Fluopyram: Optimal Application Time Point and Planting Hole Treatment to Control Meloidogyne incognita
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Nematode Inoculum and Chemical Nematicide
2.2. In Vitro Effect of Fluopyram on Meloidogyne incognita
2.3. Optimal Time Point of Fluopyram Application
Penetration Assay
2.4. Application of Fluopyram as Planting Hole Treatment
2.5. Data Analysis
3. Results and Discussion
3.1. In Vitro Effect of Fluopyram on Meloidogyne incognita
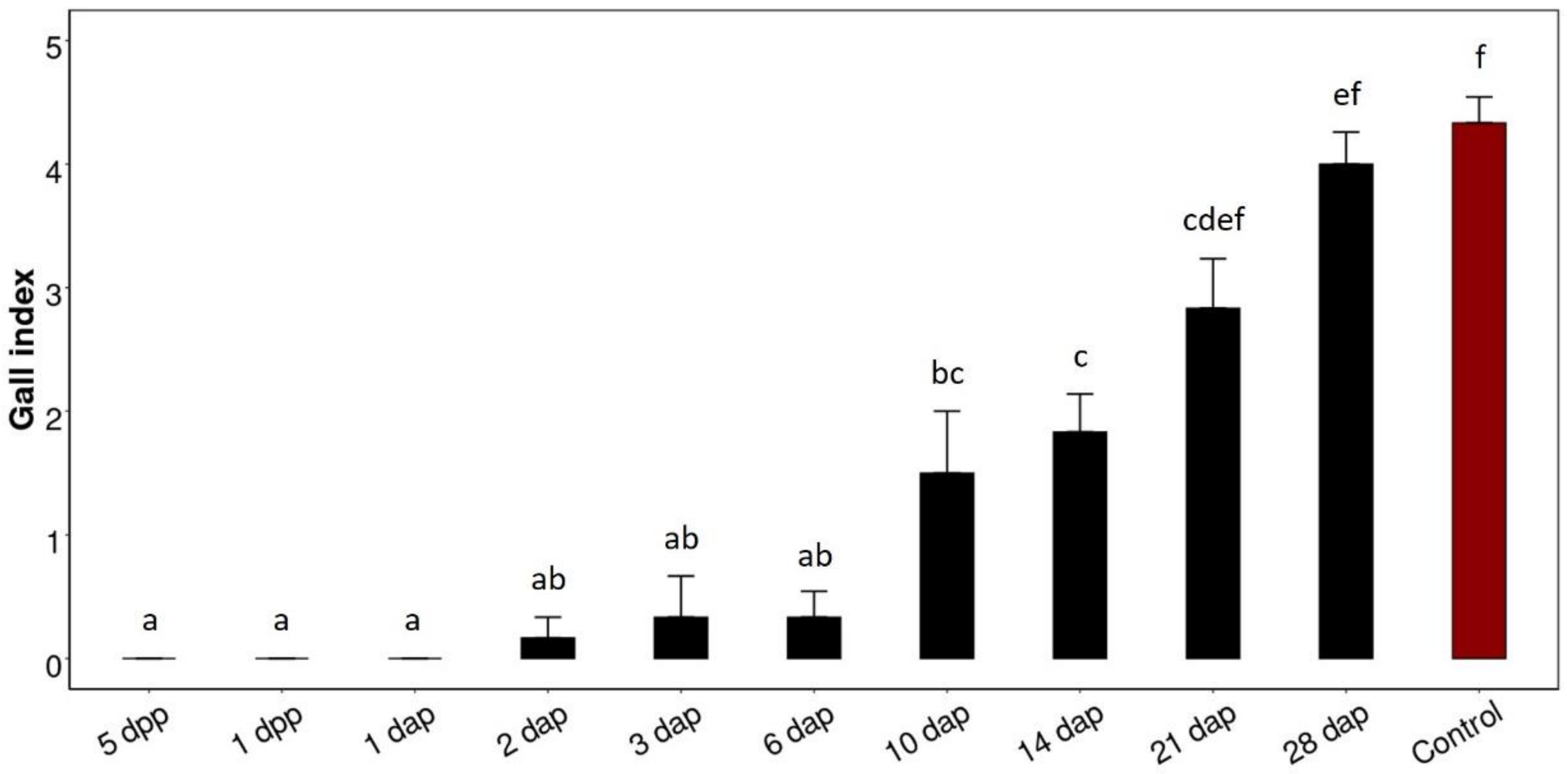
3.2. Optimal Time Point for Fluopyram Application
Penetration Assay
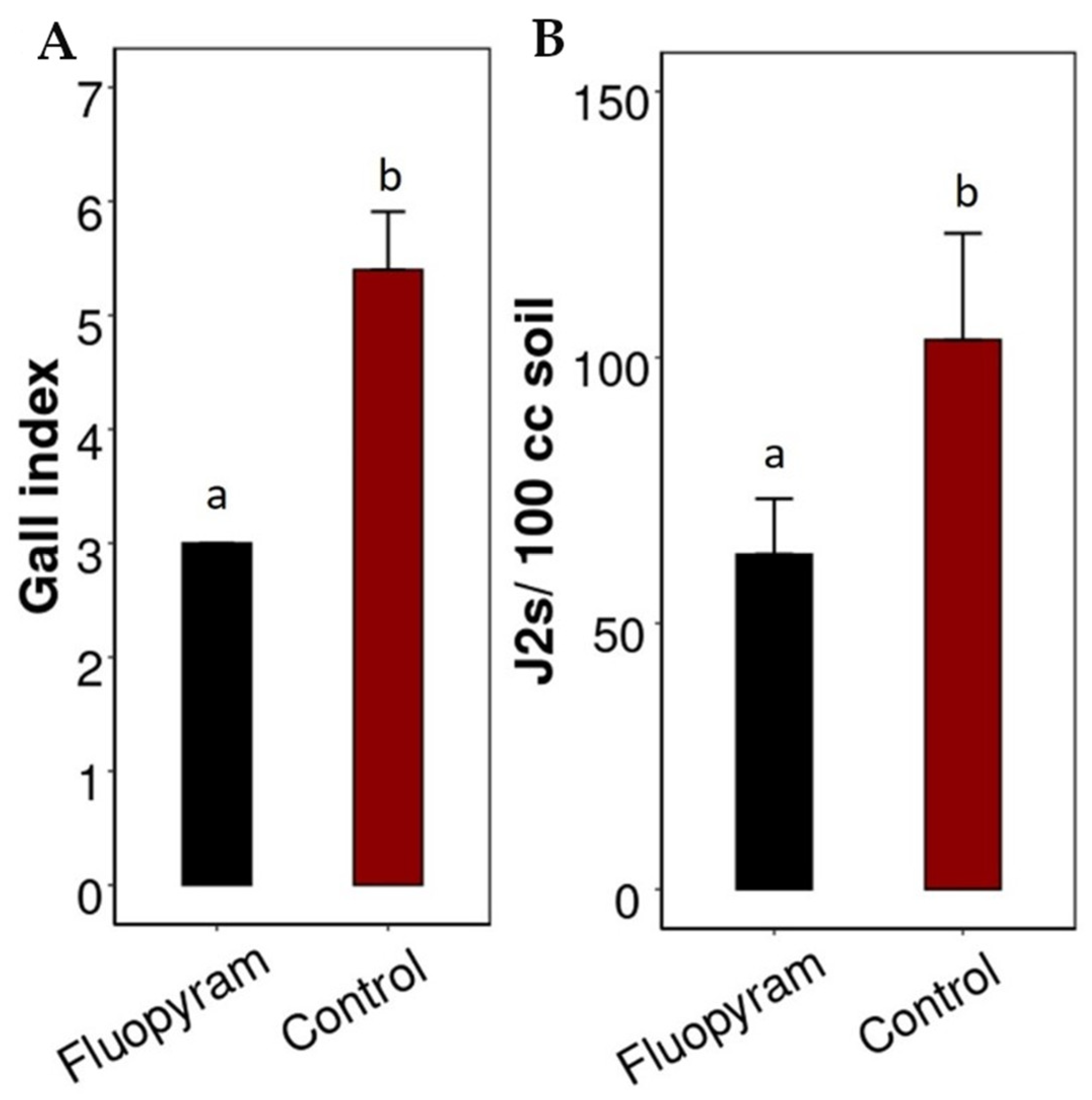
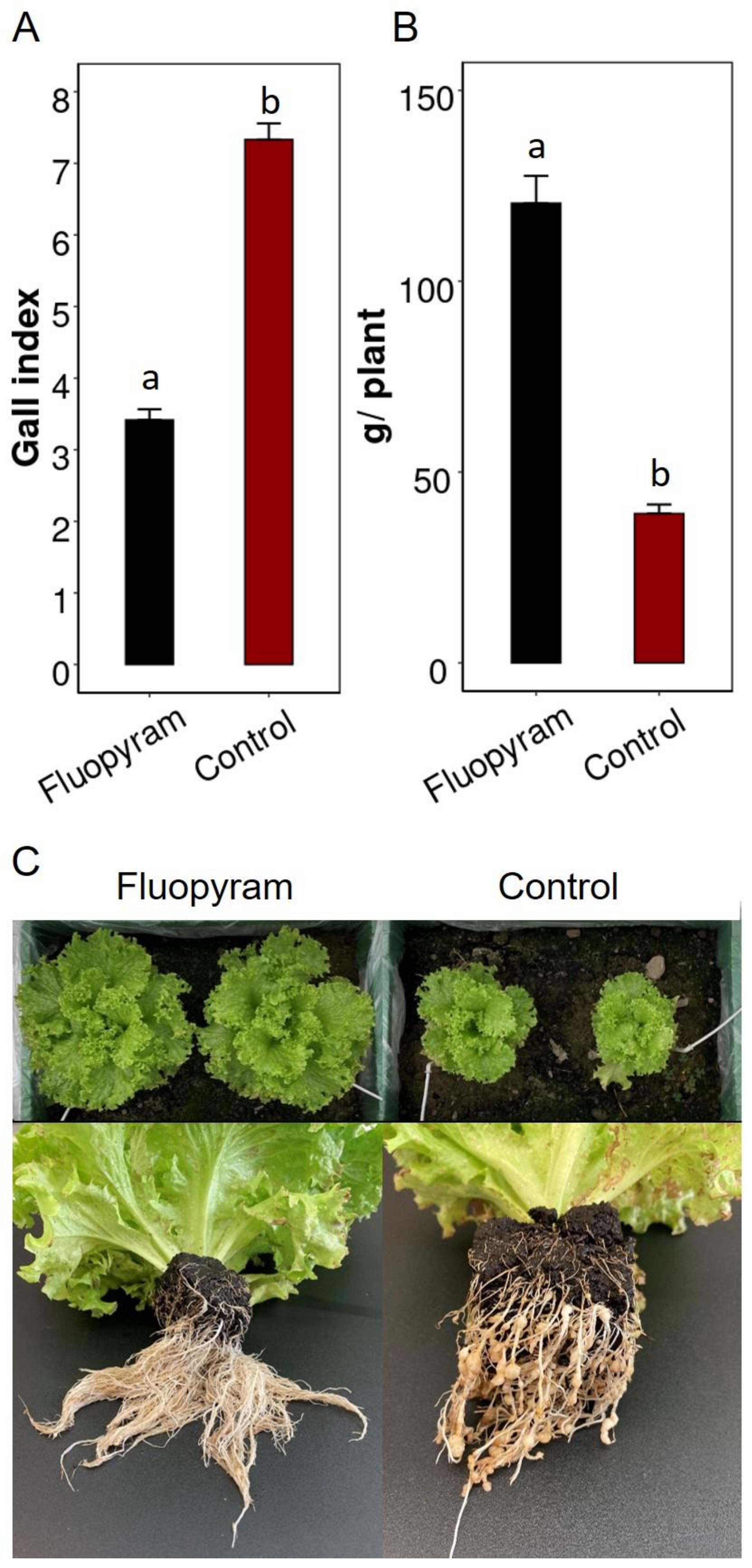
3.3. Application of Fluopyram as Planting Hole Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology: Top 10 plant-parasitic nematodes. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Thapa, B.; Shrestha, J. Root-knot nematode (Meloidogyne incognita) and its management: A review. J. Agric. Nat. Res. 2020, 3, 21–31. [Google Scholar] [CrossRef]
- Wesemael, W.; Viaene, N.; Moens, M. Root-knot nematodes (Meloidogyne spp.) in Europe. Nematology 2011, 13, 3–16. [Google Scholar] [CrossRef]
- Eisenback, J.D. Meloidogyne incognita (root-knot nematode). In Invasive Species Compendium; CABI: Wallingford, UK, 2022. [Google Scholar] [CrossRef]
- Oka, Y. Sensitivity to fluensulfone of inactivated Meloidogyne spp. second-stage juveniles. Pest Manag. Sci. 2020, 76, 2379–2387. [Google Scholar] [CrossRef]
- Sasanelli, N.; Konrat, A.; Migunova, V.; Toderas, I.; Iurcu-Straistaru, E.; Rusu, S.; Bivol, A.; Andoni, C.; Veronico, P. Review on control methods against plant parasitic nematodes applied in southern member states (C Zone) of the European Union. Agriculture 2021, 11, 602. [Google Scholar] [CrossRef]
- Oka, Y. From old-generation to next-generation nematicides. Agronomy 2020, 10, 1387. [Google Scholar] [CrossRef]
- Brannen, P.M. Chemical Nematicides for Control of Plant-Parasitic Nematodes in Georgia Vegetable Crops. Uga.edu. Available online: https://extension.uga.edu/publications/detail.html?number=B1502 (accessed on 29 April 2022).
- Khalil, M.S.; Selim, R.E. Fluorinated nematicides: Novel classes in the way. J. Plant Sci. Phytopathol. 2021, 5, 014–016. [Google Scholar] [CrossRef]
- WIPO. Fungicidal Composition Comprising a Pyridylethylbenzamide Derivative and a Compound Capable of Inhibiting the Transport of Electrons of the Respiratory Chain in Phytopathogenic Fungal Organisms. World Intellectual Property Organization WO 2005/077901 A1. Available online: https://patentimages.storage.googleapis.com/91/7f/c7/35effb198929ba/WO2005077901A1.pdf (accessed on 5 May 2022).
- Umetsu, N.; Shirai, Y. Development of novel pesticides in the 21st century. J. Pestic. Sci. 2020, 45, 54–74. [Google Scholar] [CrossRef] [Green Version]
- Rieck, H.; Coqueron, P.Y. Modern Crop Protection Compounds; Insecticides; Wiley-VCH: Weinheim, Germany, 2012; Volume 3, pp. 639–645. [Google Scholar]
- Heiken, J.A. The Effects of Fluopyram on Nematodes. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2017. [Google Scholar]
- Ludlow, K. Public Release Summary on the Evaluation of the New Active Fluopyram in Product Luna Privilege Fungicide. Australian Pesticides and Veterinary Medicines Authority. 2015. Available online: https://apvma.gov.au/node/14166 (accessed on 16 March 2022).
- Zhou, J.; Liang, S.; Cui, Y.; Rong, Y.; Song, J.; Lv, D. Study on environmental behaviour of fluopyram in different banana planting soil. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Hallmann, J.; Kiewnick, S. Virulence of Meloidogyne incognita populations and Meloidogyne enterolobii on resistant cucurbitaceous and solanaceous plant genotypes. J. Plant Dis. Prot. 2018, 125, 415–424. [Google Scholar] [CrossRef]
- Hallmann, J.; Subbotin, S.A. Methods for extraction, processing and detection of plant and soil nematodes. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 3rd ed.; Sikora, R.A., Coyne, D., Hallmann, J., Timper, P., Eds.; CABI: Wallingford, UK, 2018; pp. 87–119. [Google Scholar]
- Zeck, W.M. Rating scheme for field evaluation of root-knot nematode infestations. Pflanzenschutz Nachr. 1971, 24, 141–144. [Google Scholar]
- Bybd, D.W.; Kirkpatrick, T.; Barker, K.R. An improved technique for clearing and staining plant tissues for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar] [PubMed]
- Oostenbrink, M. Estimating nematode populations by some selected methods. Nematology 1960, 85–102. [Google Scholar]
- Finney, D.J. Probit Analysis, 2nd ed.; Cambridge University Press: New York, NY, USA, 1952; Volume 78, pp. 388–390. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Faske, T.R.; Hurd, K. Sensitivity of Meloidogyne incognita and Rotylenchulus reniformis to fluopyram. J. Nematol. 2015, 47, 316–321. [Google Scholar] [PubMed]
- Giannakou, I.O.; Kamaras, S. Comparison of a Vintage and a Recently Released Nematicide for the Control of Root-Knot Nematodes and Side Effects on Two Entomopathogenic Nematodes. Plants 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Bangash, S.H.; Lin, H.; Zeng, D.; Tang, W. Efficacy of fluopyram applied by chemigation on controlling eggplant root-knot nematodes (Meloidogyne spp.) and its effects on soil properties. PLoS ONE 2020, 15, e0235423. [Google Scholar] [CrossRef]
- Quader, M.; Riley, I.T.; Walker, G.E. Distribution pattern of root-knot nematodes (Meloidogyne spp.) in South Australian vineyards. Australas Plant Pathol. 2001, 30, 357. [Google Scholar] [CrossRef]
- East, K.E.; Moyer, M.M.; Madden, N.M.; Zasada, I.A. How low can they go? Plant-parasitic nematode distribution in a Washington vineyard. Catal. Discov Pract. 2019, 3, 31–36. [Google Scholar] [CrossRef]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated control of Meloidogyne incognita in tomatoes using fluopyram and purpureocillium lilacinum strain 251. Crop Prot. 2019, 124, 104874. [Google Scholar] [CrossRef]
- Chawla, S.; Patel, D.J.; Patel, S.H.; Kalasariya, R.L.; Shah, P.G. Behaviour and risk assessment of fluopyram and its metabolite in cucumber (Cucumis sativus) fruit and in soil. Env. Sci. Pollut. Res. Int. 2018, 25, 11626–11634. [Google Scholar] [CrossRef] [PubMed]



| Exposure Time | Concentration [µmol/L] | N [%] | A [%] | I [%] | LC50 [µmol/L] | Gall Index |
|---|---|---|---|---|---|---|
| Day 1 | Control | 95.1 | 1.9 | 3.0 | 2.15 (1.281–3.610) | 7.17 ± 0.69 a |
| 0.25 | 81.1 | 13.2 | 5.7 | 7.33 ± 0.75 a | ||
| 0.5 | 83.2 | 11.9 | 5.0 | 7.17 ± 0.69 a | ||
| 1.0 | 74.5 | 18.1 | 7.4 | 6.33 ± 0.47 abc | ||
| 2.0 | 59.7 | 28.0 | 12.3 | 5.83 ± 0.37 bcd | ||
| 4.0 | 28.2 | 39.2 | 32.6 | 5.83 ± 0.37 cd | ||
| 8.0 | 6.1 | 35.3 | 58.6 | 4.83 ± 0.69 d | ||
| Day 2 | Control | 95.0 | 2.0 | 3.0 | 0.45 (0.242–0.836) | 7.00 ± 0.58 a |
| 0.25 | 58.5 | 36.2 | 5.3 | 6.83 ± 0.69 a | ||
| 0.5 | 53.1 | 42.4 | 4.5 | 7.17 ± 0.69 a | ||
| 1.0 | 18.3 | 74.7 | 6.9 | 7.17 ± 0.69 a | ||
| 2.0 | 9.2 | 80.1 | 10.7 | 6.25 ± 0.43 ab | ||
| 4.0 | 4.9 | 70.4 | 24.6 | 6.33 ± 0.75 a | ||
| 8.0 | 0.8 | 27.5 | 71.7 | 4.83 ± 0.90 b | ||
| Day 3 | Control | 94.9 | 2.5 | 2.7 | 0.05 (0.016–0.159) | 7.00 ± 0.58 a |
| 0.25 | 27.0 | 66.7 | 6.3 | 7.00 ± 0.58 a | ||
| 0.5 | 18.9 | 75.4 | 5.7 | 7.00 ± 0.82 a | ||
| 1.0 | 4.5 | 85.2 | 10.3 | 6.83 ± 0.69 a | ||
| 2.0 | 1.7 | 87.0 | 11.3 | 6.60 ± 0.49 a | ||
| 4.0 | 0.4 | 70.7 | 28.9 | 6.67 ± 0.47 a | ||
| 8.0 | 0.0 | 31.8 | 68.2 | 4.83 ± 0.69 b | ||
| Day 14 | Control | 83.5 | 7.9 | 8.6 | 0.04 (0.024–0.080) | 5.83 ± 1.07 a |
| 0.25 | 6.0 | 66.9 | 27.1 | 5.80 ± 1.47 a | ||
| 0.5 | 1.5 | 76.9 | 21.6 | 5.50 ± 1.38 a | ||
| 1.0 | 0.7 | 79.1 | 20.2 | 5.17 ± 1.07 ab | ||
| 2.0 | 0.0 | 80.5 | 19.5 | 6.50 ± 0.50 a | ||
| 4.0 | 0.0 | 48.3 | 51.7 | 5.33 ± 0.75 ac | ||
| 8.0 | 0.0 | 23.6 | 76.4 | 3.33 ± 0.75 bc |
| Treatment | Cucumber Height [cm] | Gall Index | ||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 1 | Trial 2 | |
| Soil | 138.08 ± 51.32 a | 93.93 ± 20.95 a | 8.08 ± 0.75 a | 7.14 ± 0.64 a |
| Soil + fluopyram | 124.71 ± 15.34 b | 4.79 ± 0.56 b | ||
| Soil + roots | 95.92 ± 73.87 a | 75.50 ± 23.98 ac | 8.75 ± 1.01 a | 7.14 ± 0.52 ac |
| Soil + roots + fluopyram | 224.75 ± 20.20 b | 118.07±34.04 abd | 6.33 ± 0.47 b | 5.64 ± 0.89 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stucky, T.; Dahlin, P. Fluopyram: Optimal Application Time Point and Planting Hole Treatment to Control Meloidogyne incognita. Agronomy 2022, 12, 1576. https://doi.org/10.3390/agronomy12071576
Stucky T, Dahlin P. Fluopyram: Optimal Application Time Point and Planting Hole Treatment to Control Meloidogyne incognita. Agronomy. 2022; 12(7):1576. https://doi.org/10.3390/agronomy12071576
Chicago/Turabian StyleStucky, Tobias, and Paul Dahlin. 2022. "Fluopyram: Optimal Application Time Point and Planting Hole Treatment to Control Meloidogyne incognita" Agronomy 12, no. 7: 1576. https://doi.org/10.3390/agronomy12071576
APA StyleStucky, T., & Dahlin, P. (2022). Fluopyram: Optimal Application Time Point and Planting Hole Treatment to Control Meloidogyne incognita. Agronomy, 12(7), 1576. https://doi.org/10.3390/agronomy12071576




