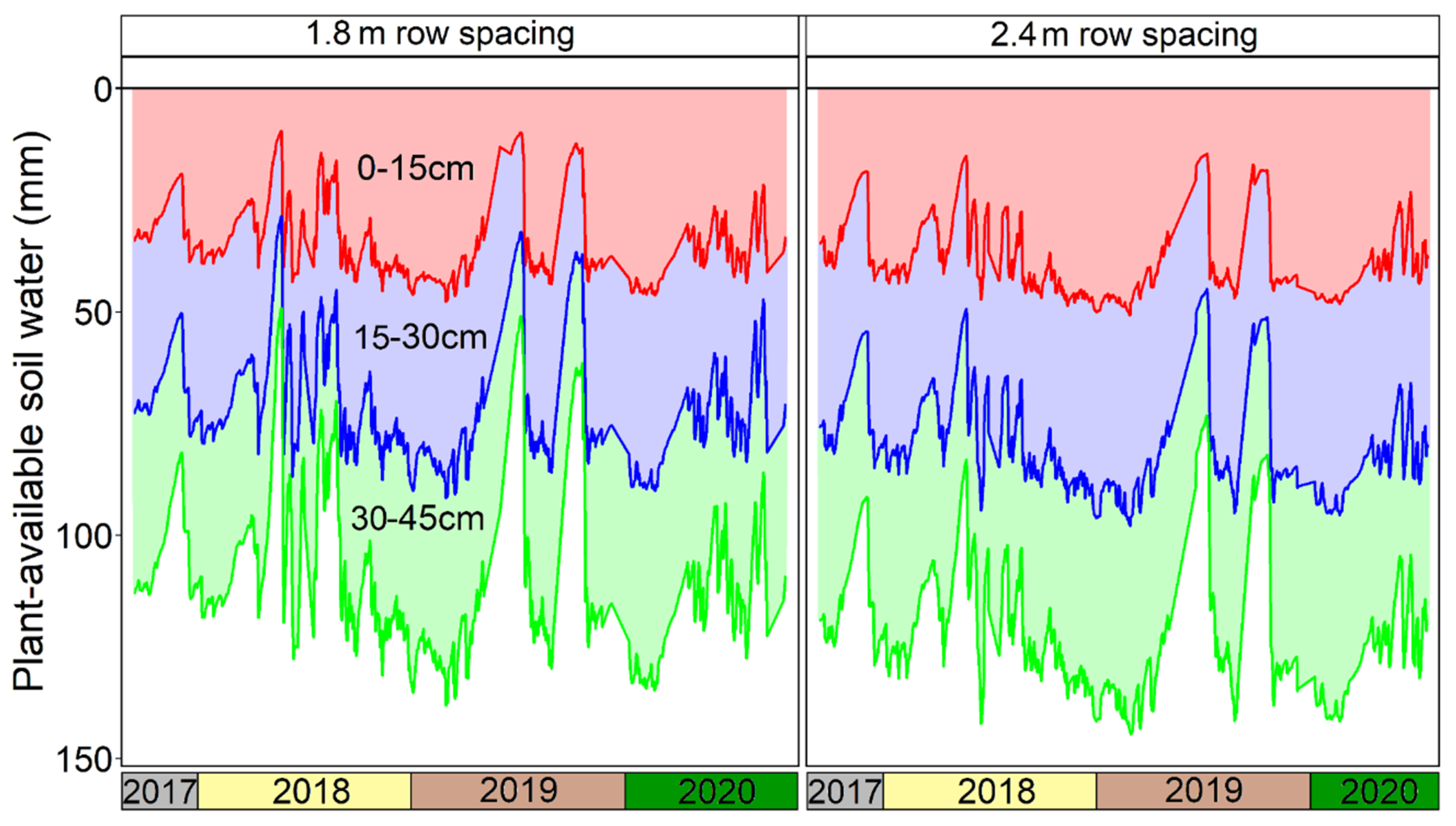
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hale, A.L.; Todd, J.R.; Gravois, K.A.; Mollov, D.; Malapi-Wight, M.; Momotaz, A.; Laborde, C.; Goenaga, R.; Kimbeng, C.; Solis, A. Sugarcane breeding programs in the USA. Sugar Tech 2022, 24, 97–111. [Google Scholar] [CrossRef]
- Richard, E., Jr.; Dunckelman, J.; Carter, C. Productivity of sugarcane on narrow rows, as affected by mechanical harvestinbg. Field Crops Res. 1991, 26, 375–386. [Google Scholar] [CrossRef]
- Deliberto, M.; Hilbun, B.; Gravois, K.; Waguespack, H. Evaluating the investment, fuel, and labor cost structure of wide-row sugarcane production in Louisiana. J. Am. Soc. Sugar Cane Technol. 2020, 40, 56–61. [Google Scholar]
- White, P.M.; Callahan, H.J., Jr.; Webber, C.L., III; Ellsworth, P.Z. The effect of row spacing on L 01-299 and HoCP 04-838 sugarcane yields in Louisiana. J. Am. Soc. Sugar Cane Technol. 2021, 41, 18–25. [Google Scholar]
- Souza, G.S.d.; Souza, Z.M.d.; Silva, R.B.d.; Barbosa, R.S.; Araújo, F.S. Effects of traffic control on the soil physical quality and the cultivation of sugarcane. Rev. Bras. Ciência Solo 2014, 38, 135–146. [Google Scholar] [CrossRef]
- Kingwell, R.; Fuchsbichler, A. The whole-farm benefits of controlled traffic farming: An Australian appraisal. Agric. Syst. 2011, 104, 513–521. [Google Scholar] [CrossRef]
- Ehsanullah, K.J.; Jamil, M.; Ghafar, A. Optimizing the row spacing and seeding density to improve yield and quality of sugarcane. Crop Environ. 2011, 2, 1–5. [Google Scholar]
- Barbieri, P.; Echarte, L.; Della Maggiora, A.; Sadras, V.; Echeverria, H.; Andrade, F. Maize evapotranspiration and water-use efficiency in response to row spacing. Agron. J. 2012, 104, 939–944. [Google Scholar] [CrossRef]
- Acreche, M.M. Nitrogen-, water-and radiation-use efficiencies affected by sugarcane breeding in Argentina. Plant Breed. 2017, 136, 174–181. [Google Scholar] [CrossRef]
- Olivier, F.; Lecler, N.; Singels, A. Increasing water use efficiency of irrigated sugarcane by means of specific agronomic practices. Water Res. Comm. Rep. 2009, document number 1577/1/09. [Google Scholar]
- Olivier, F.; Singels, A. Water use efficiency of irrigated sugarcane as affected by row spacing and variety. Proc. S. Afr. Sugarcane Technol. Assoc. 2003, 77, 347–351. [Google Scholar]
- Ullah, S.; Anjum, S.A.; Raza, M.M.; Riaz, A.; Abbas, A.; Yousif, M.M.; Ma, J.; Liu, Y.; Zhang, J.; Cheng, D. Optimizing row spacing to ameliorate the productivity of spring sugarcane (Saccharum officinarum L.). Agric. Sci. 2016, 7, 531. [Google Scholar]
- Chen, S.; Zhang, X.; Sun, H.; Ren, T.; Wang, Y. Effects of winter wheat row spacing on evapotranpsiration, grain yield and water use efficiency. Agric. Water Manag. 2010, 97, 1126–1132. [Google Scholar] [CrossRef]
- Timmons, D.; Holt, R.; Thompson, R. Effect of plant population and row spacing on evapotranspiration and water-use efficiency by soybeans. Agron. J. 1967, 59, 262–265. [Google Scholar] [CrossRef]
- Passioura, J. Roots and drought resistance. Agric. Water Manag. 1983, 7, 265–280. [Google Scholar] [CrossRef]
- Polania, J.A.; Poschenrieder, C.; Beebe, S.; Rao, I.M. Effective use of water and increased dry matter partitioned to grain contribute to yield of common bean improved for drought resistance. Front. Plant Sci. 2016, 7, 660. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Breaux, R. Breeding to enhance sucrose content of sugarcane varieties in Louisiana. Field Crops Res. 1984, 9, 59–67. [Google Scholar] [CrossRef]
- Bischoff, K.P.; Gravois, K.A. The development of new sugarcane varieties at the LSU AgCenter. J. Am. Soc. Sugar Cane Technol. 2004, 24, 142–164. [Google Scholar]
- Singels, A.; Smit, M. The effect of row spacing on an irrigated plant crop of sugarcane variety NCo376. Proc. Proc. S. Afr. Sugarcane Technol. Assoc. 2002, 76, 94–105. [Google Scholar]
- Jones, H.G. Monitoring plant and soil water status: Established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 2007, 58, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Noborio, K. Measurement of soil water content and electrical conductivity by time domain reflectometry: A review. Comput. Electron. Agric. 2001, 31, 213–237. [Google Scholar] [CrossRef]
- Topp, G.C. State of the art of measuring soil water content. Hydrol. Processes 2003, 17, 2993–2996. [Google Scholar] [CrossRef]
- Gravois, K.; Bischoff, K.; Pontif, M.; LaBorde, C.; Hoy, J.; Reagan, T.; Kimbeng, C.; Legendre, B.; Hawkins, G.; Sexton, D. Registration of ‘L 01–299’ sugarcane. J. Plant Regist. 2011, 5, 191–195. [Google Scholar] [CrossRef]
- Todd, J.; White, W.; Dufrene, E.; Tew, T.; Pan, Y.-B.; Duet, M.; Verdun, D.; Hale, A.; Dalley, C.; Grisham, M. Registration of ‘HoCP 04-838’ sugarcane. J. Plant Regist. 2018, 12, 324–332. [Google Scholar] [CrossRef]
- Saxton, K.E.; Rawls, W.J. Soil water characteristic estimates by texture and organic matter for hydrologic solutions. Soil Sci. Soc. Am. J. 2006, 70, 1569–1578. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- de Mendiburu, F.; Taseen, M. Agricolae: Statistical Procedures for Agricultural Research, 2020; R package version 1:3-5.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Team, R.C. nlme: Linear and Nonlinear Mixed Effects Models, 2021; R package version 3:1-153.
- Alessi, J.; Power, J. Effects of plant and row spacing on dryland soybean yield and water-use efficiency. Agron. J. 1982, 74, 851–854. [Google Scholar] [CrossRef]
- Chopart, J.-L.; Azevedo, M.; Le Mezo, L.; Marion, D. Sugarcane Root System Depth in Three Different Countries. In Proceedings of the 27th Congress of the International Society of Sugar Cane Technologists Congress(ISSCT), Veracruz, Mexico, 7–11 March 2010; Volume 27, pp. 1–8. [Google Scholar]
- Smith, D.; Inman-Bamber, N.; Thorburn, P. Growth and function of the sugarcane root system. Field Crops Res. 2005, 92, 169–183. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, K.; Zhao, P.; Qin, W.; Zhao, Y.; Zhu, J.; Zan, F.; Zhao, J.; Lu, X.; Wu, C. Sugarcane root distribution and growth as affected by genotype and crop cycle. Bragantia 2020, 79, 192–202. [Google Scholar] [CrossRef]
- Jackson, R.B.; Pockman, W.T.; Hoffmann, W.A. The structure and function of root systems. In Handbook of Functional Plant Ecology; Pugnaire, F.I., Valladares, F., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 195–220. [Google Scholar]
- Sinclair, T.R. Effective water use required for improving crop growth rather than transpiration efficiency. Front. Plant Sci. 2018, 9, 1442. [Google Scholar] [CrossRef] [PubMed]
- Skaggs, R.W.; Breve, M.; Gilliam, J. Hydrologic and water quality impacts of agricultural drainage. Crit. Rev. Environ. Sci. Technol. 1994, 24, 1–32. [Google Scholar] [CrossRef]
- Camp, C.; Carter, C. Sugarcane yield response to subsurface drainage for an alluvial soil. Trans. Am. Soc. Agric. Eng. 1983, 26, 1112–1116. [Google Scholar] [CrossRef]
- Carter, C.E.; Camp, C.R. Subsurface drainage of an alluvial soil increased sugarcane yields. Trans. Am. Soc. Agric. Eng. 1983, 26, 426–429. [Google Scholar] [CrossRef]
- Gayle, G.; Skaggs, R.; Carter, C. Effects of excessive soil water conditions on sugarcane yields. Trans. Am. Soc. Agric. Eng. 1987, 30, 993–997. [Google Scholar] [CrossRef]
- Carter, C.E.; Irvine, J.; McDaniel, V.; Dunckelman, J. Yield response of sugarcane to stalk density and subsurface drainage treatments. Trans. Am. Soc. Agric. Eng. 1985, 28, 172–178. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Zhu, X.-G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Eyland, D.; van Wesemael, J.; Lawson, T.; Carpentier, S. The impact of slow stomatal kinetics on photosynthesis and water use efficiency under fluctuating light. Plant Physiol. 2021, 186, 998–1012. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol 2009, 181, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Von Caemmerer, S.; Furbank, R.T. Modeling C4 photosynthesis. In C4 Plant Biology; Sage, R.F., Monson, R.K., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 173–211. [Google Scholar]
- Jackson, P.; Basnayake, J.; Inman-Bamber, G.; Lakshmanan, P.; Natarajan, S.; Stokes, C. Genetic variation in transpiration efficiency and relationships between whole plant and leaf gas exchange measurements in Saccharum spp. and related germplasm. J. Exp. Bot. 2015, 67, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, O. How can we breed for more water use-efficient sugarcane? J. Exp. Bot. 2016, 67, 557–559. [Google Scholar] [CrossRef]
- Long, S.P.; Zhu, X.-G.; Naidu, S.L.; Ort, D.R. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- Li, C.; Jackson, P.; Lu, X.; Xu, C.; Cai, Q.; Basnayake, J.; Lakshmanan, P.; Ghannoum, O.; Fan, Y. Genotypic variation in transpiration efficiency due to differences in photosynthetic capacity among sugarcane-related clones. J. Exp. Bot. 2017, 68, 2377–2385. [Google Scholar] [CrossRef][Green Version]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef]

| Depth | Row Spacing | Variety | VWC †,‡ | Min. VWC | Max. VWC | PAW (mm) † | Min. PAW | Max. PAW | T (°C) †,* | Min. T | Max. T |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 cm | 1.8 m | L 01-299 | 33.0 ± 12.5 | 12.5 | 44.8 | 35.3 ± 9.6 | 4.6 | 52.9 | 20.5 ± 5 | 5.5 | 29.9 |
| 1.8 m | HoCP 04-838 | 32.1 ± 12.0 | 12.0 | 45.9 | 34.0 ± 10.7 | 3.9 | 54.6 | 20.4 ± 5 | 4.9 | 29.7 | |
| 2.4 m | L 01-299 | 33.4 ± 13.4 | 13.4 | 45.5 | 36.0 ± 9.4 | 5.9 | 54.0 | 20.7 ± 5.1 | 4.3 | 30.6 | |
| 2.4 m | HoCP 04-838 | 34.1 ± 13.1 | 13.1 | 46.4 | 36.9 ± 9.7 | 5.5 | 55.4 | 20.9 ± 5 | 5.4 | 30.5 | |
| 30 cm | 1.8 m | L 01-299 | 36.7 ± 12.1 | 12.1 | 44.0 | 38.2 ± 7.4 | 1.4 | 49.3 | 20.5 ± 4.7 | 7.5 | 29.1 |
| 1.8 m | HoCP 04-838 | 35.4 ± 18.1 | 18.1 | 45.7 | 36.4 ± 6.4 | 10.3 | 51.8 | 20.5 ± 4.7 | 7.1 | 29.1 | |
| 2.4 m | L 01-299 | 37.2 ± 23.4 | 23.4 | 47.5 | 39.0 ± 6.6 | 18.3 | 54.5 | 20.9 ± 4.6 | 6.0 | 29.7 | |
| 2.4 m | HoCP 04-838 | 38.6 ± 23.1 | 23.1 | 47.1 | 41.1 ± 4.8 | 17.8 | 53.9 | 20.7 ± 4.8 | 7.4 | 29.6 | |
| 45 cm | 1.8 m | L 01-299 | 37.5 ± 12.4 b | 12.4 | 48.8 | 37.4 ± 7.7 b | 0.0 | 54.3 | 20.4 ± 4.5 | 8.6 | 28.7 |
| 1.8 m | HoCP 04-838 | 39.3 ± 15.1 ab | 15.1 | 47.6 | 40.2 ± 8.8 ab | 3.9 | 52.6 | 20.6 ± 4.4 | 8.5 | 28.6 | |
| 2.4 m | L 01-299 | 42.8 ± 29.6 a | 29.6 | 50.1 | 45.4 ± 6.7 a | 25.6 | 56.3 | 20.8 ± 4.5 | 7.5 | 29.1 | |
| 2.4 m | HoCP 04-838 | 39.7 ± 21.0 ab | 21.0 | 49.2 | 40.7 ± 5.2 ab | 12.7 | 54.9 | 20.6 ± 4.5 | 8.6 | 29.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellsworth, P.Z.; White, P.M., Jr. Row Spacing and the Use of Plant-Available Water in Sugarcane Cultivation in Water-Abundant Louisiana. Agronomy 2022, 12, 1586. https://doi.org/10.3390/agronomy12071586
Ellsworth PZ, White PM Jr. Row Spacing and the Use of Plant-Available Water in Sugarcane Cultivation in Water-Abundant Louisiana. Agronomy. 2022; 12(7):1586. https://doi.org/10.3390/agronomy12071586
Chicago/Turabian StyleEllsworth, Patrick Z., and Paul M. White, Jr. 2022. "Row Spacing and the Use of Plant-Available Water in Sugarcane Cultivation in Water-Abundant Louisiana" Agronomy 12, no. 7: 1586. https://doi.org/10.3390/agronomy12071586
APA StyleEllsworth, P. Z., & White, P. M., Jr. (2022). Row Spacing and the Use of Plant-Available Water in Sugarcane Cultivation in Water-Abundant Louisiana. Agronomy, 12(7), 1586. https://doi.org/10.3390/agronomy12071586







