Humate Combined with Film-Mulched Ridge-Furrow Tillage Improved Carbon Sequestration in Arid Fluvo-Aquic Soil
Abstract
:1. Introduction
2. Methods
2.1. Experimental Sites
2.2. Study Design
2.3. Soil Sampling and Measurements
2.3.1. Soil Bulk Density (BD)
2.3.2. Soil Aggregate
2.3.3. Soil Organic Carbon (SOC)
2.3.4. Soil Microbial Biomass Nitrogen and Carbon (MBN and MBC)
2.4. Crop Residue C Input
2.5. Statistical Analyses
3. Results
3.1. Soil Organic Carbon
3.1.1. SOC Concentrations
3.1.2. SOC Stocks
3.2. Soil Aggregates
3.2.1. Aggregate Size Distribution
3.2.2. Mean Weight Diameter (MWD) and Mean Geometric Diameter (GMD)
3.2.3. Aggregate-Associated Carbon
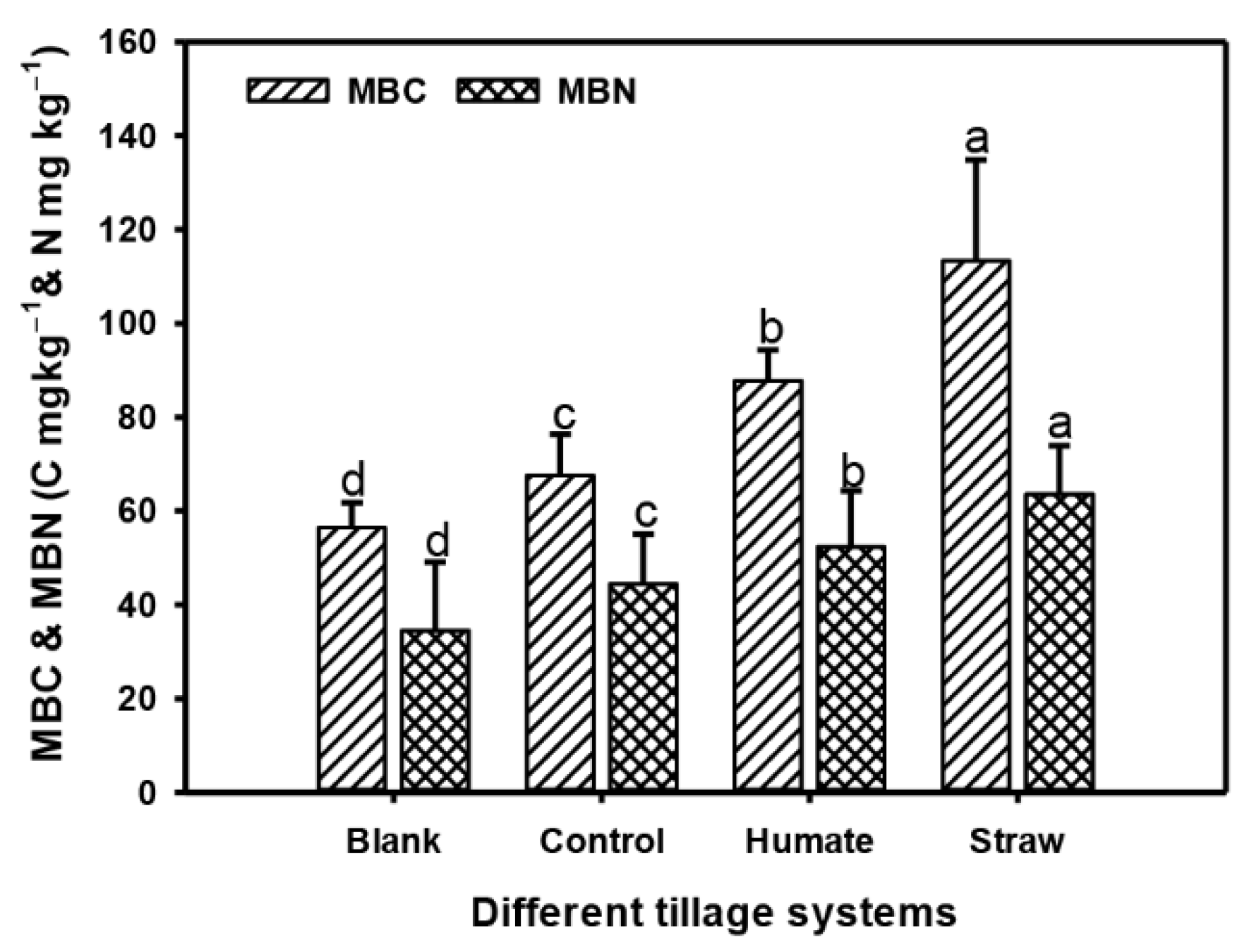
3.3. Soil Microbial Biomass Carbon and Nitrogen
3.4. Contribution of the Aggregate-Associated C, MWD, GMD, MBC, MBN, CR-C, and Total Biomass Yield to Soil Carbon Storage
4. Discussion
4.1. Humate and Straw Affects Carbon Concentration in the Soil
4.2. Aggregate-Associated C and Physical Protection Are Major Contributors to C Sequestration
4.3. Regulation of Microbial Diversity and Function Related to C Sequestration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, W.N.; Grant, B.B.; Campbell, C.A.; McConkey, B.G.; Desjardins, R.L.; Kröbel, R.; Malhi, S.S. Crop Residue Removal Effects on Soil Carbon: Measured and Inter-Model Comparisons. Agric. Ecosyst. Environ. 2012, 161, 27–38. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A. Amendments with Humified Compost Effectively Sequester Organic Carbon in Agricultural Soils. Land Degrad. Dev. 2020, 31, 1206–1216. [Google Scholar] [CrossRef]
- Dikgwatlhe, S.B.; Chen, Z.D.; Lal, R.; Zhang, H.L.; Chen, F. Changes in Soil Organic Carbon and Nitrogen as Affected by Tillage and Residue Management under Wheat-Maize Cropping System in the North China Plain. Soil Tillage Res. 2014, 144, 110–118. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Drury, C.F.; Baldock, J.A. Changes in Soil Carbon under Long-Term Maize in Monoculture and Legume-Based Rotation. Can. J. Soil Sci. 2001, 81, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, C.; Lal, R.; Kundu, S.; Babu, M.B.B.P.; Venkateswarlu, B.; Singh, A.K. Soil Carbon Sequestration in Rainfed Production Systems in the Semiarid Tropics of India. Sci. Total Environ. 2014, 487, 587–603. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, T.; Li, Y.; Wang, K.; Jia, Z.; Han, Q.; Ren, X. Effects of Straw Incorporation on the Stratification of the Soil Organic C, Total N and C:N Ratio in a Semiarid Region of China. Soil Tillage Res. 2015, 153, 28–35. [Google Scholar] [CrossRef]
- Assunção, S.A.; Pereira, M.G.; Rosset, J.S.; Berbara, R.L.L.; García, A.C. Carbon Input and the Structural Quality of Soil Organic Matter as a Function of Agricultural Management in a Tropical Climate Region of Brazil. Sci. Total Environ. 2019, 658, 901–911. [Google Scholar] [CrossRef]
- Devêvre, O.C.; Horwáth, W.R. Decomposition of Rice Straw and Microbial Carbon Use Efficiency under Different Soil Temperatures and Moistures. Soil Biol. Biochem. 2000, 32, 1773–1785. [Google Scholar] [CrossRef]
- Henriksen, T.M.; Breland, T.A. Carbon Mineralization, Fungal and Bacterial Growth, and Enzyme Activities as Affected by Contact between Crop Residues and Soil. Biol. Fertil. Soils 2002, 35, 41–48. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Hu, G.; Shao, S.; He, H.; Zhang, W.; Zhang, X.; Li, L. Dynamic Contribution of Microbial Residues to Soil Organic Matter Accumulation Influenced by Maize Straw Mulching. Geoderma 2019, 333, 35–42. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Fan, X.D.; Ren, C.J.; Zhang, L.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of the Organic Carbon Content and Stability of Soil Aggregates Affected by Soil Bacterial Community after Afforestation. Catena 2018, 171, 622–631. [Google Scholar] [CrossRef]
- Duiker, S.W.; Lal, R. Crop Residue and Tillage Effects on Carbon Sequestration in a Luvisol in Central Ohio. Soil Tillage Res. 1999, 52, 73–81. [Google Scholar] [CrossRef]
- Malhi, S.S.; Nyborg, M.; Goddard, T.; Puurveen, D. Long-Term Tillage, Straw and N Rate Effects on Quantity and Quality of Organic C and N in a Gray Luvisol Soil. Nutr. Cycl. Agroecosyst. 2011, 90, 1–20. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, X.; Zhang, S.; Xing, Y.; Wang, R.; Liang, W. Organic Amendment Effects on Aggregate-Associated Organic C, Microbial Biomass C and Glomalin in Agricultural Soils. Catena 2014, 123, 188–194. [Google Scholar] [CrossRef]
- Mirzaei Aminiyan, M.; Safari Sinegani, A.A.; Sheklabadi, M. Aggregation Stability and Organic Carbon Fraction in a Soil Amended with Some Plant Residues, Nanozeolite, and Natural Zeolite. Int. J. Recycl. Org. Waste Agric. 2015, 4, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Jia, Z.; Liang, L.; Yang, B.; Ding, R.; Nie, J.; Wang, J. Maize Straw Effects on Soil Aggregation and Other Properties in Arid Land. Soil Tillage Res. 2015, 153, 131–136. [Google Scholar] [CrossRef]
- Singh, P.; Heikkinen, J.; Ketoja, E.; Nuutinen, V.; Palojärvi, A.; Sheehy, J.; Esala, M.; Mitra, S.; Alakukku, L.; Regina, K. Tillage and Crop Residue Management Methods Had Minor Effects on the Stock and Stabilization of Topsoil Carbon in a 30-Year Field Experiment. Sci. Total Environ. 2015, 518–519, 337–344. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhu, A.N.; Yang, W.L.; Zhang, J.B. Accumulation of Organic Components and Its Association with Macroaggregation in a Sandy Loam Soil Following Conservation Tillage. Plant Soil 2017, 416, 1–15. [Google Scholar] [CrossRef]
- Liu, C.A.; Li, F.R.; Zhou, L.M.; Feng, Q.; Li, X.; Pan, C.C.; Wang, L.; Chen, J.L.; Li, X.G.; Jia, Y.; et al. Effects of Water Management with Plastic Film in a Semi-Arid Agricultural System on Available Soil Carbon Fractions. Eur. J. Soil Biol. 2013, 57, 9–12. [Google Scholar] [CrossRef]
- Ma, D.; Chen, L.; Qu, H.; Wang, Y.; Misselbrook, T.; Jiang, R. Impacts of Plastic Film Mulching on Crop Yields, Soil Water, Nitrate, and Organic Carbon in Northwestern China: A Meta-Analysis. Agric. Water Manag. 2018, 202, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and Fulvic Acids as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Fan, W.; Wu, J.; Li, J.; Hu, J. Comparative Effects of Different Maize Straw Returning Modes on Soil Humus Composition and Humic Acid Structural Characteristics in Northeast China. Chem. Ecol. 2018, 34, 355–370. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Goh, T.B. Changes in Macroaggregation and Associated Characteristics in Mine Tailings Amended with Humic Substances. Commun. Soil Sci. Plant Anal. 2004, 35, 1905–1922. [Google Scholar] [CrossRef]
- Zingaretti, D.; Lominchar, M.A.; Verginelli, I.; Santos, A.; Baciocchi, R. Humic Acids Extracted from Compost as Amendments for Fenton Treatment of Diesel-Contaminated Soil. Environ. Sci. Pollut. Res. 2020, 27, 22225–22234. [Google Scholar] [CrossRef]
- Padbhushan, R.; Das, A.; Rakshit, R.; Sharma, R.P.; Kohli, A.; Kumar, R. Long-Term Organic Amendment Application Improves Influence on Soil Aggregation, Aggregate Associated Carbon and Carbon Pools under Scented Rice-Potato-Onion Cropping System after the 9th Crop Cycle. Commun. Soil Sci. Plant Anal. 2016, 47, 2445–2457. [Google Scholar] [CrossRef]
- Kumar, R.; Rawat, K.S.; Singh, J.; Singh, A.; Rai, A. Soil Aggregation Dynamics and Carbon Sequestration. J. Appl. Nat. Sci. 2013, 5, 250–267. [Google Scholar] [CrossRef]
- Yilmaz, E.; Sönmez, M. The Role of Organic/Bio–Fertilizer Amendment on Aggregate Stability and Organic Carbon Content in Different Aggregate Scales. Soil Tillage Res. 2017, 168, 118–124. [Google Scholar] [CrossRef]
- Gao, X.; Gu, F.; Mei, X.; Hao, W.; Li, H.; Gong, D. Carbon Exchange of a Rainfed Spring Maize Cropland under Plastic Film Mulching with Straw Returning on the Loess Plateau, China. Catena 2017, 158, 298–308. [Google Scholar] [CrossRef]
- Dong, W.; Si, P.; Liu, E.; Yan, C.; Zhang, Z.; Zhang, Y. Influence of Film Mulching on Soil Microbial Community in a Rainfed Region of Northeastern China. Sci. Rep. 2017, 7, 8468. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-E.; Li, X.G.; Hai, L.; Wang, Y.P.; Fu, T.-T.; Turner, N.C.; Li, F.M. Film-Mulched Ridge-Furrow Management Increases Maize Productivity and Sustains Soil Organic Carbon in a Dryland Cropping System. Soil Sci. Soc. Am. J. 2014, 78, 1434–1441. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Fu, T.; Wang, L.; Turner, N.C.; Siddique, K.H.M.; Li, F.M. Multi-Site Assessment of the Effects of Plastic-Film Mulch on the Soil Organic Carbon Balance in Semiarid Areas of China. Agric. For. Meteorol. 2016, 228–229, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wang, X.; Liusui, Y.; Han, C.; Zhao, C.; Liu, H. Variation of Soil Aggregation and Intra-Aggregate Carbon by Long-Term Fertilization with Aggregate Formation in a Grey Desert Soil. Catena 2017, 149, 437–445. [Google Scholar] [CrossRef]
- NRCS Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014.
- FAO/Unesco Soil Map of the World, Revised Legend. World Soil Resources Report 60; FAO: Roma, Italy, 1990. [Google Scholar]
- McCarty, G.W.; Lyssenko, N.N.; Starr, J.L. Short-Term Changes in Soil Carbon and Nitrogen Pools during Tillage Management Transition. Soil Sci. Soc. Am. J. 1998, 62, 1564–1571. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Jia, X.; Shao, M. Accumulation of Soil Organic Carbon in Aggregates after Afforestation on Abandoned Farmland. Biol. Fertil. Soils 2013, 49, 637–646. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil Carbon Stocks and Land Use Change: A Meta Analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of OM and Nutrients Stored in Soils under Contrasting Management. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Shar, A.G.; Li, S.; Chen, Y.; Shi, J.; Zhang, X.; Tian, X. Effect of Straw Return Mode on Soil Aggregation and Aggregate Carbon Content in an Annual Maize-Wheat Double Cropping System. Soil Tillage Res. 2018, 175, 178–186. [Google Scholar] [CrossRef]
- Han, X.; Xu, C.; Dungait, J.A.J.; Bol, R.; Wang, X.; Wu, W.; Meng, F. Straw Incorporation Increases Crop Yield and Soil Organic Carbon Sequestration but Varies under Different Natural Conditions and Farming Practices in China: A System Analysis. Biogeosciences 2018, 15, 1933–1946. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.; Xu, M.; Wang, W.; Sun, X.; Zhao, K. Return Rate of Straw Residue Affects Soil Organic C Sequestration by Chemical Fertilization. Soil Tillage Res. 2011, 113, 70–73. [Google Scholar] [CrossRef]
- Gan, Y.; Siddique, K.H.M.; Turner, N.C.; Li, X.G.; Niu, J.Y.; Yang, C.; Liu, L.; Chai, Q. Chapter Seven-Ridge-Furrow Mulching Systems—An Innovative Technique for Boosting Crop Productivity in Semiarid Rain-Fed Environments; Sparks, D.L., Ed.; Advances in Agronomy; Elsevier Academic Press Inc.: Amsterdam, The Netherlands, 2013; Volume 118, ISBN 9780124059429. [Google Scholar] [CrossRef]
- Liu, G.; Zuo, Y.; Zhang, Q.; Yang, L.; Zhao, E.; Liang, L.; Tong, Y.A. Ridge-Furrow with Plastic Film and Straw Mulch Increases Water Availability and Wheat Production on the Loess Plateau. Sci. Rep. 2018, 8, 6503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlinden, G.; Pycke, B.; Mertens, J.; Debersaques, F.; Verheyen, K.; Baert, G.; Bries, J.; Haesaert, G. Application of Humic Substances Results in Consistent Increases in Crop Yield and Nutrient Uptake. J. Plant Nutr. 2009, 32, 1407–1426. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, J.; Dai, H.; Wang, D.; Li, D. Effect of Ridge-Furrow and Plastic-Mulching Planting Patterns on Yield Formation and Water Movement of Potato in a Semi-Arid Area. Agric. Water Manag. 2014, 131, 87–94. [Google Scholar] [CrossRef]
- Potthoff, M.; Dyckmans, J.; Flessa, H.; Beese, F.; Joergensen, R.G. Decomposition of Maize Residues after Manipulation of Colonization and Its Contribution to the Soil Microbial Biomass. Biol. Fertil. Soils 2008, 44, 891–895. [Google Scholar] [CrossRef]
- Kou, T.J.; Zhu, P.; Huang, S.; Peng, X.X.; Song, Z.W.; Deng, A.X.; Gao, H.J.; Peng, C.; Zhang, W.J. Effects of Long-Term Cropping Regimes on Soil Carbon Sequestration and Aggregate Composition in Rainfed Farmland of Northeast China. Soil Tillage Res. 2012, 118, 132–138. [Google Scholar] [CrossRef]
- Benbi, D.K.; Senapati, N. Soil Aggregation and Carbon and Nitrogen Stabilization in Relation to Residue and Manure Application in Rice–Wheat Systems in Northwest India. Nutr. Cycl. Agroecosyst. 2010, 87, 233–247. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Liu, C.; Yao, Z.; Zhang, W.; Han, S. Influences of Observation Method, Season, Soil Depth, Land Use and Management Practice on Soil Dissolvable Organic Carbon Concentrations: A Meta-Analysis. Sci. Total Environ. 2018, 631–632, 105–114. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.Y.; Ma, B.L.; Xiong, Y.C.; Qiang, S.C.; Wang, C.L.; Liu, C.A.; Li, F.M. Ridge-Furrow with Full Plastic Film Mulching Improves Water Use Efficiency and Tuber Yields of Potato in a Semiarid Rainfed Ecosystem. Field Crops Res. 2014, 161, 137–148. [Google Scholar] [CrossRef]
- Padbhushan, R.; Rakshit, R.; Das, A.; Sharma, R.P. Effects of Various Organic Amendments on Organic Carbon Pools and Water Stable Aggregates under a Scented Rice–Potato–Onion Cropping System. Paddy Water Environ. 2016, 14, 481–489. [Google Scholar] [CrossRef]
- Zhu, G.; Shangguan, Z.; Deng, L. Soil Aggregate Stability and Aggregate-Associated Carbon and Nitrogen in Natural Restoration Grassland and Chinese Red Pine Plantation on the Loess Plateau. Catena 2017, 149, 253–260. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, T.; Jia, Z.; Han, Q.; Ren, X.; Li, Y. Effects of Straw Incorporation on Soil Organic Matter and Soil Water-Stable Aggregates Content in Semiarid Regions of Northwest China. PLoS ONE 2014, 9, e92839. [Google Scholar] [CrossRef] [PubMed]
- Cates, A.M.; Ruark, M.D. Soil Aggregate and Particulate C and N under Corn Rotations: Responses to Management and Correlations with Yield. Plant Soil 2017, 415, 521–533. [Google Scholar] [CrossRef]
- Sheehy, J.; Regina, K.; Alakukku, L.; Six, J. Impact of No-till and Reduced Tillage on Aggregation and Aggregate-Associated Carbon in Northern European Agroecosystems. Soil Tillage Res. 2015, 150, 107–113. [Google Scholar] [CrossRef]
- Shi, P.; Arter, C.; Liu, X.; Keller, M.; Schulin, R. Soil Aggregate Stability and Size-Selective Sediment Transport with Surface Runoff as Affected by Organic Residue Amendment. Sci. Total Environ. 2017, 607–608, 95–102. [Google Scholar] [CrossRef]
- He, Y.T.; Zhang, W.J.; Xu, M.G.; Tong, X.G.; Sun, F.X.; Wang, J.Z.; Huang, S.M.; Zhu, P.; He, X.H. Long-Term Combined Chemical and Manure Fertilizations Increase Soil Organic Carbon and Total Nitrogen in Aggregate Fractions at Three Typical Cropland Soils in China. Sci. Total Environ. 2015, 532, 635–644. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching Practices Alter the Bacterial-Fungal Community and Network in Favor of Soil Quality in a Semiarid Orchard System. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, H.; Xie, W.; Yang, Z.; Lv, Q. Long-Term Effects of Maize Straw Return and Manure on the Microbial Community in Cinnamon Soil in Northern China Using 16S RRNA Sequencing. PLoS ONE 2021, 16, e0249884. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.; Zhang, W.; Li, F.; Qi, J. Ridge–Furrow Mulched with Plastic Film Increases Little in Carbon Dioxide Efflux but Much Significant in Biomass in a Semiarid Rainfed Farming System. Agric. For. Meteorol. 2017, 244–245, 33–41. [Google Scholar] [CrossRef]



| Soil Layer (cm) | Soil Texture | pH | SOC (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | AK (mg kg−1) | TN (g kg−1) | BD (g cm−3) | >0.25-mm Soil Aggregates by Dry Sieving (%) | Carbonate Content (g kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | Clay loam | 8.5 | 7.1 a | 24.3 a | 25.6 a | 167.6 a | 0.80 a | 1.42 a | 64.5 | 90–180 (CaCO3) |
| 20–40 | Sandy loam | 8.5 | 6.8 b | 14.4 b | 4.1 b | 114.0 b | 0.57 b | 1.47 b | 69.4 | |
| 40–60 | Sandy loam | 8.5 | 4.0 c | 13.0 c | 1.5 c | 91.2 c | 0.45 c | 1.62 c | 60.3 |
| Item | Treatments | 0–20 cm | 20–40 cm | 40–60 cm | 0–60 cm |
|---|---|---|---|---|---|
| SOC (g kg−1) | Blank | 6.86 c | 3.89 c | 3.04 b | 4.61 d |
| Control | 6.90 c | 4.41 b | 3.34 ab | 4.91 c | |
| Humate | 7.3 b | 4.59 b | 3.59 a | 5.14 b | |
| Straw | 7.7 a | 5.21 a | 3.53 a | 5.44 a | |
| SOC stock (Mg ha−1) | Blank | 22.0 b | 10.8 b | 10.3 a | 43.1 b |
| Control | 22.3 ab | 10.9 b | 10.4 a | 43.5 b | |
| Humate | 22.7 ab | 11.1 ab | 10.6 a | 44.4 ab | |
| Straw | 23.3 a | 11.6 a | 10.8 a | 45.4 a | |
| SOC sequestration (Mg ha−1 y−1) | Blank | - | - | - | - |
| Control | 0.3 (-) | 0.0 (-) | 0.1 (-) | 0.4 (-) | |
| Humate | 0.7 (0.4) | 0.3 (0.2) | 0.3 (0.2) | 1.3 (0.9) | |
| Straw | 1.3 (1.0) | 0. 8 (0.7) | 0.5 (0.4) | 2.3 (1.9) |
| Treatments | MWD (mm) | GWD (mm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 0–60 cm Average | 0–20 cm | 20–40 cm | 40–60 cm | 0–60 cm Average | |
| Blank | 2.6 ± 0.3 b | 2.8 ± 0.2 a | 2.6 ± 0.4 b | 2.7 ± 0.02 c | 1.8 ± 0.2 b | 2.0 ± 0.1 ab | 1.7 ± 0.4 b | 1.8 ± 0.1 c |
| Control | 2.8 ± 0.1 ab | 2.9 ± 0.2 a | 2.9 ± 0.2 ab | 2.9 ± 0.01 b | 2.0 ± 0.1 ab | 2.0 ± 0.2 ab | 2.0 ± 0.2 ab | 2.0 ± 0.1 bc |
| Humate | 2.8 ± 0.1 ab | 2.9 ± 0.3 a | 3.0 ± 0.2 ab | 2.9 ± 0.01 b | 2.0 ± 0.0 ab | 2.1 ± 0.2 ab | 2.1 ± 0.2 ab | 2.0 ± 0.0 b |
| Straw | 3.1 ± 0.2 a | 3.1 ± 0.3 a | 3.2 ± 0.0 a | 3.1 ± 0.01 a | 2.3 ± 0.2 a | 2.2 ± 0.3 a | 2.4 ± 0.1 a | 2.3 ± 0.1 a |
| Tillage | SOC Stock (Mg ha−1) | Soil Mass (Mg ha−1) | Tadd (cm) | SOC Stock in Additional Soil Layer (Mg ha−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| >5 mm | 2–5 mm | 1–2 mm | 0.25–1 mm | <0.25 mm | Total | >5 mm | 2–5 mm | 1–2 mm | 0.25–1 mm | <0.25 mm | Total | |||
| Straw | 9.4 ± 0.4 a | 9.6 ± 0.5 a | 9.6 ± 0.7 a | 8.8 ± 0.6 a | 11.3 ± 1.2 a | 48.7 ± 2.6 a | 8900.0 ± 511.6 c | 2.1 | 0.6 | 0.5 | 0.6 | 0.7 | 0.9 | 3.3 |
| Humate | 9.2 ± 0.2 a | 9.4 ± 0.4 a | 9.1 ± 0.3 b | 8.4 ± 0.7 b | 10.8 ± 1.0 ab | 46.7 ± 1.9 ab | 9066.7 ± 213.9 c | 1.6 | 0.6 | 0.6 | 0.4 | 0.4 | 0.2 | 2.3 |
| Control | 8.9 ± 0.8 c | 9.0 ± 1.2 | 8.7 ± 0.9 b | 8.0 ± 1.3 b | 10.5 ± 0.6 b | 45.1 ± 4.2 b | 9206. 7 ± 273.0 b | 1.0 | 0.6 | 0.4 | 0.2 | 0.3 | 0.1 | 1.6 |
| Blank | 8.1 ± 1.0 d | 8.5 ± 1.3 d | 8.2 ± 1.0 c | 7.8 ± 0.5 c | 10.1 ± 0.8 b | 43.1 ± 4.3 c | 9740.0 ± 156.2 a | - | - | - | -- | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; He, B.; Zhang, G. Humate Combined with Film-Mulched Ridge-Furrow Tillage Improved Carbon Sequestration in Arid Fluvo-Aquic Soil. Agronomy 2022, 12, 1398. https://doi.org/10.3390/agronomy12061398
Yang F, He B, Zhang G. Humate Combined with Film-Mulched Ridge-Furrow Tillage Improved Carbon Sequestration in Arid Fluvo-Aquic Soil. Agronomy. 2022; 12(6):1398. https://doi.org/10.3390/agronomy12061398
Chicago/Turabian StyleYang, Fengke, Baolin He, and Guoping Zhang. 2022. "Humate Combined with Film-Mulched Ridge-Furrow Tillage Improved Carbon Sequestration in Arid Fluvo-Aquic Soil" Agronomy 12, no. 6: 1398. https://doi.org/10.3390/agronomy12061398
APA StyleYang, F., He, B., & Zhang, G. (2022). Humate Combined with Film-Mulched Ridge-Furrow Tillage Improved Carbon Sequestration in Arid Fluvo-Aquic Soil. Agronomy, 12(6), 1398. https://doi.org/10.3390/agronomy12061398





