Abstract
Productive water use can be an effective adaptation strategy for improving crop performance. A 2-year field study was undertaken in 2018 and 2019 to investigate the effect of sowing date and genotype on water-use efficiency of lentils grown in diverse locations in Australia. Above-ground dry matter accumulation, grain yield, soil evaporation, water use, and water-use efficiency (WUE) were measured and/or calculated at crop maturity. Early sowing (SD1/mid-April), late maturity and supplementary irrigation increased water use. The long growth cycle resulting from early sowing influenced WUE for dry matter production and grain yield. WUE ranged from 10.5 to 18.8 kg dry matter ha−1 mm−1 (WUEET (evapotranspiration)) and 17.1 to 28.3 kg dry matter ha−1 mm−1 (WUET (transpiration)) for dry matter production. For grain yield, WUE ranged from 2.11 to 5.65 kg grain ha−1 mm−1 (WUEET) and 4.71 to 9.19 kg grain ha−1 mm−1 (WUET). There was more water loss through soil evaporation in SD1 compared to the other sowing dates. Excessive or limited availability of water did not translate to more dry matter accumulation and grain yield. The study concluded that SD1 gives the maximum water productivity for biomass, and SD2 (end of April) and SD3 (mid-May) for grain yield.
1. Introduction
Water is an important component in agricultural production, but it is becoming scarce due to climate change causing variable rainfall patterns and drought events [1]. Agriculture consumes over 80% of available freshwater, with a large proportion of this used for cropping [2], but it faces competition for the water from other industries and households. This limited water availability challenges the agricultural industry’s ability to produce more food to feed the increasing world population. Therefore, there is a growing need to produce higher yields from limited amounts of water, and research approaches have focused and committed resources on breeding drought-tolerant and/or water use efficient crops. Advances in agronomy and management practices are complementing these efforts.
Water-use efficiency (WUE), also commonly referred to as water productivity, can be an effective adaptation strategy for improving crop performance by producing ‘more crop per drop of water [3,4,5]. It is broadly defined in crop science as the ratio of above-ground biomass or economic grain yield to the evapotranspiration or total water use by the crop [6,7,8,9]. Overall, it is an important determinant of grain yield especially under water-limited conditions, with the water use efficient crops potentially yielding better.
A number of factors such as climate, soil type, sowing time, crop and land management practices, the intensity of tillage, and crop sequence influence WUE [10,11,12]. Genetically, it can be influenced by inter and intra crop variation due to differences in growth habit and architecture, maturity class, rooting pattern, osmotic adjustment, transpiration efficiency, and assimilate redistribution, which facilitates different drought coping mechanisms [7,10,11,13]. Physiological processes including transpiration, osmotic potential, turgor potential and photosynthesis, influence water use, WUE and the overall crop water balance [14]. Vigorous early growth will shade the soil surface, reduce soil evaporation, evapotranspiration and soil temperature and potentially increase the availability of soil water in the post-flowering period and WUE [15,16,17]. However, the larger canopy would likely lead to more water loss through transpiration, necessitating a need to strike a balance between water loss and saving it through shading. Deeper roots will ensure that water at depth is not wasted through drainage but is available to the crop to improve crop performance and WUE [7]. Drought tolerant genotypes have been found to produce higher root density and/or deeper and more vigorous roots, while susceptible ones tend to have shallow rooting systems and suffer water stress, especially during later stages of growth as the surface soil dries up due to high temperature-induced evaporation and rainfall decrease in Mediterranean environments [13,14]. The rooting depth for lentils is around 60–90 cm [18,19,20], and the depth of soil moisture capture can be used as a surrogate for root depth [21]. While root characteristics are largely genetically determined, they are also significantly influenced by the environment. For example, hostile soils limit root penetration and prevent moisture capture at lower subsoil layers.
For sustainable grain crop productivity, sufficient water is required during the vegetative stage, early in the season, and at the later stages of grain and/or pod filling. Early maturing genotypes will tend to use less water during the vegetative phase and retain more water for the reproductive phase, which improves grain yield and WUE [14]. When leaf areas and evaporative demand are small early in the season, water use is minimal. Excess water is stored in the soil profile or is lost through evaporation. Use of large amounts of water early in the season might result in the production of excessive biomass, which would not be converted to grain yield due to water scarcity during crucial grain/pod filling phases later in the season [22]. This source-sink imbalance has been observed in chickpea sowing date studies [23]. Synchronising the timing of key growth phases and their duration to expected and predicted seasonal conditions is an important step towards increasing adaptation and productivity across a range of environments through using available water at key growth phases. Agronomic management approaches such as adjusting the planting calendar and seeding rates, efficient irrigation methods, soil and weed management can all be used to alleviate the severity of water stress and/or improve crop WUE [24]. Early sown crops generally have a longer life cycle which extends the growing season duration and might allow for root growth and development of deeper root systems.
A meta-analysis study has shown that summer crops have higher average WUE (3.23 kg m−3) than winter crops (1.03 kg m−3), and that legumes have lower WUE (0.42 kg m−3) compared to cereals (2.37 kg m−3), oilseeds (0.69 kg m−3), and fibre crops (0.45 kg m−3) [11]. This is because it takes less energy to produce starch, which is a dominant component in cereals than to produce protein and oil, which are dominant in legumes and oilseeds, respectively. Lentil is more water efficient compared to the other legumes [11]. For example, the WUE for dry matter (WUEdm) and seed yield (WUEgr) was 13.7 and 3.8 kg ha−1 mm−1, respectively, compared to chickpea, whose WUEdm and WUEgr, were 8.7 and 3.2 kg ha−1 mm−1 [20]. However, for chickpea, higher values (22–29 kg dry matter ha−1 mm−1 and 10–13 kg seed yield ha−1 mm−1) have also been reported [25].
In Australia, lentils are grown mostly under dryland conditions in areas with low and variable seasonal rainfall and as a result, commonly suffer moisture stress, especially in arid years. Production is mostly concentrated in Victoria and the mid-north of South Australia on mostly alkaline soils, and yields are on average 1200 kg ha−1 [26,27]. Overall, lentils are cultivated on an area of over 412,381 ha, and produce about 525,848 t total grain per annum [27]. In 2020, Australia contributed approximately 8% of global lentil production, with Canada being the largest producer at 43% [27]. Grain yield in the Mediterranean-type dryland cropping regions of southern Australia is largely dependent on the growing season rainfall (GSR) and the available soil water in the shallow (0.10–0.60 m) subsoil [13,19]. If supplemental irrigation is to be used, there is a need for information on optimal irrigation strategies to achieve high WUE in lentils and combat drought effects. The adaptation and response of lentils to different levels of rainfall and supplementary irrigation has not been extensively explored, as evidenced by very few published papers on lentil WUE in Australia. The aim of this study was to understand the impact of water parameters on lentil dry matter accumulation and grain yield. It focused on soil evaporation, water use, and WUE of lentil varieties grown under diverse environments in southern and western NSW, Australia, and with different sowing times.
2. Materials and Methods
2.1. Experimental Details
A chickpea companion paper includes detailed experimental information, including designs and climatic conditions [28]. Briefly, field experiments were conducted between April and November in 2018 and 2019, at the New South Wales Department of Primary Industries (NSW DPI) research facilities located at Trangie Agricultural Research Centre (TARC; 31.99° S, 147.95° E) in central-western NSW, Wagga Wagga Agricultural Institute (WWAI; 35.05° S, 147.35° E), Leeton Field station (LFS; 34.59° S, 146.36° E), Yanco Agricultural Institute (YAI; 34.61° S, 146.41° E) in southern NSW, Australia (Figure 1). The YAI experiment was conducted in 2018 only and is 6 km from LFS. The soils at the individual sites are Red Chromosol at TARC, Kandosol at WWAI, and Brown Chromosol at both YAI and LFS. Eight diverse lentil genotypes consisting of released varieties (Table 1), were used in this study across four sowing dates, two years and four locations. A split-block design with three replicates was used with sowing date as main plot and genotypes randomised within plots. The four sowing dates were mid-April (SD1), end of April (SD2), mid-May (SD3) and end of May (SD4). In both years, at TARC 85 kg ha−1 of GranulockZ Extra (N 9.86: P 16.83: K 0.0:S 4.59: Zn 1.70) was applied, while at WWAI 100 kg ha−1 of Granulock Z Soygran (N 5.5: P 15.3: K 0.0: S 7.5) was applied as fertiliser. At LFS no fertiliser was applied in 2018, while in 2019 55 kg ha−1 Utiliser pulse mix (N 13.5: P 13.5: K 0.0: S 9.5) was applied. At YAI 80 kg ha−1 of Energiser Plus was applied. The experiments were sprayed with recommended chemicals to keep them weed, pest and disease free.
Figure 1.
Location of the field experiments conducted in 2018 and 2019 at New South Wales Department of Primary Industries (NSW DPI) research facilities.

Table 1.
Lentil genotypes and their classification/characteristics evaluated in the 2018 and 2019 seasons.
Two harvest cuts to calculate total above ground biomass/dry matter (kg ha−1) and grain yield (kg ha−1) were taken from a m2 area of the inner rows, excluding border rows at least 1 m from the ends of each plot. Water parameters (soil evaporation, water use (WU) and water use efficiency (WUE) were calculated at the end of the season. All the experiments (location by year combination) experienced below-average growing season rainfall.
The season duration, in-crop rainfall, and irrigation applied were recorded (Table 2). Daily weather (maximum temperature (MaxT), minimum temperature (MinT), and rainfall and solar radiation) parameters were obtained from the Scientific Information for Land Owners website (SILO) (https://legacy.longpaddock.qld.gov.au/silo/about.html (accessed 20 February 2021); [29]).

Table 2.
Growing season duration across the sowing dates (SD), and water received during 2018 and 2019 at the experimental sites. TARC = Trangie Agricultural Research Centre; WWAI = Wagga Wagga Agricultural Institute; LFS = Leeton Field station; YAI = Yanco Agricultural Institute.
The mean temperatures for the 2018 and 2019 years were recorded (Table 3). Detailed climatic data including long term rainfall and temperature data, have previously been published [28] and are therefore provided as Supplementary Figure S1.

Table 3.
Mean temperature at the experimental sites during the years in which the experiments were conducted. For site abbreviations, see text.
2.2. Soil Water Balance Parameters
Rainfall, evapotranspiration (ET), runoff and drainage are important water parameters [30] that influence water availability to crops [31,32]. Temperature, rainfall, initial soil water and soil water at harvest were measured, and these data were used as the basis for calculating water parameters for this study [33,34] following the procedure of [35] to determine the soil water balance. The farming systems model APSIM [36] version 7.10 was used to calculate the hydraulic parameters (starting and ending daily soil water content, water use, runoff, drainage and soil evaporation). The parameters in the Soil Water module of APSIM are the same as those used in [33,34]. We assumed that the initial soil water was equal to LL15 (water content at 15 bar suction) on 1 January 2009. This LL15 value was determined in the Wagga Wagga Agricultural Institute soil moisture analysis laboratory. The APSIM model was then run continuously until the end of 2019 (31 December), without resetting soil water conditions, in order to obtain the “initial soil water at sowing” and the “soil water at harvest” for each of the lentil experiments [35]. The APSIM crop sequence used in the 10-year run-up period before the lentil experiments was a typical one for the lentil growing regions in Australia: wheat(W)-canola(C)-barley(B)-W-B-C-W-C-B-W.
Total water use (WU) expressed as ET was calculated by subtracting the water remaining at the end of the season from the initial water at the beginning. This was added to water received from any irrigation and rainfall events using the soil water balance Equation (1).
ET = P + SWs − SWh − R − D
Transpiration, which does not include soil evaporation, was calculated using the soil water balance Equation (2).
T = P + SWs − SWh − R − D − E
In both Equations (1) and (2) P, R and D are cumulative rainfall, runoff and deep drainage from the day of sowing to harvesting and SWs, SWh are soil water at the date of sowing and harvest. In Equation (2), E is the cumulative soil evaporation from the day of sowing to harvesting.
All variables in Equations (1) and (2) are in units of millimetres.
Water use efficiency (WUEgr), Equations (3) and (4), for grain yield, is defined as grain yield per unit of water (kg ha−1 mm−1) lost by evapotranspiration (ET) or transpiration (T).
WUEgr ET = GR/ET forET
WUEgr T = GR/ET for T
Similarly, water use efficiency (WUEdm), Equations (5) and (6), for dry matter (biomass accumulation) is defined as dry matter per unit of water (kg ha−1 mm−1) kg, ha−1) lost by ET or T.
WUEdm ET = DM/ET for ET
WUEdm T = DM/ET for T
2.3. Statistical Analysis
For each of the eight variables: ET (mm), T (mm), grain yield (GR) (kg ha−1), above-ground dry matter (DM) (kg ha−1), WUEET,gr, WUET,gr, WUEET,dm and WUET,dm, a linear mixed model was fitted using R software v4.1.3 running under the RStudio IDE [37]. Cultivar and sowing time, and the interaction between them, were considered as fixed factors. Site and year were considered as random factors. The goodness of fit was measured using the conditional R2 proposed by [38], which considers the effects of both fixed and random factors. Nipper and SD1 were used as references for comparison of genotypes and sowing dates.
The following packages in R were used to generate the outputs presented: lme4 [39] for fitting mixed models; lmerTest [40] for generating the p-values under the mixed models; sjstats [41] for the conditional R2; emmeans [42], multcomp and multcompView (multcompView: Visualizations of Paired Comparisons version 0.1–8 from CRAN (rdrr.io)) [43] for the multiple pairwise comparisons and the compact display of significance. The overall means across sowing time and cultivars within each variable were compared using Tukey’s post-hoc tests at a family-wise error rate of 5%.
3. Results
3.1. Summary of Overall Statistical Results
Interaction effects between cultivar and sowing time were statistically insignificant in all the models considered and were thus removed from the models. The estimated coefficients and model summaries are reported in Table 4. The linear mixed models were able to explain a large proportion of the variances of the response variables considered, with conditional R2 values ranging from 0.776 to 0.909 (Table 4). In all models, statistically significant differences were found among cultivars and sowing times. The estimated means under each combination of cultivar and sowing time are reported in Table 5, Table 6 and Table 7.

Table 4.
Estimates and F-ratios of fixed factors, variances of random factors, and conditional R2 of mixed models for each variable. Values in parentheses indicate p-values. Ref = the reference level for that factor against which the other levels of that factor are tested.

Table 5.
Calculated values of grain yield (GR) and dry matter (biomass) accumulation (DM) from the fitted linear mixed models. Different letters indicate significantly different values. The values for the genotypes at each sowing date (SD) are the means of three replicates.

Table 6.
Calculated values (mm) of evapotranspiration (ET) and transpiration (T) from the fitted linear mixed models. Different letters indicate significantly different values. The values for the genotypes at each sowing date (SD) are the means of three replicates.

Table 7.
Calculated values of water use efficiencies for grain yield and biomass accumulation from the fitted linear mixed models. Different letters indicate significantly different values. The values for the genotypes at each sowing date (SD) are the means of three replicates.
3.2. Dry Matter and Grain Yield
Statistically significant (p < 0.001) genotypic and sowing time effects were observed for dry matter production (Table 5). PBA Ace accumulated the most above-ground biomass at a value of 4210 kg ha−1 while PBA Blitz accumulated the least (3396 kg ha−1) (Table 5). The above-ground biomass for PBA Ace was significantly higher than those for PBA Blitz, PBA Greenfield, PBA Hurricane and PBA Jumbo2 (Table 5). Total above ground biomass, when averaged over all varieties, progressively decreased as the time of sowing was delayed from mid-April to end of May (SD1 > SD2 > SD3 > SD4; Table 5). The mean accumulated biomasses among different sowing times were significantly different, with values of 4532 kg ha−1 and 2849 kg ha−1 at SD1 and SD4, respectively. Statistically significant (p < 0.001) genotypic and sowing time effects were also observed for grain yield (Table 5). PBA Bolt was the highest yielding variety (1205 kg ha−1), and PBA Greenfield (745 kg ha−1) was the lowest yielding one (Table 5). The grain yields obtained for PBA Bolt were similar to the other six genotypes. Early (SD1) and late sowing (SD4) resulted in grain yield penalties, with the SD2 and SD3 showing significantly higher yield (Table 5).
3.3. Water Use
Application of irrigation influenced total crop water use measured as ET and T (Table 6). Water use decreased as sowing was delayed. ET differed between sowing dates, with total crop water use varying from 234 mm (SD4) to 261 mm (SD1) (Table 6). PBA Greenfield used 255 mm, statistically more water than PBA Blitz, PBA Bolt, PBA Hurricane and PBA Jumbo2 with water use ranging between 244 to 246 mm (Table 6). When water use was measured as T, there were no genotypic differences observed. However, it ranged from 146 mm (SD4) to 173 mm (SD1), with SD3 and SD4 statistically similar (Table 6).
The water use efficiency benchmarking approach of [44] was generated (Figure 2a,b) for lentils. The graph shows the relationship between water use measured as ET with grain yield and dry matter. The French and Schultz approach is a rainfall-based benchmark, and they proposed a rule of soil evaporation as 60 per cent of the seasonal rainfall but it does not account for the timing of rainfall. In pulse crops, deficit rainfall or soil water deficit during the reproductive stage can cause large reductions in yield and water use efficiency [45]. In our lentil experiments, there was large season-to-season variation in soil evaporation and rainfall and early sowing resulted in more dry matter accumulation and higher water use (Table 5 and Table 6). However, higher or lesser water use did not necessarily translate to higher grain yield. Greatest grain yield was obtained at intermediate sowing dates, when intermediate amounts of water were consumed.
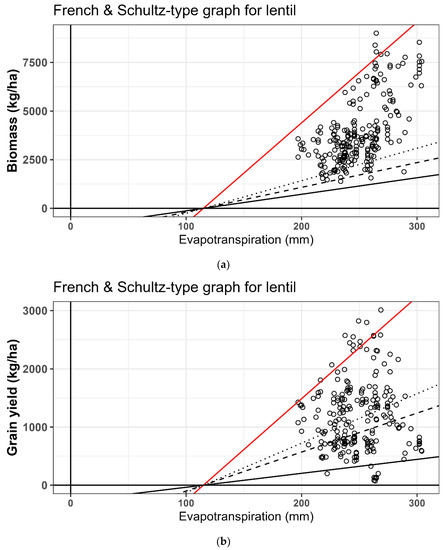
Figure 2.
(a,b). French and Schultz [44] type graph showing the relationship between total water use during the season and biomass accumulation and grain yield. The x-intercepts (115 mm) are based on [17]. Slopes of the three drawn lines are based on values provided in [17,20] (top to bottom lines: maximum, mean and minimum WUE). The red lines were fitted by eye as estimated upper boundaries of this data, and to contrast them with other published values.
3.4. Water Use Efficiency
For grain yield, PBA Greenfield had the lowest WUET and WUEET, which were significantly different from all other genotypes except PBA Blitz (Table 7). WUEET ranged from 2.97 kg grain ha−1 mm−1 (PBA Greenfield) to 4.99 kg grain ha−1 mm−1 (PBA Bolt). Correspondingly, WUET ranged from 4.77 kg grain ha−1 mm−1 (PBA Greenfield) to 7.93 kg grain ha−1 mm−1 (PBA Bolt). For grain yield, SD3 resulted in the highest WUE (WUET = 8.20 kg grain ha−1 mm−1 and WUEET = 5.04 kg grain ha−1 mm−1), while early sowing (SD1) had the lowest WUE (WUET = 5.28 kg grain ha−1 mm−1 and WUEET = 3.51 kg grain ha−1 mm−1 (Table 7). For grain yield, SD2 and SD3 resulted in the highest WUE, but SD2 was also not significantly different from SD4. The trend for WUE corresponds and aligns to overall grain yield observation where low yielding factors (SD1) and genotypes (PBA Greenfield) also have overall low WUE (Table 5 and Table 7).
PBA Ace accumulated the most biomass, with PBA Greenfield and PBA Blitz accumulating the least. Correspondingly, PBA Greenfield had the lowest WUEETdm for biomass accumulation (13.4 kg dm ha−1 mm−1), but it was not significantly different from PBA Blitz. PBA Ace (16.8 kg dm ha−1 mm−1), which was not significantly different from Nipper, had the highest dry matter WUE (Table 7). PBA Hallmark, PBA Bolt, PBA Blitz, Nipper, PBA Hurricane and PBA Jumbo2 had similar WUE for biomass accumulation. Similarly, PBA Greenfield had the lowest WUETdm for biomass accumulation (21.1 kg dm ha−1 mm−1), Still, it was not significantly different from PBA Blitz. In contrast, PBA Ace (26.3 kg dm ha−1 mm−1), which was not significantly different from Nipper, PBA Hurricane, PBA Hallmark and PBA Bolt, had the highest WUE for biomass.
More biomass was accumulated due to early sowing (Table 5 and Table 7) and gradually decreased as sowing was delayed. Correspondingly, early sowing (SD1 resulted in the highest WUE for biomass (WUETdm = 25.7 kg dm ha−1 mm−1; WUEEtdm = 17.1 kg dm ha−1 mm−1), which decreased as sowing was delayed. However, SD1 was not statistically significant from SD2. SD4 had the lowest WUE (WUETdm = 19.7 kg dm ha−1 mm−1; WUEEtdm = 12.2 kg dm ha−1 mm−1).
4. Discussion
The growing season environments at the diverse experimental locations differed in climatic conditions. Temperature, rainfall, evapotranspiration (ET), runoff and drainage are important factors for crop growth, and all determine the soil water holding capacity and the amount of water available to the crop. The two years of study were classified as dry and received rainfall that was below the long-term average (Supplementary Figure S1). The long-term average growing season rainfall at Trangie is 200 mm, but only 137 mm and 45 mm was received in 2018 and 2019, respectively. At Wagga Wagga the long-term rainfall is 322 mm, but only 153 mm and 193 mm was received in 2018 and 2019, respectively. At Leeton and Yanco the long-term rainfall is 193 mm, but 87 mm and 160 mm was received in 2018 and 2019, respectively. The sites differ in soil characteristics, affecting water holding capacity and root perforations. In general, sandy soils have poor holding capacity though encouraging adventurous rooting behaviour. Under moisture stress, the crops are forced to develop deeper roots searching for soil water and nutrients. On average, lentils extract soil moisture to a depth of 80 cm [8]. However, it has been observed that irrespective of soil type, grain yield appears not to be linked with available soil moisture at sowing, as crops grown under low starting soil water maintained their yield potential and had high water-use efficiency, but in contrast, higher available soil water at sowing did not necessarily translate to higher yields [19].
The loss of water due to evaporation ranges from 30 to 170 mm in diverse soils [44]. The exact amount of water lost depends on rainfall distribution, crop species and canopy type as well as soil type and surface residue. In this study, the French and Schultz approach [44], a rainfall-based benchmark of crop productivity, was adopted for lentil grain yield and biomass accumulation. Due to large season-to-season variation in soil evaporation and rainfall in the diverse experiments, differences in yield and biomass were observed, and were attributed to the effects of both genotypes and sowing dates. Commercially available lentil cultivars have variable architecture and growth habits which range from erect to prostrate, but largely have thin open canopies, which would allow for more soil evaporation. There is bound to be more evaporation in plots of late maturing varieties as evaporation would happen over a more protracted period. In addition, late varieties would mature at the onset of summer when temperatures are conducive to greater soil evaporation. However, early sowing despite a longer growing season might limit soil evaporation because the longer vegetative phase would allow for biomass accumulation which shaded the soil, as was observed in this study. Early establishment, vigour, ground cover, and shading of soil surface have been shown to effectively reduced soil evaporation [15,16].
Lentils do not have a dominant tap root nor a more fibrous root system, consequently, they tend not to dry the soil and thus leave more residual moisture for subsequent crops [20]. The effective water use leads to differences in the pattern and depth of water extraction and overall crop water requirements at different growth phases. The relationship between water use and biomass or grain production is known and was extensively reported [20,44]. The amounts of water used (197–304 mm) measured in our study were consistent with other studies which showed that a fully irrigated lentil crop uses 332 mm in New Zealand [46], 194–278 mm in Nepal [47], 174–273 mm in Western Australia [17], 188–317 mm in Victoria and South Australia [19]. A multi-site/year study in Australia showed that lentil yields ranging from 400–2500 kg ha−1 are obtainable in areas with growing season rainfall of 118–229 mm [19]. The lentils in this study were not fully irrigated, potentially explaining the slightly lower amount of water use. Overall, to produce grain yields of 900–3000 kg ha−1, a lentil crop required between 100 and 450 mm of water [48,49], and 475 mm in South Australia for maximum yield (French 1978, quoted in [44]). The time of sowing and soil water content at sowing have both been noted to have a big impact on grain yield, with stored water being more efficient in producing biomass and grain yield than in-season rainfall because it is less prone to run off and/or evaporation loss as well as being prevented from going into the root system by the canopy itself [44]. The importance of season duration is demonstrated where the late-maturing genotype PBA Greenfield used the most water, while there was overlap between the mid/late maturing PBA Hallmark and other mid maturing varieties such as Nipper, PBA Ace and PBA Jumbo2. Equally, there was similarity in the amount of water used by the early maturing PBA Blitz, which used the least water, compared to other early and/or early early mid varieties such as PBA Bolt and PBA Hurricane, PBA Ace and PBA Jumbo2. Water use progressively decreased as sowing was delayed, with more water being used in SD1 which was accompanied by a longer growing season and bigger biomass. WUE for dry matter and grain yield for cultivars and at different sowing times mirrored these respective traits.
The WUE values obtained in this study are comparable to others reported in literature with ranges of 1.9–8.5 kg grain ha−1 mm−1 for WUEgr and 2.4–16.7 kg dry matter ha−1 mm−1 for WUEdm [17,19,20]. However, the differences across studies reflect the differences in environments due to different soil types, climate and soil moisture. Moreover, rapid growth, as was observed in early sowing, could limit the loss of water through evaporation and thus change the total water use and the calculated WUE. Most studies assume there is no drainage or runoff, but these were calculated in this study, possibly accounting for differences between studies.
Late maturing PBA Greenfield had low WUE efficiency regarding biomass accumulation and grain yield, but the maturity class was not the sole driver, as it had similar biomass WUE to the early variety PBA Blitz. WUE might also be partly confounded by possible differences in varietal tolerance to acidic soils in NSW. It has been suggested that crop WUE tends to be higher under drought stress and reduced water supply and in conditions characterised by dry soils and high air temperatures [11,50,51]. However, in chickpea, higher WUE efficiency was observed under irrigated conditions [10]. The differences are a manifestation of the diversity of environments and/or management practices, as it has been observed that a range of management practices such as crop sequence [12], the intensity of tillage [10] and stubble retention [52] influence available soil water and WUE. Early sowing was observed (companion paper) to favour a longer vegetative period and greater biomass accumulation, which is reflected by higher biomass WUE. However, this did not translate to higher WUE for grain yield. This could partly be due to the ‘haying off’ effect, where plants use most of the water to produce biomass and thus suffer moisture limitations during the grain filling phase [22]. This reflects a disconnection between an abundantly available source (biomass) not being converted to sinks (grain). This may also suggest that most of the water is extracted during the vegetative phase, favouring biomass accumulation, with little left to be extracted during grain filling. Crop response to moisture stress is very complex [53], and the impact depends on the crop growth phase. During the vegetative phase it reduces crop growth, biomass accumulation and transpiration, resulting in low leaf expansion, early leaf senescence and a shorter growing season duration [54]. However, if water stress occurs at the reproductive phase, yield and quality penalties are suffered, resulting in small and shriveled grains, with altered chemical composition [55].
An effect of sowing date on WUE efficiency has been reported in other legumes such as chickpea, where delaying sowing decreased WUE by up to 80% for seed yield [25], and this study reports the importance of sowing time on managing water and water parameters in lentils. Here we demonstrated the link between ET and WUE in lentils in different southern and western NSW environments and the importance of starting soil water content, season duration and crop maturity class. However, the 2018 and 2019 seasons in southern and central western NSW were atypical and lentil crops experienced low autumn and winter rainfall, below average seasonal rainfall, and low temperatures early in the season. Despite these obstacles, our results show that water-use efficiency in lentils can be significantly improved by management practices, such as cultivar choice, and optimal sowing time, to ensure sufficient biomass accumulation and its efficient partitioning into grain yield.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12071542/s1, Figure S1: Climatic conditions at the experimental locations in 2018 and 2019.
Author Contributions
L.M., M.R.A. and M.F.R. conceived and conceptualised the topic. M.F.R. acquired funding and oversaw the whole study. L.M. and M.F.R. collected the field data. M.R.A., D.J.L., R.H.L.I. and Y.S.C. did the data modelling and statistical analysis. All authors contributed to writing, editing, revision and approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was a joint investment between the NSW Department of Primary Industries and Grains Research and Development Corporation (GRDC) under the Grains Agronomy and Pathology Partnership (GAPP), with project title, ‘The adaptation of profitable pulses in the central and southern zones of the northern grains region’. Project code: BLG112, March 2018 to June 2020, awarded to MFR.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Tony Napier, Leigh Jenkins and their technical staff at the Yanco Agricultural Institute and Trangie Agricultural Research Centre respectively, for their assistance in conducting and maintaining experiments. Also, thank you to Neroli Graham, Andrew Carmichael and Tania Moore for providing useful comments and edits.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Srinivasan, V.; Lambin, E.F.; Gorelick, S.M.; Thompson, B.H.; Rozelle, S. The nature and causes of the global water crisis: Syndromes from a meta-analysis of coupled human-water studies. Water Resour. Res. 2012, 48, 1–16. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving water use in crop production. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Drought resistance, water-use efficiency, and yield potential: Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L. Adaptation strategies for agricultural water management under climate change in Europe. Agric. Water Manag. 2015, 155, 113–124. [Google Scholar] [CrossRef]
- Molden, D.; Murray-Rust, H.; Sakthivadivel, R.; Makin, I. A Water-productivity Framework for Understanding and Action. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; Kijne, J.W., Barker, R., Molden, D., Eds.; Comprehensive Assessment of Water Management in Agriculture Series 1; CABI: Wallingford, UK; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2003; pp. 1–18. [Google Scholar]
- Bramley, H.; Turner, N.; Siddique, K. Water Use Efficiency. In Genomics and Breeding for Climate-Resilient Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 225–268. [Google Scholar]
- Condon, A.G. Drying times: Plant traits to improve crop water use efficiency and yield. J. Exp. Bot. 2020, 71, 2239–2252. [Google Scholar] [CrossRef]
- Oweis, T.; Hachum, A.; Pala, M. Lentil production under supplemental irrigation in a Mediterranean environment. Agric. Water Manag. 2004, 68, 251–265. [Google Scholar] [CrossRef]
- Sharma, B.; Molden, D.; Cook, S. Water use efficiency in agriculture: Measurement, current situation and trends. In Managing Water and Fertiliser for Sustainable Intensification; Drechsel, P., Heffer, P., Magan, H., Mikkelsen, R., Wichlens, D., Eds.; International Fertiliser Association: Paris, France, 2015; pp. 39–64. [Google Scholar]
- Kaloki, P.; Trethowan, R.; Tan, D.K.Y. Genetic and environmental influences on chickpea water-use efficiency. J. Agron. Crop. Sci. 2019, 205, 470–476. [Google Scholar] [CrossRef]
- Mbava, N.; Mutema, M.; Zengeni, R.; Shimelis, H.A.; Chaplot, V. Factors affecting crop water use efficiency: A worldwide meta-analysis. Agric. Water Manag. 2020, 228, 105878. [Google Scholar] [CrossRef]
- Merrill, S.D.; Tanaka, D.L.; Krupinsky, J.M.; Liebig, M.A.; Hanson, J.D. Soil water depletion and recharge under ten crop species and applications to the principles of dynamic cropping systems. J. Agron. 2007, 99, 931–938. [Google Scholar] [CrossRef]
- Turner, N.C.; Asseng, S. Productivity, sustainability, and rainfall-use efficiency in Australian rainfed Mediterranean agricultural systems. Aust. J. Agric. Res. 2005, 56, 1123–1136. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Ul-Allah, S.; Siddique, K.H.M. Physiological and agronomic approaches for improving water-use efficiency in crop plants. Agric. Water Manag. 2019, 219, 95–108. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Richards, R.A. Genetic improvement of early vigour in wheat. Aust. J. Agric. Res. 1999, 50, 291–302. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Regan, K.L.; Tennant, D.; Thomson, B.D. Water use and water use efficiency of cool season grain legumes in low rainfall Mediterranean-type environments. Eur. J. Agron. 2001, 15, 267–280. [Google Scholar] [CrossRef]
- Armstrong, R.; Fitzpatrick, J.; Rab, A.; Abuzar, M.; Fisher, P.; O’Leary, G. Advances in precision agriculture in south-eastern Australia. III. Interactions between soil properties and water use help explain spatial variability of crop production in the Victorian Mallee. Crop Pasture Sci. 2009, 60, 870–884. [Google Scholar] [CrossRef]
- Nuttall, J.G.; Armstrong, R.D. Impact of subsoil physicochemical constraints on crops grown in the Wimmera and Mallee is reduced during dry seasonal conditions. Soil Res. 2010, 48, 125–139. [Google Scholar] [CrossRef]
- Zhang, H.; Pala, M.; Oweis, T.; Harris, H. Water use and water-use efficiency of chickpea and lentil in a Mediterranean environment. Aust. J. Agric. Res. 2000, 51, 295–304. [Google Scholar] [CrossRef]
- Rachaputi, R.C.N.; Sands, D.; McKenzie, K.; Lehane, J.; Agius, P.; Seyoum, S.; Peak, A. Water extraction patterns of mungbean (Vigna radiata) in diverse subtropical environments. Agric. Water Manag. 2019, 219, 109–116. [Google Scholar] [CrossRef]
- van Herwaarden, A.F.; Farquhar, G.D.; Angus, J.F.; Richards, R.A.; Howe, G.N. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser. I. Biomass, grain yield, and water use. Aust. J. Agric. Res. 1998, 49, 1067–1082. [Google Scholar] [CrossRef]
- Richards, M.F.; Maphosa, L.; Preston, A.L. Impact of sowing time on Chickpea (Cicer arietinum L.) biomass accumulation and yield. Agronomy 2022, 12, 160. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.R.; McKenzie, B.A.; Hill, G.D. Water-use efficiency and the effect of water deficits on crop growth and yield of Kabuli chickpea (Cicer arietinum L.) in a cool-temperate subhumid climate. J. Agric. Sci. 2003, 141, 285–301. [Google Scholar] [CrossRef]
- Sadras, V.O.; Rosewarne, G.M.; Lake, L. Australian lentil breeding between 1988 and 2019 has delivered greater yield gain under stress than under high-yield conditions. Front. Plant Sci. 2021, 830, 1–10. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. 2022. Available online: https://www.fao.org/faostat/en/#search/lentil (accessed on 20 June 2022).
- Richards, M.F.; Preston, A.L.; Napier, T.; Jenkins, L.; Maphosa, L. Sowing date affects the timing and duration of key Chickpea (Cicer arietinum L.) growth phases. Plants 2020, 9, 1257. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.J.; Carter, J.O.; Moodie, K.B.; Beswick, A.R. Using spatial interpolation to construct a comprehensive archive of Australian climate data. Environ. Model. Softw. 2001, 16, 309–330. [Google Scholar] [CrossRef]
- Zhang, L.; Walker, G.R.; Dawes, W.R. Water balance modelling: Concepts and applications. In Regional Water and Soil Assessment for Managing Sustainable Agriculture in China and Australia, ACIAR Monograph; McVicar, T.R., Rui, L., Walker, J., Fitzpatrick, R.W., Changming, L., Eds.; Australian Centre for International Agricultural Research (ACIAR): Canberra, Australia, 2002; Volume 84, pp. 31–47. [Google Scholar]
- Eitzinger, J.; Štastná, M.; Žalud, Z.; Dubrovský, M. A simulation study of the effect of soil water balance and water stress on winter wheat production under different climate change scenarios. Agric. Water Manag. 2003, 61, 195–217. [Google Scholar] [CrossRef]
- Hochman, Z.; Gobbett, D.; Holzworth, D.; McClelland, T.; van Rees, H.; Marinoni, O.; Garcia, J.N.; Horan, H. Quantifying yield gaps in rainfed cropping systems: A case study of wheat in Australia. Field Crops Res. 2012, 136, 85–96. [Google Scholar] [CrossRef]
- Anwar, M.R.; Chauhan, Y.S.; Luckett, D.J.; Ip, R.H.L.; Maphosa, L.; Simpson, M.; Raman, R.; Richards, M.F.; Preston, A.; Pengilley, G.; et al. Manipulating flowering time in chickpeas to minimise frost risk, water and heat stress. In Proceedings of the 20th Australian Agronomy Conference. Australian Society for Agronomy, Toowoomba, Australia, 18–22 September 2022; pp. 1–4. [Google Scholar]
- Anwar, M.R.; Luckett, D.J.; Chauhan, Y.S.; Ip, R.H.L.; Maphosa, L.; Simpson, M.; Warren, A.; Raman, R.; Richards, M.F.; Pengilley, G.; et al. Modelling the effects of cold temperature during the reproductive stage on the yield of chickpea (Cicer arietinum L.). Int. J. Biometeorol. 2022, 66, 111–125. [Google Scholar] [CrossRef]
- He, D.; Wang, E. On the relation between soil water holding capacity and dryland crop productivity. Geoderma 2019, 353, 11–24. [Google Scholar] [CrossRef]
- Holzworth, D.P.; Huth, N.I.; deVoil, P.G.; Zurcher, E.J.; Herrmann, N.I.; McLean, G.; Chenu, K.; van Oosterom, E.J.; Snow, V.; Murphy, C.; et al. APSIM—Evolution towards a new generation of agricultural systems simulation. Environ. Model Softw. 2014, 62, 327–350. [Google Scholar] [CrossRef]
- RStudio, Integrated Development for R. RStudio, 2019, PBC, Boston. Available online: http://www.rstudio.com (accessed on 30 January 2022).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lüdecke, D. sjstats: Statistical Functions for Regression Models, Version 0.17.2; Zenodo: Genève, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lenth, R. Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.4.2. 2019. Available online: https://CRAN.Rproject.org/package=emmeans (accessed on 30 January 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- French, R.J.; Schultz, J.E. Water use efficiency of wheat in a Mediterranean-type environment. I. The relation between yield, water use and climate. Aust. J. Agric. Res. 1984, 35, 743–764. [Google Scholar] [CrossRef]
- French, R.J. The risk of vegetative water deficit in early-sown faba bean (Vicia faba L.) and its implications for crop productivity in a Mediterranean-type environment. Crop Pasture Sci. 2010, 61, 566–577. [Google Scholar] [CrossRef]
- McKenzie, B.A.; Hill, G.D. Growth, yield and water use of lentils (Lens culinaris) in Canterbury, New Zealand. J. Agric. Sci. 1990, 114, 309–320. [Google Scholar] [CrossRef]
- Shrestha, R.; Siddique, K.; Turner, N.; Turner, D.; Berger, J. Growth and seed yield of lentil (Lens culinaris Medikus) genotypes of West Asian and South Asian origin and crossbreds between the two under rainfed conditions in Nepal. Aust. J. Agric. Res. 2005, 56, 971–981. [Google Scholar] [CrossRef]
- Prasad, S.N.; Singh, R.; Rao, D.H. Effect of pre-sowing irrigation and soil mulch on yield attributes, yield and wateruse of chickpea (Cicer arietinum), linseed (Linum usitatissimum) and Indian mustard (Brassica juncea). Indian J. Agric. Sci. 2013, 69, 6. [Google Scholar]
- Strong, W.M.; Dalal, R.C.; Cooper, J.E.; Doughton, J.A.; Weston, E.J.; McNamara, G.T. Sustaining productivity of a Vertisol at Warra, Queensland, with fertilisers, no-tillage or legumes 4. Nitrogen fixation, water use and yield of chickpea. Aust. J. Exp. Agric. 1997, 37, 667–676. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crops Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Chibarabada, T.P.; Modi, A.T.; Mabhaudhi, T. Water use characteristics of a bambara groundnut (Vigna subterranea L. Verdc) landrace during seedling establishment. Water SA 2015, 41, 472–482. [Google Scholar] [CrossRef]
- Van Duivenbooden, N.; Pala, M.; Studer, C.; Bielders, C.L.; Beukes, D.J. Cropping systems and crop complementarity in dryland agriculture to increase soil water use efficiency: A review. NJAS Wagening. J. Life Sci. 2000, 48, 213–236. [Google Scholar] [CrossRef]
- Chenu, K.; Deihimfard, R.; Chapman, S.C. Large-scale characterization of drought pattern: A continent-wide modelling approach applied to the Australian wheatbelt--spatial and temporal trends. New Phytol. 2013, 198, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 37–61. [Google Scholar]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).