Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
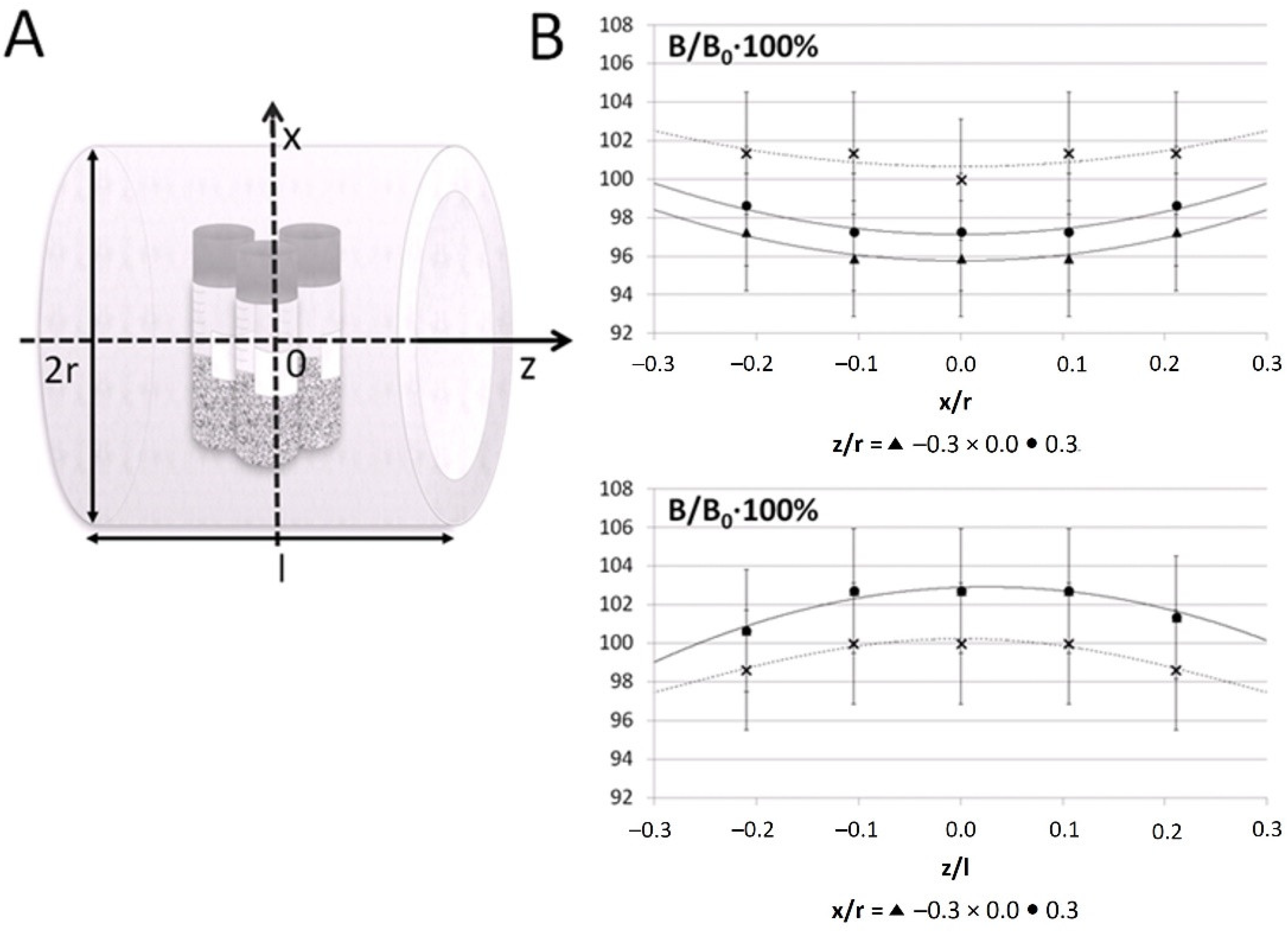
2.2. Exposure to Electromagnetic Field
2.3. Germination Assay
GI = (|([Tx + 1] − T1) × N1| + |([Tx + 1] − T2) × N2| +…+ |([Tx + 1] − Tx) × Nx|)/S)
CVG = 100 [(N1 + N2 +...+ Nx)/(N1T1 + N2T2 +…+ NxTx)]
t50 = Ti + [(([N + 1]/2 − Ni) × (Tj − Ti))/Nj − Ni]
2.4. Determination of Phytohormones
2.4.1. Sample Extraction
2.4.2. Sample Purification
2.4.3. Sample Analysis
2.5. Statistical Analysis
3. Results
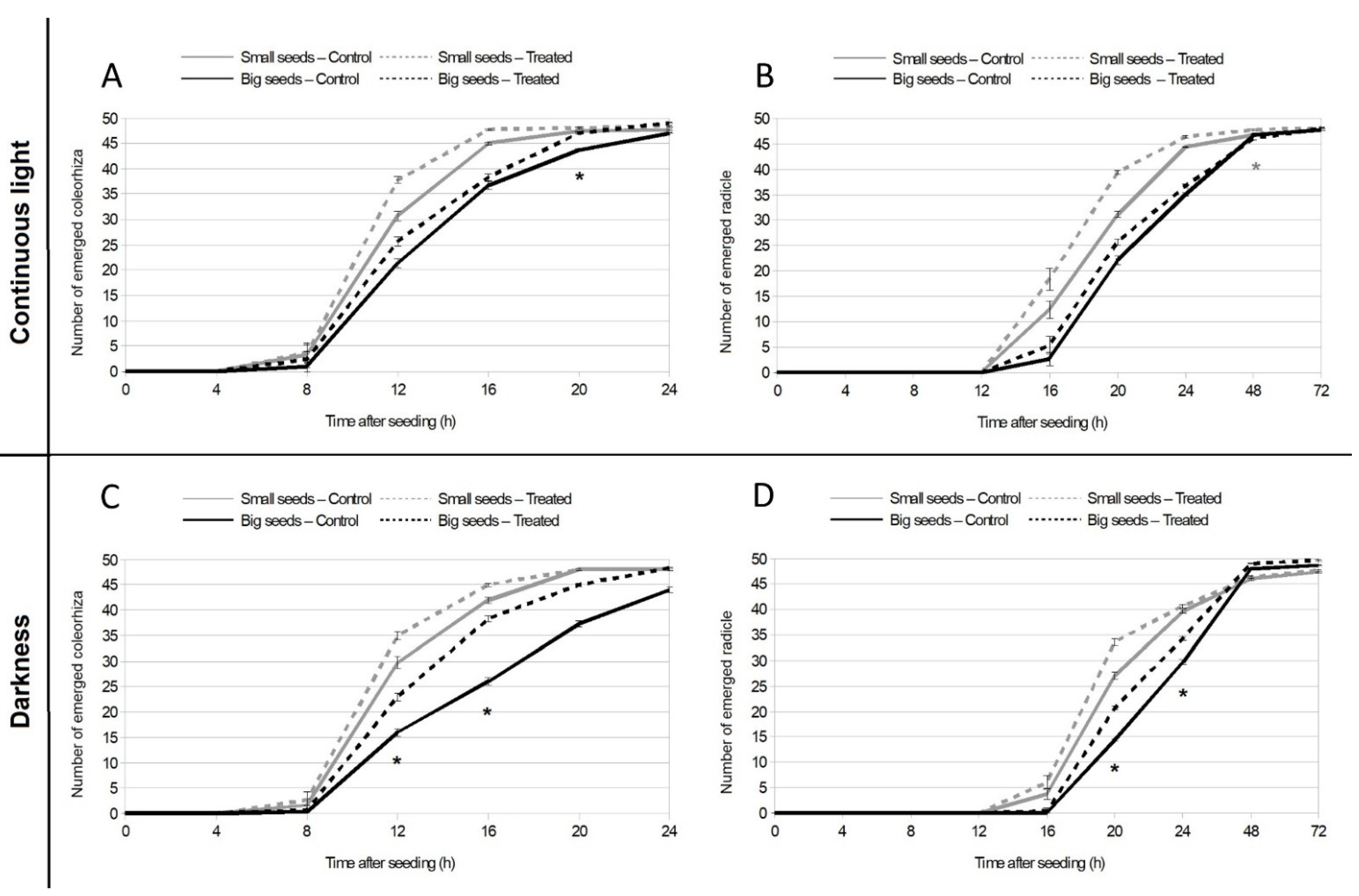
3.1. EMF Effect on Germination under Continuous Light and Continuous Dark Conditions
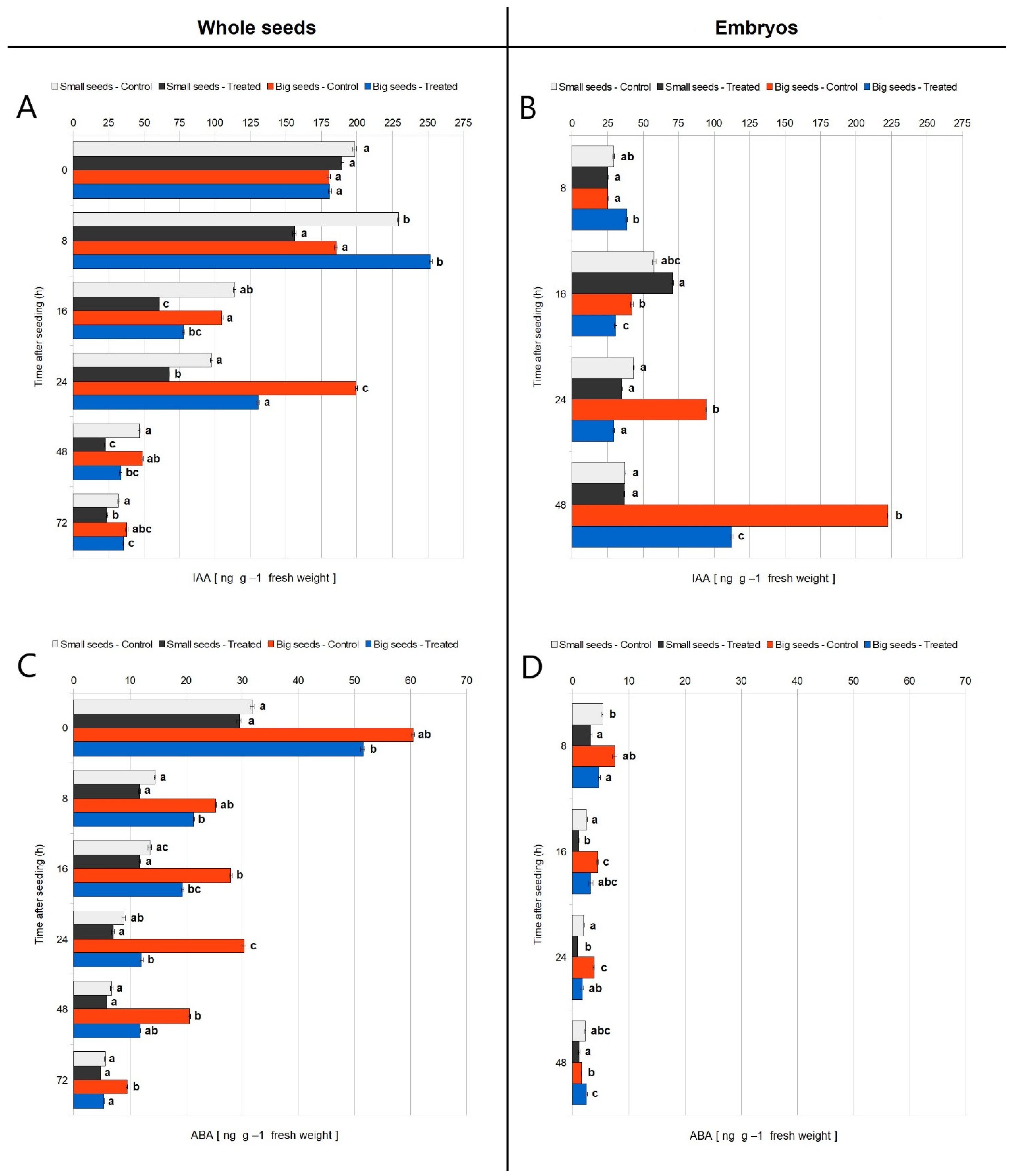
3.2. IAA and ABA Concentration in Germinating Small and Big Seeds, in Control Conditions, and after EMF Treatment
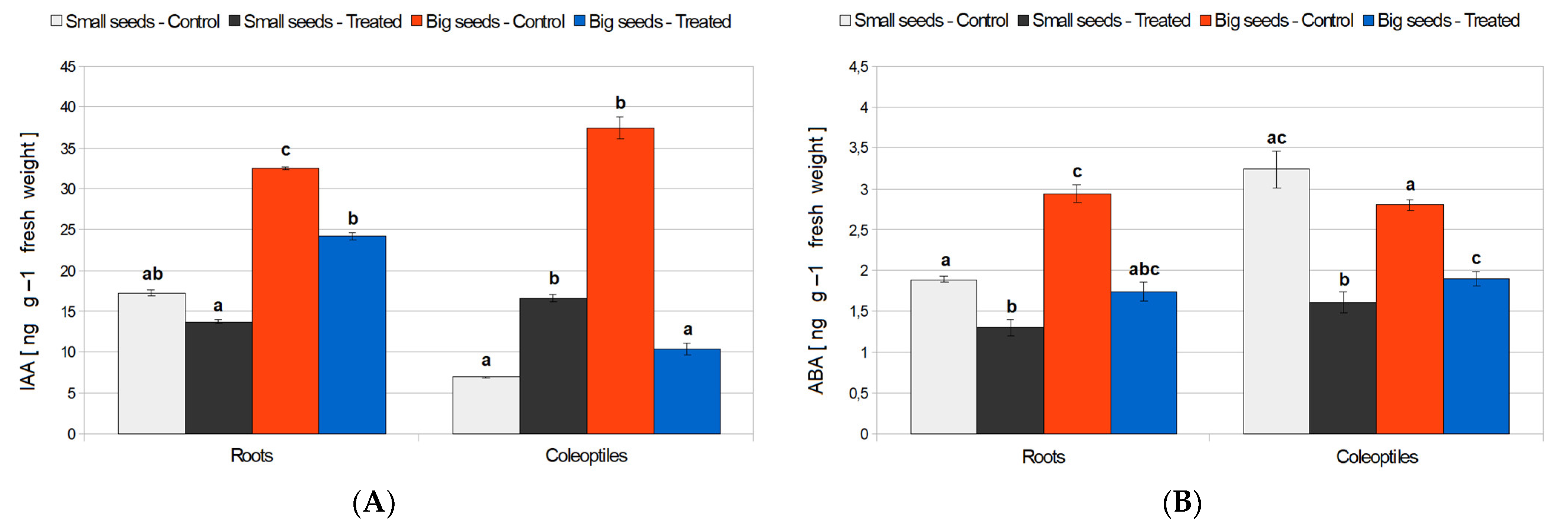
3.3. Seedling Growth Parameters and Concentration of IAA and ABA in Young Organs of Wheat, in Control, and Treatment Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, L.; Ma, M.; Wu, L.; Zhou, M.; Li, M.; Wu, B.; Li, L.; Liu, X.; Jing, R.; Chen, W.; et al. Modified expression of TaCYP78A5 enhances grain weight with yield potential by accumulating auxin in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2021, 20, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, V. Control of seed size in plants. Proc. Natl. Acad. Sci. USA 2005, 102, 17887–17888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, G. Molecular prospective on the wheat grain development. Crit. Rev. Biotechnol. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zareian, A.; Hamidi, A.; Sadeghi, H.; Jazaeri, M.R. Effect of Seed Size on Some Germination Characteristics, Seedling Emergence Percentage and Yield of Three Wheat (Triticum Aestivum L.) Cultivars in Laboratory and Field. Middle-East J. Sci. Res. 2013, 13, 1126–1131. [Google Scholar] [CrossRef]
- Bera, K.; Dutta, P.; Sadhukhan, S. Seed priming with non-ionizing physical agents: Plant responses and underlying physiological mechanisms. Plant Cell Rep. 2021, 41, 53–73. [Google Scholar] [CrossRef]
- Ramesh, B.; Kavitha, G.; Gokiladevi, S.; Balachandar, R.K.; Kavitha, K.; Gengadharan, A.C.; Puvanakrishnan, R. Effect of Extremely Low Power Time-Varying Electromagnetic Field on Germination and Other Characteristics in Foxtail Millet (Setaria italica) Seeds. Bioelectromagnetics 2020, 41, 526–539. [Google Scholar] [CrossRef]
- Shabrangy, A.; Ghatak, A.; Zhang, S.; Priller, A.; Chaturvedi, P.; Weckwerth, W. Magnetic Field Induced Changes in the Shoot and Root Proteome of Barley (Hordeum vulgare L.). Front. Plant Sci. 2021, 12, 622795. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of Plasma-Seed Treatments as a Potential Seed Processing Technology. Front. Phys. 2021, 9, 174. [Google Scholar] [CrossRef]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, C.H.; Dominguez-Pacheco, A.; Carballo, A.; Cruz-Orea, A.; Ivanov, R.; Luis, J.; Pastor, J. Alternating Magnetic Field Irradiation Effects on Three Genotype Maize Seed Field Performance. Acta Agroph. 2009, 14, 7–17. [Google Scholar]
- Cakmak, T.; Dumlupinar, R.; Erdal, S. Acceleration of germination and early growth of wheat and bean seedlings grown under various magnetic field and osmotic conditions. Bioelectromagnetics 2010, 31, 120–129. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusià, J.; Martínez, B.; Fontcuberta, J. Diamagnetic Susceptibility and Root Growth Responses to Magnetic Fields in Lens culinaris, Glycine soja, and Triticum aestivum. Electromagn. Biol. Med. 2004, 23, 97–112. [Google Scholar] [CrossRef]
- Marks, N.; Szecowka, P.S. Impact of Variable Magnetic Field Stimulation on Growth of Aboveground Parts of Potato Plants. Int. Agrophys. 2010, 24, 165–170. [Google Scholar]
- Vashisth, A.; Joshi, D.K. Growth characteristics of maize seeds exposed to magnetic field: Effect of Magnetic Field on Maize Crop. Bioelectromagnetics 2017, 38, 151–157. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Karkanis, A.; Papastylianou, P.; Triantafyllidis, V.; Travlos, I.; Bilalis, D.J. Effects of Presowing Pulsed Electromagnetic Treatment of Tomato Seed on Growth, Yield, and Lycopene Content. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- De Souza, A.; García, D.; Sueiro, L.; Gilart, F. Improvement of the seed germination, growth and yield of onion plants by extremely low frequency non-uniform magnetic fields. Sci. Hortic. 2014, 176, 63–69. [Google Scholar] [CrossRef]
- Luo, X.; Li, D.; Tao, Y.; Wang, P.; Yang, R.; Han, Y. Effect of static magnetic field treatment on the germination of brown rice: Changes in α-amylase activity and structural and functional properties in starch. Food Chem. 2022, 383, 132392. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kumari, B.D.R. Pulsed magnetic field: A contemporary approach offers to enhance plant growth and yield of soybean. Plant Physiol. Biochem. 2012, 51, 139–144. [Google Scholar] [CrossRef]
- Florez, M.; Martinez, E.; Carbonell, V. Germination and initial growth of triticale seeds under stationary magnetic fields. J. Adv. Agric. 2014, 2, 72–79. [Google Scholar] [CrossRef]
- Konefał-Janocha, M.; Banaś-Ząbczyk, A.; Bester, M.; Bocak, D.; Budzik, S.; Górny, S.; Larsen, S.; Majchrowski, K.; Cholewa, M. The Effect of Stationary and Variable Electromagnetic Fields on the Germination and Early Growth of Radish (Raphanus sativus). Pol. J. Environ. Stud. 2018, 28, 709–715. [Google Scholar] [CrossRef]
- Pauzaite, G.; Malakauskienė, A.; Naučienė, Z.; Žūkienė, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildaziene, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1700068. [Google Scholar] [CrossRef]
- Podleśny, J.; Pietruszewski, S.; Podleśna, A. Efficiency of the Magnetic Treatment of Broad Bean Seeds Cultivated under Experimental Plot Conditions. Int. Agrophys. 2004, 18, 65–71. [Google Scholar]
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Jin, Y.; Guo, W.; Hu, X.; Liu, M.; Xu, X.; Hu, F.; Lan, Y.; Lv, C.; Fang, Y.; Liu, M.; et al. Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci. Rep. 2019, 9, 14384. [Google Scholar] [CrossRef] [Green Version]
- Pietruszewski, S.; Martínez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophys. 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Muszyński, S.; Dziwulska, A. Electromagnetic Fields and Electromagnetic Radiation as Non-Invasive External Stimulants for Seeds (Selected Methods and Responses). Int. Agrophys. 2007, 21, 95–100. [Google Scholar]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Wójcik-Gront, E.; Iwańska, M.; Wnuk, A.; Oleksiak, T. The Analysis of Wheat Yield Variability Based on Experimental Data from 2008–2018 to Understand the Yield Gap. Agriculture 2021, 12, 32. [Google Scholar] [CrossRef]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A meta-analysis of projected global food demand and population at risk of hunger for the period 2010–2050. Nat. Food 2021, 2, 494–501. [Google Scholar] [CrossRef]
- Pittman, U.J. Magnetism and plant growth: I. effect on germination and early growth of cereal seeds. Can. J. Plant Sci. 1963, 43, 513–518. [Google Scholar] [CrossRef]
- Fischer, G.; Tausz, M.; Köck, M.; Grill, D. Effects of weak 16 2/3 Hz magnetic fields on growth parameters of young sunflower and wheat seedlings. Bioelectromagnetics 2004, 25, 638–641. [Google Scholar] [CrossRef]
- Martinez, E.; Carbonell, M.V.; Flórez, M. Magnetic Biostimulation of Initial Growth Stages of Wheat (Triticum Aestivum, L.). Electromagn. Biol. Med. 2002, 21, 43–53. [Google Scholar] [CrossRef]
- Dannehl, D. Effects of electricity on plant responses. Sci. Hortic. 2018, 234, 382–392. [Google Scholar] [CrossRef]
- Bienkowski, P.; Wyszkowska, J. Technical aspects of exposure to magnetic fields of extremely low frequencies (elf) in biomedical research/ techniczne aspekty ekspozycji na pole magnetyczne ekstremalnie niskich czestotliwosci (elf) w badaniach biomedycznych. Med. Pract. 2015, 66, 185–198. [Google Scholar]
- Barampuram, S.; Allen, G.; Krasnyanski, S. Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 118, 179–185. [Google Scholar] [CrossRef]
- Ranal, M.A.; De Santana, D.G. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Scott, S.J.; Jones, R.A.; Williams, W.A. Review of Data Analysis Methods for Seed Germination1. Crop Sci. 1984, 24, 1192–1199. [Google Scholar] [CrossRef]
- Pu, C.-H.; Lin, S.-K.; Chuang, W.-C.; Shyu, T.-H. Modified QuEChERS method for 24 plant growth regulators in grapes using LC-MS/MS. J. Food Drug Anal. 2018, 26, 637–648. [Google Scholar] [CrossRef] [Green Version]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Barrero, J.M.; Talbot, M.J.; White, R.G.; Jacobsen, J.V.; Gubler, F. Anatomical and Transcriptomic Studies of the Coleorhiza Reveal the Importance of This Tissue in Regulating Dormancy in Barley. Plant Physiol. 2009, 150, 1006–1021. [Google Scholar] [CrossRef] [Green Version]
- Holloway, T.; Steinbrecher, T.; Pérez, M.; Seville, A.; Stock, D.; Nakabayashi, K.; Leubner-Metzger, G. Coleorhiza-enforced seed dormancy: A novel mechanism to control germination in grasses. New Phytol. 2021, 229, 2179–2191. [Google Scholar] [CrossRef]
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Shu, K.; Liu, X.-D.; Xie, Q.; He, Z.-H. Two Faces of One Seed: Hormonal Regulation of Dormancy and Germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Novitskii, Y.I.; Novitskaya, G.V.; Serdyukov, Y.A. Lipid utilization in radish seedlings as affected by weak horizontal extremely low frequency magnetic field. Bioelectromagnetics 2014, 35, 91–99. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.O.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Kornarzyński, K. Magnetic Biostimulation of Wheat Seeds. Int. Agrophys. 1999, 13, 497–501. [Google Scholar]
- Pietruszewski, S.; Kornarzyński, K.; Łacek, R. Germination of Wheat Grain in an Alternating Magnetic Field. Int. Agrophys. 2001, 15, 269–271. [Google Scholar]
- Aksyonov, S.I.; Bulychev, A.A.; Grunina, T.Y.; Goryachev, S.N.; Turovetsky, V.B. Effects of Elf-Emf Treatment on Wheat Seeds at Different Stages of Germination and Possible Mechanisms of Their Origin. Electro- Magn. 2001, 20, 231–253. [Google Scholar] [CrossRef]
- Payez, A.; Ghanati, F.; Behmanesh, M.; Abdolmaleki, P.; Hajnorouzi, A.; Rajabbeigi, E. Increase of seed germination, growth and membrane integrity of wheat seedlings by exposure to static and a 10-KHz electromagnetic field. Electromagn. Biol. Med. 2013, 32, 417–429. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Kania, K. Effect of Magnetic Field on Germination and Yield of Wheat. Int. Agrophys. 2010, 24, 297–302. [Google Scholar]
- Reina, F.G.; Pascual, L.A. Influence of a stationary magnetic field on water relations in lettuce seeds. Part I: Theoretical considerations. Bioelectromagnetics 2001, 22, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Matwijczuk, A.; Kornarzyński, K.; Pietruszewski, S. Effect of magnetic field on seed germination and seedling growth of sunflower. Int. Agrophys. 2012, 26, 271–278. [Google Scholar] [CrossRef]
- Rochalska, M.; Grabowska-Topczewska, K.; Mackiewicz, A. Influence of Alternating Low Frequency Magnetic Field on Improvement of Seed Quality. Int. Agrophys. 2011, 25, 265–269. [Google Scholar]
- Liu, X.D.; Zhang, H.; Zhao, Y.; Feng, Z.Y.; Li, Q.; Yang, H.-Q.; Luan, S.; Li, J.M.; He, Z.-H. Auxin controls seed dormancy through stimulation of abscisic acid signaling by inducing ARF-mediated ABI3 activation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 15485–15490. [Google Scholar] [CrossRef] [Green Version]
- Ramaih, S.; Guedira, M.; Paulsen, G.M. Relationship of indoleacetic acid and tryptophan to dormancy and preharvest sprouting of wheat. Funct. Plant Biol. 2003, 30, 939–945. [Google Scholar] [CrossRef]
- Matsuura, T.; Mori, I.C.; Himi, E.; Hirayama, T. Plant hormone profiling in developing seeds of common wheat (Triticum aestivum L.). Breed. Sci. 2019, 69, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Oracz, K.; Karpiński, S. Phytohormones Signaling Pathways and ROS Involvement in Seed Germination. Front. Plant Sci. 2016, 7, 864. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Guo, G.; Lv, D.; Hu, Y.; Li, J.; Li, X.; Yan, Y. Transcriptome analysis during seed germination of elite Chinese bread wheat cultivar Jimai 20. BMC Plant Biol. 2014, 14, 20. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef] [Green Version]
- Podleśny, J.; Podleśna, A.; Gładyszewska, B.; Bojarszczuk, J. Effect of Pre-Sowing Magnetic Field Treatment on Enzymes and Phytohormones in Pea (Pisum sativum L.) Seeds and Seedlings. Agronomy 2021, 11, 494. [Google Scholar] [CrossRef]
- Mildaziene, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common Sunflower (Helianthus annus L.) Seeds with Radio-frequency Electromagnetic Field and Cold Plasma Induces Changes in Seed Phytohormone Balance, Seedling Development and Leaf Protein Expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Mildaziene, V.; Ivankov, A.; Pauzaite, G.; Naucienė, Z.; Zukiene, R.; Degutyte-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Process. Polym. 2020, 18, 2000160. [Google Scholar] [CrossRef]


| Time after Seeding (h) | Coleorhiza Emergence—Continuous Light | Coleorhiza Emergence—Continuous Darkness | ||
|---|---|---|---|---|
| Small Seeds—Control | Big Seeds—Control | Small Seeds—Control | Big Seeds—Control | |
| 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 8 | 3 ± 1.83 a | 1 ± 1 a | 2 ± 1.29 a | 0 ± 0.58 a |
| 12 | 31 ± 0.97 a | 21 ± 0.92 a | 30 ± 1.18 a | 16 ± 0.75 a |
| 16 | 45 ± 0.31 a | 37 ± 0.81 ab | 42 ± 0.56 a | 26 ± 0.74 b |
| 20 | 47 ± 0.1 a | 44 ± 0.2 a | 48 ± 0.14 a | 37 ± 0.61 a |
| 24 | 48 ± 0.05 a | 47 ± 0.08 a | 48 ± 0.14 a | 44 ± 0.57 a |
| Time after Seeding (h) | Radicle Emergence—Continuous Light | Radicle Emergence—Continuous Darkness | ||
|---|---|---|---|---|
| Small Seeds—Control | Big Seeds—Control | Small Seeds—Control | Big Seeds—Control | |
| 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 12 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 16 | 12.33 ± 1.77 a | 2.67 ± 1.34 a | 3.67 ± 0.97 a | 0 ± 0 a |
| 20 | 31 ± 0.58 a | 22 ± 0.86 b | 27 ± 0.68 ab | 14.33 ±0.23 b |
| 24 | 44.33 ± 0.13 a | 35 ± 0.26 b | 39.67 ± 0.37 ab | 29.67 ± 0.43 c |
| 48 | 46.67 ± 0.13 a | 46.67 ± 0.13 a | 46 ± 0.23 a | 48 ± 0.22 a |
| 72 | 47.67 ± 0.17 a | 47.67 ± 0.13 a | 47.33 ± 0.13 a | 48.67 ± 0.13 a |
| Germination Parameters | Coleorhiza Emergence—Continuous Light | Coleorhiza Emergence—Continuous Darkness | ||||||
|---|---|---|---|---|---|---|---|---|
| Small Seeds—Control | Small Seeds—Treated | Big Seeds—Control | Big Seeds—Treated | Small Seeds—Control | Small Seeds—Treated | Big Seeds—Control | Big Seeds—Treated | |
| G (%) | 95.34 ± 0.05 a | 96.66 ± 0.17 a | 94 ± 0.08 a | 98 ± 0.08 a | 96 ± 0.14 a | 96 ± 0.22 a | 88 ± 0.57 a | 96.66 ± 0.13 a |
| GI (h) | 11.69 ± 0.25 a | 12.54 ± 0.18 a | 9.72 ± 0.27 b | 10.25 ± 0.27 c | 11.09 ± 0.25 a | 11.89 ± 0.19 a | 8.19 ± 0.15 b | 9.84 ± 0.2 c |
| CVG (%/h) | 7.58 ± 0.18 a | 8.07 ± 0.14 b | 6.58 ± 0.14 ac | 6.82 ± 0.15 c | 7.24 ± 0.16 ab | 7.67 ± 0.13 c | 5.96 ± 0.06 a | 6.62 ± 0.1 b |
| MGT (h) | 13.31 ± 0.23 a | 12.46 ± 0.18 a | 15.28 ± 0.22 b | 14.75 ± 0.22 c | 13.91 ± 0.23 a | 13.11 ± 0.18 a | 16.81 ± 0.1 b | 15.16 ± 0.16 c |
| t50 (h) | 11.09 ± 0.21 a | 10.34 ± 0.13 a | 12.87 ± 0.30 a | 12.33 ± 0.3 a | 11.62 ± 0.29 a | 10.77 ± 0.15 a | 14.67 ± 0.17 b | 12.57 ± 0.23 c |
| CVt (%) | 22.1 ± 0.19 ab | 18.99 ± 0.12 a | 25.52 ± 0.22 b | 25.8 ± 0.65 ab | 21.17 ± 0.26 a | 19.35 ± 0.74 ab | 27.86 ± 0.21 b | 25.57 ± 0.36 b |
| Germination Parameters | Radicle Emergence—Continuous Light | Radicle Emergence—Continuous Darkness | ||||||
|---|---|---|---|---|---|---|---|---|
| Small Seeds—Control | Small Seeds—Treated | Big Seeds—Control | Big Seeds—Treated | Small Seeds—Control | Small Seeds—Treated | Big Seeds—Control | Big Seeds—Treated | |
| G (%) | 95.34 ± 0.17 a | 96 ± 0.14 a | 95.34 ± 0.13 a | 96 ± 0.08 a | 94.66 ± 0.13 a | 95.34 ± 0.19 a | 97.34 ± 0.13 a | 99.34 ± 0.05 a |
| GI (h) | 50.49 ± 0.18 a | 52.77 ± 0.12 a | 44.19 ± 0.21 b | 44.95 ± 0.19 b | 46.99 ± 0.23 ac | 48.06 ± 0.26 a | 40.45 ± 0.2 b | 42.97 ± 0.21 c |
| CVG (%/h) | 4.47 ± 0.13 a | 4.96 ± 0.09 a | 3.49 ± 0.09 b | 3.58 ± 0.09 b | 3.87 ± 0.12 a | 4.05 ± 0.15 a | 3.08 ± 0.07 b | 3.34 ± 0.08 a |
| MGT (h) | 22.51 ± 0.27 a | 20.23 ± 0.19 a | 28.81 ± 0.26 b | 28.05 ± 0.24 b | 26.01 ± 0.31 ac | 24.94 ± 0.36 a | 32.55 ± 0.23 b | 30.03 ± 0.25 c |
| t50 (h) | 18.94 ± 0.19 ab | 16.64 ± 0.24 a | 20.47 ± 0.16 b | 19.90 ± 0.18 b | 19.60 ± 0.1 ab | 18.68 ± 0.1 a | 22.84 ± 0.07 c | 21.42 ± 0.12 bc |
| CVt (%) | 31.43 ± 0.65 a | 28.06 ± 0.95 a | 49.51 ± 0.23 a | 55.75 ± 0.77 a | 40.25 ± 0.16 a | 40.12 ± 0.32 ab | 44.58 ± 0.2 ab | 47.04 ± 0.12 b |
| Growth Parameters | Control | Treated | |
|---|---|---|---|
| Root length (mm) | Small seeds | 71.11 ± 1.45 ac | 71.19 ± 1.34 a |
| Big seeds | 63.94 ± 1.30 b | 68.32 ± 1.03 c | |
| Coleoptile length (mm) | Small seeds | 34.65 ± 0.65 a | 34.85 ± 0.73 b |
| Big seeds | 26.55 ± 0.82 c | 28.84 ± 0.73 d | |
| Fresh weight (mg) | Small seeds | 104.11 ± 1.63 a | 100.32 ± 1.44 b |
| Big seeds | 128.70 ± 1.78 c | 132.76 ± 1.59 c | |
| Dry weight (mg) | Small seeds | 24.83 ± 0.08 a | 24.06 ± 0.13 a |
| Big seeds | 42.61 ± 0.08 b | 41.66 ± 0.20 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecchetti, D.; Pawełek, A.; Wyszkowska, J.; Antoszewski, M.; Szmidt-Jaworska, A. Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size. Agronomy 2022, 12, 1423. https://doi.org/10.3390/agronomy12061423
Cecchetti D, Pawełek A, Wyszkowska J, Antoszewski M, Szmidt-Jaworska A. Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size. Agronomy. 2022; 12(6):1423. https://doi.org/10.3390/agronomy12061423
Chicago/Turabian StyleCecchetti, Daniele, Agnieszka Pawełek, Joanna Wyszkowska, Marcel Antoszewski, and Adriana Szmidt-Jaworska. 2022. "Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size" Agronomy 12, no. 6: 1423. https://doi.org/10.3390/agronomy12061423
APA StyleCecchetti, D., Pawełek, A., Wyszkowska, J., Antoszewski, M., & Szmidt-Jaworska, A. (2022). Treatment of Winter Wheat (Triticum aestivum L.) Seeds with Electromagnetic Field Influences Germination and Phytohormone Balance Depending on Seed Size. Agronomy, 12(6), 1423. https://doi.org/10.3390/agronomy12061423







