Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Treatments and Foliar N-Fertilization (FNf)
2.3. Evaluation Time and Leaf Sampling
2.4. Ammonium Analysis
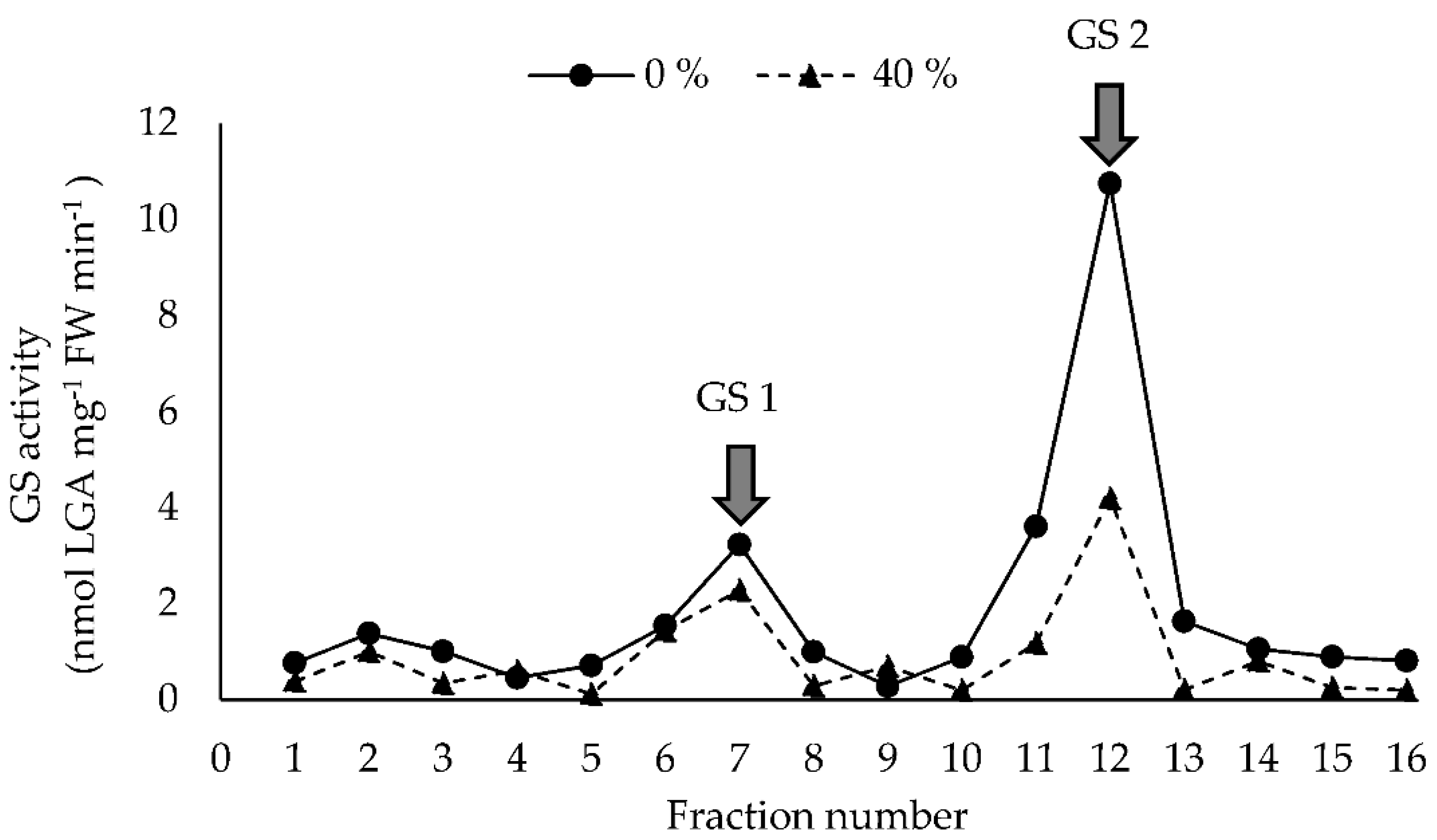
2.5. Total Glutamine Synthetase and Specific GS1 and GS2 Activity
2.6. Urease Activity
2.7. Total-N and Amino Acid Concentrations
2.8. Gas Exchange Parameters
2.9. Statistical Analysis
3. Results
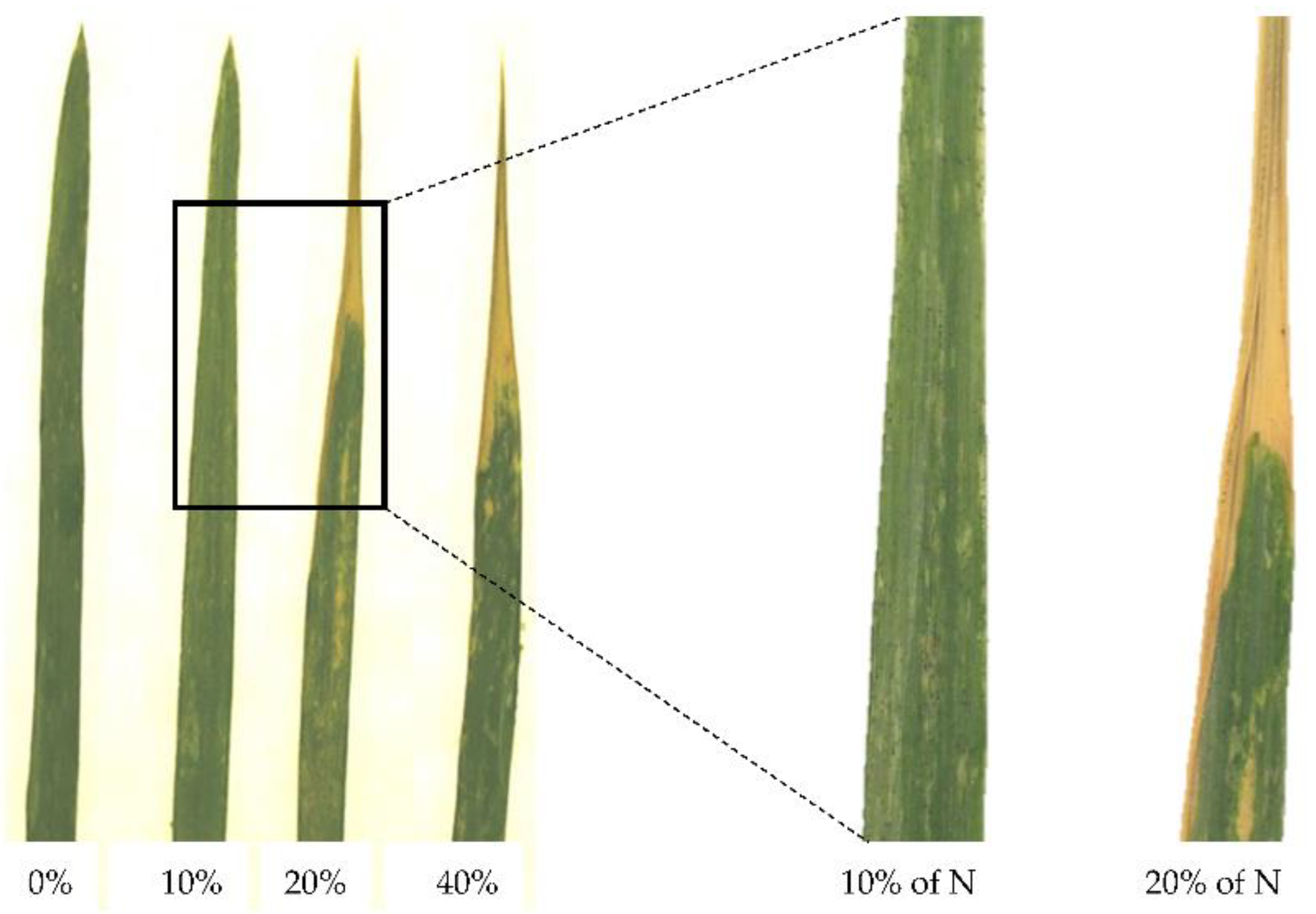
3.1. Leaf Scorching Characterization
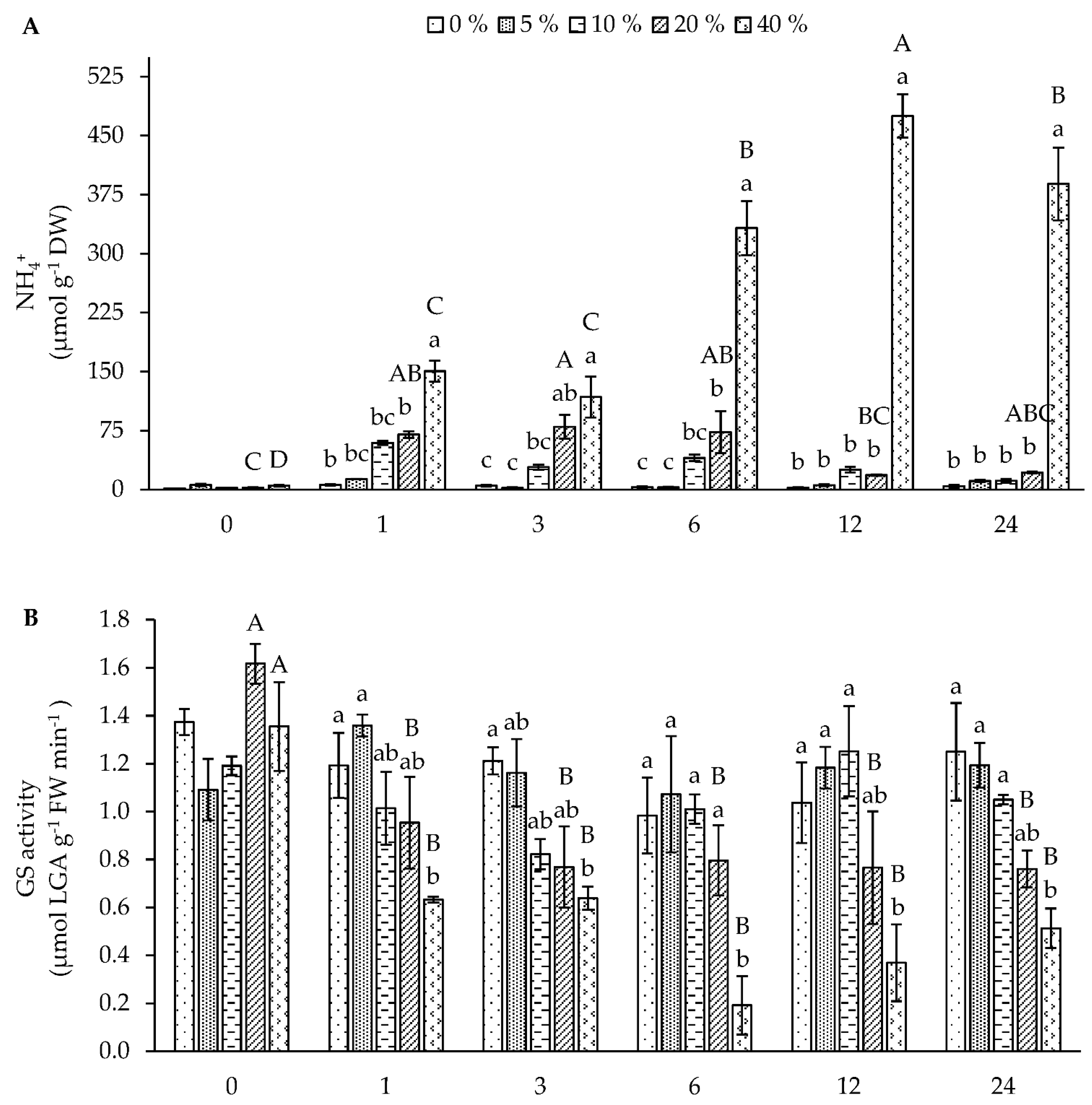
3.2. Absorption and Assimilation of Foliar Applied Nitrogen
3.2.1. Short-Term Foliar N-Fertilization Responses (First Experiment)
3.2.2. Long-Term Foliar N-Fertilization Responses (Second Experiment)
4. Discussion
4.1. Leaf Scorching Is Promoted by NH4+ Accumulation
4.2. Future Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Statistics Division. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 March 2022).
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Reeves, T.; Thomas, G.; Ramsay, G. (Eds.) Save and Grow in Practice: Maize, Rice and Wheat - A Guide to Sustainable Cereal Production; FAO: Rome, Italy, 2016; ISBN 978-92-5-108519-6. [Google Scholar]
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef] [Green Version]
- Senapati, N.; Semenov, M.A. Assessing yield gap in high productive countries by designing wheat ideotypes. Sci. Rep. 2019, 9, 5516. [Google Scholar] [CrossRef] [PubMed]
- Guberac, V.; Marić, S.; Drezner, G.; Petrović, S.; Dvojković, K.; Brandić, V. Interrelationships of important agronomic traits and kernel yield in winter wheat. In Proceedings of the 11th International Wheat Genetics Symposium, Sydney, Australia; 2008; pp. 681–684. [Google Scholar]
- Oladosu, G.; Msangi, S. Biofuel-food market interactions: A review of modeling approaches and findings. Agriculture 2013, 3, 53–71. [Google Scholar] [CrossRef] [Green Version]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Townsend, T.J.; Roy, J.; Wilson, P.; Tucker, G.A.; Sparkes, D.L. Food and bioenergy: Exploring ideotype traits of a dual-purpose wheat cultivar. F. Crop. Res. 2017, 201, 210–221. [Google Scholar] [CrossRef]
- Copeland, J.; Turley, D. National and regional supply/demand balance for agricultural straw in Great Britain. Natl. Non-Food Crops Cent. 2008, 17p. [Google Scholar]
- Jørgensen, H.; van Hecke, J.; Zhang, H.; Malik, P.L.; Felby, C.; Schjoerring, J.K. Wheat as a dual crop for biorefining: Straw quality parameters and their interactions with nitrogen supply in modern elite cultivars. GCB Bioenergy 2019, 11, 400–415. [Google Scholar] [CrossRef]
- Lv, X.; Ding, Y.; Long, M.; Liang, W.; Gu, X.; Liu, Y.; Wen, X. Effect of foliar application of various nitrogen forms on starch accumulation and grain filling of wheat (Triticum aestivum L.) under drought stress. Front. Plant Sci. 2021, 12, 645379. [Google Scholar] [CrossRef]
- Mandic, V.; Krnjaja, V.; Tomic, Z.; Bijelic, Z.; Simic, A.; Muslic, D.R.; Gogic, M. Nitrogen fertilizer influence on wheat yield and use efficiency under different environmental conditions. Chil. J. Agric. Res. 2015, 75, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Pask, A.J.D.; Sylvester-Bradley, R.; Jamieson, P.D.; Foulkes, M.J. Quantifying how winter wheat crops accumulate and use nitrogen reserves during growth. F. Crop. Res. 2012, 126, 104–118. [Google Scholar] [CrossRef]
- Xue, C.; Erley, G.S.A.; Rossmann, A.; Schuster, R.; Koehler, P.; Mühling, K.H. Split nitrogen application improves wheat baking quality by influencing protein composition rather than concentration. Front. Plant Sci. 2016, 7, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossmann, A.; Buchner, P.; Savill, G.P.; Hawkesford, M.J.; Scherf, K.A.; Mühling, K.H. Foliar N application at anthesis alters grain protein composition and enhances baking quality in winter wheat only under a low N fertiliser regimen. Eur. J. Agron. 2019, 109, 125909. [Google Scholar] [CrossRef]
- Varga, B.; Svečnjak, Z. The effect of late-season urea spraying on grain yield and quality of winter wheat cultivars under low and high basal nitrogen fertilization. Field Crops Res. 2006, 96, 125–132. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.J.; Griffiths, S. Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr. Opin. Plant Biol. 2019, 49, 35–42. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.R. Analysing nitrogen responses of cereals to prioritize routes to the improvement of nitrogen use efficiency. J. Exp. Bot. 2009, 60, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Walsh, O.S.; Shafian, S.; Christiaens, R.J. Nitrogen fertilizer management in dryland wheat cropping systems. Plants 2018, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Dick, C.D.; Thompson, N.M.; Epplin, F.M.; Arnall, D.B. Managing late-season foliar nitrogen fertilization to increase grain protein for winter wheat. Agron. J. 2016, 108, 2329–2338. [Google Scholar] [CrossRef]
- Wyatt, E.C.; Bushong, J.T.; Macnack, N.E.; Mullock, J.L.; Taylor, R.; Raun, W.R. Influence of droplet size of foliar-applied nitrogen on grain protein content of hard red winter wheat. Crop Forage Turfgrass Manag. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Abad, A.; Lloveras, J.; Michelena, A. Nitrogen fertilization and foliar urea effects on durum wheat yield and quality and on residual soil nitrate in irrigated Mediterranean conditions. Field Crops Res. 2004, 87, 257–269. [Google Scholar] [CrossRef]
- Woodard, H.J.; Bly, A. Relationship of nitrogen management to winter wheat yield and grain protein in South Dakota. J. Plant Nutr. 1998, 21, 217–233. [Google Scholar] [CrossRef]
- Gooding, M.J.; Davies, W.P. Foliar urea fertilization of cereals: A review. Fertil. Res. 1992, 32, 209–222. [Google Scholar] [CrossRef]
- Woolfolk, C.W.; Raun, W.R.; Johnson, G.V.; Thomason, W.E.; Mullen, R.W.; Wynn, K.J.; Freeman, K.W. Influence of Late-Season Foliar Nitrogen Applications on Yield and Grain Nitrogen in Winter Wheat. Agron. J. 2002, 94, 429–434. [Google Scholar] [CrossRef] [Green Version]
- Brown, B.; Westcott, M.; Christensen, N.; Pan, B.; Stark, J. Nitrogen Management for Hard Wheat Protein Enhancement. Available online: https://www.extension.uidaho.edu/publishing/pdf/PNW/PNW0578.pdf (accessed on 31 August 2021).
- Husted, S.; Hebbern, C.A.; Mattsson, M.; Schjoerring, J.K. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant. 2000, 109, 167–179. [Google Scholar] [CrossRef]
- ISO 11732:2005(en); Water Quality—Determination of Ammonium Nitrogen—Method by Flow Analysis (CFA and FIA) and Spectrometric Detection. ISO: Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11732:ed-2:v1:en (accessed on 31 August 2021).
- Husted, S.; Schjoerring, J.K. Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiol. 1995, 109, 1453–1460. [Google Scholar] [CrossRef]
- Bernard, S.M.; Møller, A.L.B.; Dionisio, G.; Kichey, T.; Jahn, T.P.; Dubois, F.; Baudo, M.; Lopes, M.S.; Tercé-Laforgue, T.; Foyer, C.H.; et al. Gene expression, cellular localisation and function of glutamine synthetase isozymes in wheat (Triticum aestivum L.). Plant Mol. Biol. 2008, 67, 89–105. [Google Scholar] [CrossRef]
- Seiler, N.; Reid, J.; Knödgen, B. A sensitive method for the assay of glutamine synthetase. Neurochem. Res. 1990, 15, 301–305. [Google Scholar] [CrossRef]
- Hogan, M.E.; Swift, I.E.; Done, J. Urease assay and ammonia release from leaf tissues. Phytochemistry 1983, 22, 663–667. [Google Scholar] [CrossRef]
- McCullough, H. The determination of ammonia in whole blood by a direct colorimetric method. Clin. Chim. Acta 1967, 17, 297–304. [Google Scholar] [CrossRef]
- ISO 16634-2:2016(en); Food Products—Determination of the Total Nitrogen Content by Combustion According to the Dumas Principle and Calculation of the Crude Protein Content—Part 2: Cereals, Pulses and Milled Cereal Products. ISO: Geneva, Switzerland. Available online: https://www.iso.org/obp/ui/#iso:std:iso:16634:-2:ed-1:v1:en (accessed on 31 August 2021).
- van Wandelen, C.; Cohen, S.A. Using quaternary high-performance liquid chromatography eluent systems for separating 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate-derivatized amino acid mixtures. J. Chromatogr. A 1997, 763, 11–22. [Google Scholar] [CrossRef]
- Dahl-Lassen, R.; van Hecke, J.; Jørgensen, H.; Bukh, C.; Andersen, B.; Schjoerring, J.K. High-throughput analysis of amino acids in plant materials by single quadrupole mass spectrometry. Plant Methods 2018, 14, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendiburu, F. De Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 26 May 2022).
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. Available online: https://github.com/taiyun/corrplot (accessed on 26 May 2022).
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis. Available online: https://cran.r-project.org/web/packages/candisc/index.html (accessed on 20 May 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Project for statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hawkesford, M.J.; Riche, A.B. Impacts of G x E x M on nitrogen use efficiency in wheat and future prospects. Front. Plant Sci. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From plant surface to plant metabolism: The uncertain fate of foliar-applied nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Dal Cortivo, C.; Panozzo, A.; Barion, G.; Visioli, G.; Giannelli, G.; Vamerali, T. Comparing soil vs. foliar nitrogen supply of the whole fertilizer dose in common wheat. Agronomy 2021, 11, 2138. [Google Scholar] [CrossRef]
- Gonçalves, A.Z.; Oliveira, P.M.R.; Neto, A.A.C.; Mercier, H. Thinking of the leaf as a whole plant: How does N metabolism occur in a plant with foliar nutrient uptake? Environ. Exp. Bot. 2020, 178, 104163. [Google Scholar] [CrossRef]
- Krogmeier, M.J.; McCarty, G.W.; Bremner, J.M. Phytotoxicity of foliar-applied urea. Proc. Natl. Acad. Sci. 1989, 86, 8189–8191. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.W.; Ikeda, H.; Oda, M. Absorption, translocation, and assimilation of foliar-applied urea compared with nitrate and ammonium in tomato plants. Soil Sci. Plant Nutr. 1999, 45, 609–616. [Google Scholar] [CrossRef]
- Clapp, J.G.; Parham, T.M. Properties and uses of liquid urea-triazone-based nitrogen fertilizers. Fertil. Res. 1991, 28, 229–232. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, G.; Kronzucker, H.J.; Baluška, F.; Shi, W. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Cheng, L.; Scagel, C.F.; Fuchigami, L.H. Nitrogen absorption, translocation and distribution from urea applied in autumn to leaves of young potted apple (Malus domestica) trees. Tree Physiol. 2002, 22, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Stiegler, J.C.; Richardson, M.D.; Karcher, D.E. Foliar nitrogen uptake following urea application to putting green turfgrass species. Crop Sci. 2011, 51, 1253–1260. [Google Scholar] [CrossRef]
- Bowman, D.C.; Paul, J.L. Volatilization and rapid depletion of urea spray-applied to Kentucky bluegrass turf. J. Plant Nutr. 1990, 13, 1335–1344. [Google Scholar] [CrossRef]
- Trivelin, P.C.O.; de Carvalho, J.G.; da Silva, A.Q.; Primavesi, A.C.P.A.; Camacho, E.; Eimori, I.E.; Guilherme, M.R. Adubação foliar de cana-de-açúcar (Saccharum spp.): Absorção e translocação de uréia-15N. Energ. Nucl. Agric. 1988, 9, 52–65. [Google Scholar]
- Ruan, J.; Gerendás, J. Absorption of foliar-applied urea- 15 N and the impact of low nitrogen, potassium, magnesium and sulfur nutritional status in tea (Camellia sinensis L.) plants. Soil Sci. Plant Nutr. 2015, 61, 653–663. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; Freney, J.R.; Sherlock, R.R.; Galbally, I.E. The fate of urea nitrogen applied in a foliar spray to wheat at heading. Fertil. Res. 1991, 28, 129–138. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar water and solute absorption: An update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Heinen, R.B.; Bienert, G.P.; Cohen, D.; Chevalier, A.S.; Uehlein, N.; Hachez, C.; Kaldenhoff, R.; Le Thiec, D.; Chaumont, F. Expression and characterization of plasma membrane aquaporins in stomatal complexes of Zea mays. Plant Mol. Biol. 2014, 86, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Goldsmith, G.R. The value of wet leaves. New Phytol. 2018, 219, 1156–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-H.; Köhler, B.; Cao, F.-Q.; Liu, L.-H. Molecular and physiological aspects of urea transport in higher plants. Plant Sci. 2008, 175, 467–477. [Google Scholar] [CrossRef]
- Eichert, T.; Burkhardt, J. Quantification of stomatal uptake of ionic solutes using a new model system. J. Exp. Bot. 2001, 52, 771–781. [Google Scholar] [CrossRef] [Green Version]
- Witte, C.-P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Jian, S.; Liao, Q.; Song, H.; Liu, Q.; Lepo, J.E.; Guan, C.; Zhang, J.; Ismail, A.M.; Zhang, Z. NRT1.1-Related NH4+ toxicity is associated with a disturbed balance between NH4+ uptake and assimilation. Plant Physiol. 2018, 178, 1473–1488. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Mineral Nutrition of Higher Plants; Marschner, P., Ed.; 2012; pp. 135–189. ISBN 978-0-12-384905-2. [Google Scholar]
- Zhang, Z.; Xiong, S.; Wei, Y.; Meng, X.; Wang, X.; Ma, X. The role of glutamine synthetase isozymes in enhancing nitrogen use efficiency of N-efficient winter wheat. Sci. Rep. 2017, 7, 1000. [Google Scholar] [CrossRef] [Green Version]
- Németh, E.; Nagy, Z.; Pécsváradi, A. Chloroplast glutamine synthetase, the key regulator of nitrogen metabolism in wheat, performs its role by fine regulation of Enzyme activity via negative cooperativity of its subunits. Front. Plant Sci. 2018, 9, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. Nitrogen acquisition, PEP carboxylase, and cellular pH homeostasis: New views on old paradigms. Plant Cell Environ. 2005, 28, 1396–1409. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.J.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- Oddy, J.; Raffan, S.; Wilkinson, M.D.; Elmore, J.S.; Halford, N.G. Stress, nutrients and genotype: Understanding and managing asparagine accumulation in wheat grain. CABI Agric. Biosci. 2020, 1, 10. [Google Scholar] [CrossRef]
- Hildebrandt, T.M. Synthesis versus degradation: Directions of amino acid metabolism during Arabidopsis abiotic stress response. Plant Mol. Biol. 2018, 98, 121–135. [Google Scholar] [CrossRef]
- Wong, H.-K.; Chan, H.-K.; Coruzzi, G.M.; Lam, H.-M. Correlation of ASN2 gene expression with ammonium metabolism in Arabidopsis. Plant Physiol. 2004, 134, 332–338. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, T.; Suzuki, A. Light-independent nitrogen assimilation in plant leaves: Nitrate incorporation into glutamine, glutamate, aspartate, and asparagine traced by 15N. Plants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Maillard, A.; Diquélou, S.; Billard, V.; Laîné, P.; Garnica, M.; Prudent, M.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Leaf mineral nutrient remobilization during leaf senescence and modulation by nutrient deficiency. Front. Plant Sci. 2015, 6, 317. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.T.B.; de Souza, W.R.; da Cunha, B.A.D.B.; Basso, M.F.; de Oliveira, N.G.; Vinecky, F.; Martins, P.K.; de Oliveira, P.A.; Arenque-Musa, B.C.; de Souza, A.P.; et al. Characterization of sugarcane (Saccharum spp.) leaf senescence: Implications for biofuel production. Biotechnol. Biofuels 2016, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhang, L.; Wang, M.; Di, D.; Kronzucker, H.J.; Shi, W. The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity. J. Exp. Bot. 2019, 70, 1375–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podgórska, A.; Szal, B. The role of reactive oxygen species under ammonium nutrition. In Reactive Oxygen and Nitrogen Species Signaling and Communication in Plants; Gupta, K.J., Igamberdiev, A.U., Eds.; Springer International Publishing: London, UK, 2015; pp. 133–154. ISBN 978-3-319-10079-1. [Google Scholar]
- Yang, S.; Hao, D.; Jin, M.; Li, Y.; Liu, Z.; Huang, Y.; Chen, T.; Su, Y. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir, R.; Hussain, S.; Noor-ul-Ain, H.; Hussain, A.; Yun, B.-W. ROS Mediated plant defense against abiotic stresses. In Plant Biotechnology: Progress in Genomic Era; Springer Singapore: Singapore, 2019; pp. 481–515. ISBN 9789811384998. [Google Scholar]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barneix, A.J. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J. Plant Physiol. 2007, 164, 581–590. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef]
- Guan, M.; de Bang, T.C.; Pedersen, C.; Schjoerring, J.K. Cytosolic glutamine synthetase Gln1;2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant Physiol. 2016, 171, 1921–1933. [Google Scholar] [CrossRef] [Green Version]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.N.S.; da Silva Júnior, G.B.; de Mello Prado, R.; de David, C.H.O.; de Souza Junior, J.P.; Teodoro, P.E. Silicon mitigates ammonium toxicity in plants. Agron. J. 2020, 112, 635–647. [Google Scholar] [CrossRef]
- Barreto, R.F.; de Mello Prado, R.; Lúcio, J.C.B.; López-Díaz, I.; Carrera, E.; Carvalho, R.F. Ammonium toxicity alleviation by silicon is dependent on cytokinins in tomato cv. micro-tom. J. Plant Growth Regul. 2021, 41, 417–428. [Google Scholar] [CrossRef]
- de Souza Junior, J.P.; de Mello Prado, R.; de Morais, T.C.B.; Frazão, J.J.; dos Santos Sarah, M.M.; de Oliveira, K.R.; de Paula, R.C. Silicon fertigation and salicylic acid foliar spraying mitigate ammonium deficiency and toxicity in Eucalyptus spp. clonal seedlings. PLoS ONE 2021, 16, e0250436. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiong, S.; Zhang, Z.; Meng, X.; Wang, L.; Zhang, X.; Yu, M.; Yu, H.; Wang, X.; Ma, X. Localization, gene expression, and functions of glutamine synthetase isozymes in wheat grain (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 580405. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.-C.; Chung, T.-Y.; Wu, H.-Y.; Juo, Y.-A.; Hsieh, M.-H. Exogenous glutamate rapidly induces the expression of genes involved in metabolism and defense responses in rice roots. BMC Genom. 2017, 18, 186. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Soares, J.N.; Reichardt, K.; Neto, D.D. Seed and foliar application of amino acids improve variables of nitrogen metabolism and productivity in soybean crop. Front. Plant Sci. 2018, 9, 396. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.J.; Fan, X.; Shen, Q.; Smith, S.J. Amino acids and nitrate as signals for the regulation of nitrogen acquisition. J. Exp. Bot. 2007, 59, 111–119. [Google Scholar] [CrossRef]






| N Concentration (%) | Total-N (mg g−1 DW) | Amino Acid (mg g−1 DW) | GS Activity (µmol LGA g−1 FW min−1) | |||
| 0% | 35.7 | a | 188.7 | a | 1.16 | a |
| 2% | 26.8 | b | 138.6 | ab | 0.83 | b |
| 4% | 28.3 | ab | 157.2 | ab | 0.98 | ab |
| 6% | 21.5 | b | 115.9 | b | 1.04 | ab |
| 9% | 27.4 | b | 144.0 | ab | 1.18 | a |
| Mean | 27.9 | 148.9 | 1.04 | |||
| N Concentration (%) | NH4+ (µg g−1 FW) | Glx Concentration (mg g−1 DW) | Urease Activity (µmol NH4+ g−1 FW h−1) | |||
| 0% | 5.0 | cd | 20.6 | a | 1083.6 | a |
| 2% | 3.2 | d | 15.3 | ab | 894.6 | a |
| 4% | 9.8 | ab | 17.1 | ab | 998.5 | a |
| 6% | 7.4 | bc | 12.7 | b | 886.1 | a |
| 9% | 11.6 | a | 16.1 | ab | 909.8 | a |
| Mean | 7.4 | 16.4 | 954.5 | |||
| Pearson Cor./ p-Value | N Concentration % | Urease Activity | GS Activity | NH4+ | Total-N | Glx Concentration | Amino Acid |
|---|---|---|---|---|---|---|---|
| N concentration, % | - | −0.43 | 0.24 | 0.72 *** | −0.51 * | −0.39 | −0.42 |
| Urease activity | 0.06 | - | 0.48 * | −0.23 | 0.63 ** | 0.63 ** | 0.65 ** |
| GS activity | 0.31 | 0.03 | - | 0.31 | 0.24 | 0.27 | 0.27 |
| NH4+ | 0.00 | 0.33 | 0.19 | - | −0.27 | −0.21 | −0.21 |
| Total-N | 0.02 | 0.00 | 0.30 | 0.26 | - | 0.95 *** | 0.96 *** |
| Glx concentration | 0.09 | 0.00 | 0.25 | 0.38 | 0.00 | - | 0.99 *** |
| 0.06 | 0.00 | 0.24 | 0.37 | 0.00 | 0.00 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, S.A.Q.d.; Kichey, T.; Persson, D.P.; Schjoerring, J.K. Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity. Agronomy 2022, 12, 1405. https://doi.org/10.3390/agronomy12061405
Castro SAQd, Kichey T, Persson DP, Schjoerring JK. Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity. Agronomy. 2022; 12(6):1405. https://doi.org/10.3390/agronomy12061405
Chicago/Turabian StyleCastro, Saulo Augusto Quassi de, Thomas Kichey, Daniel Pergament Persson, and Jan Kofod Schjoerring. 2022. "Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity" Agronomy 12, no. 6: 1405. https://doi.org/10.3390/agronomy12061405
APA StyleCastro, S. A. Q. d., Kichey, T., Persson, D. P., & Schjoerring, J. K. (2022). Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity. Agronomy, 12(6), 1405. https://doi.org/10.3390/agronomy12061405






