Evaluation of Single-Cropping under Reduced Water Supply in Strawberry Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Site, Experimental Design and Treatments
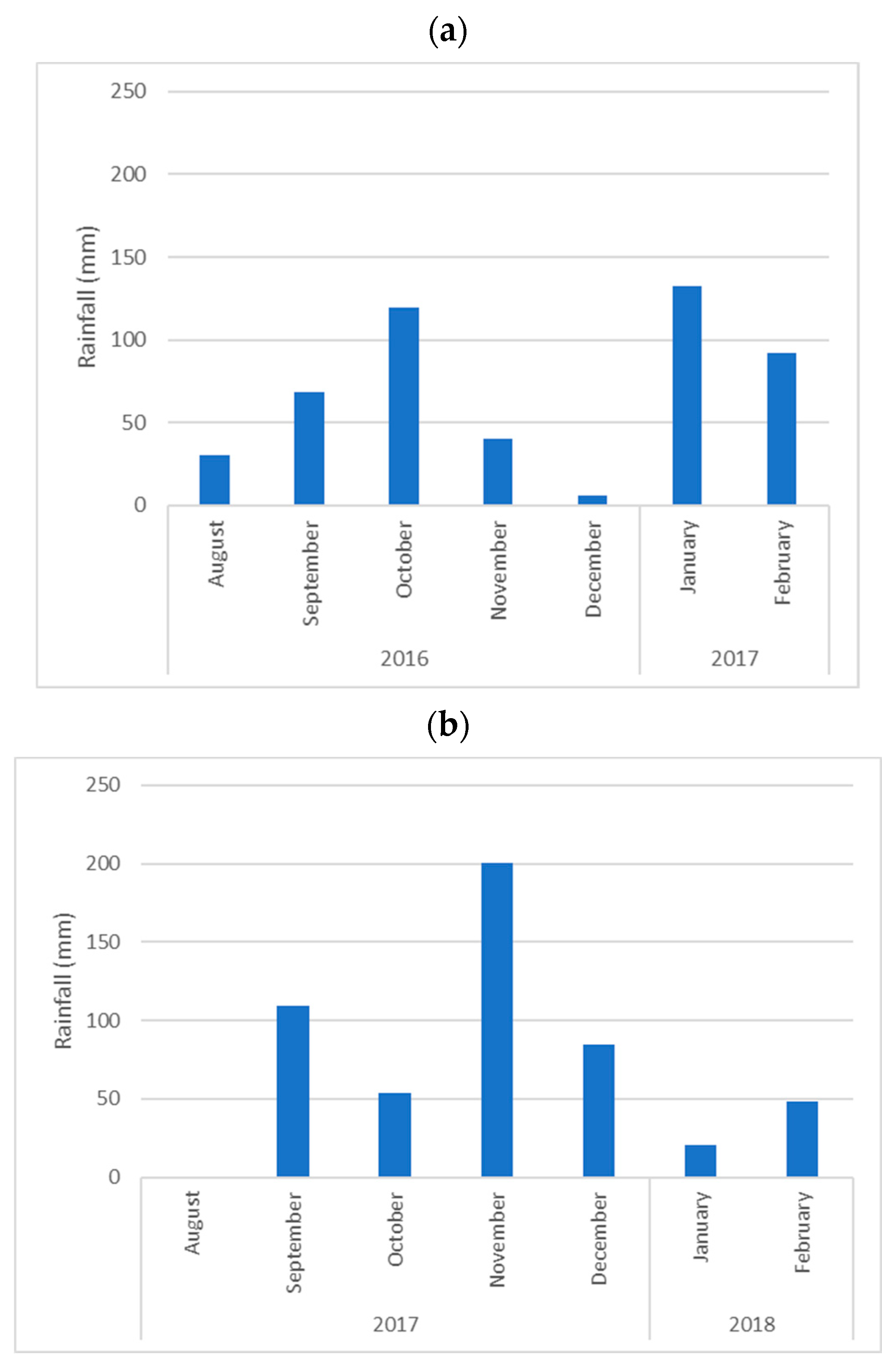
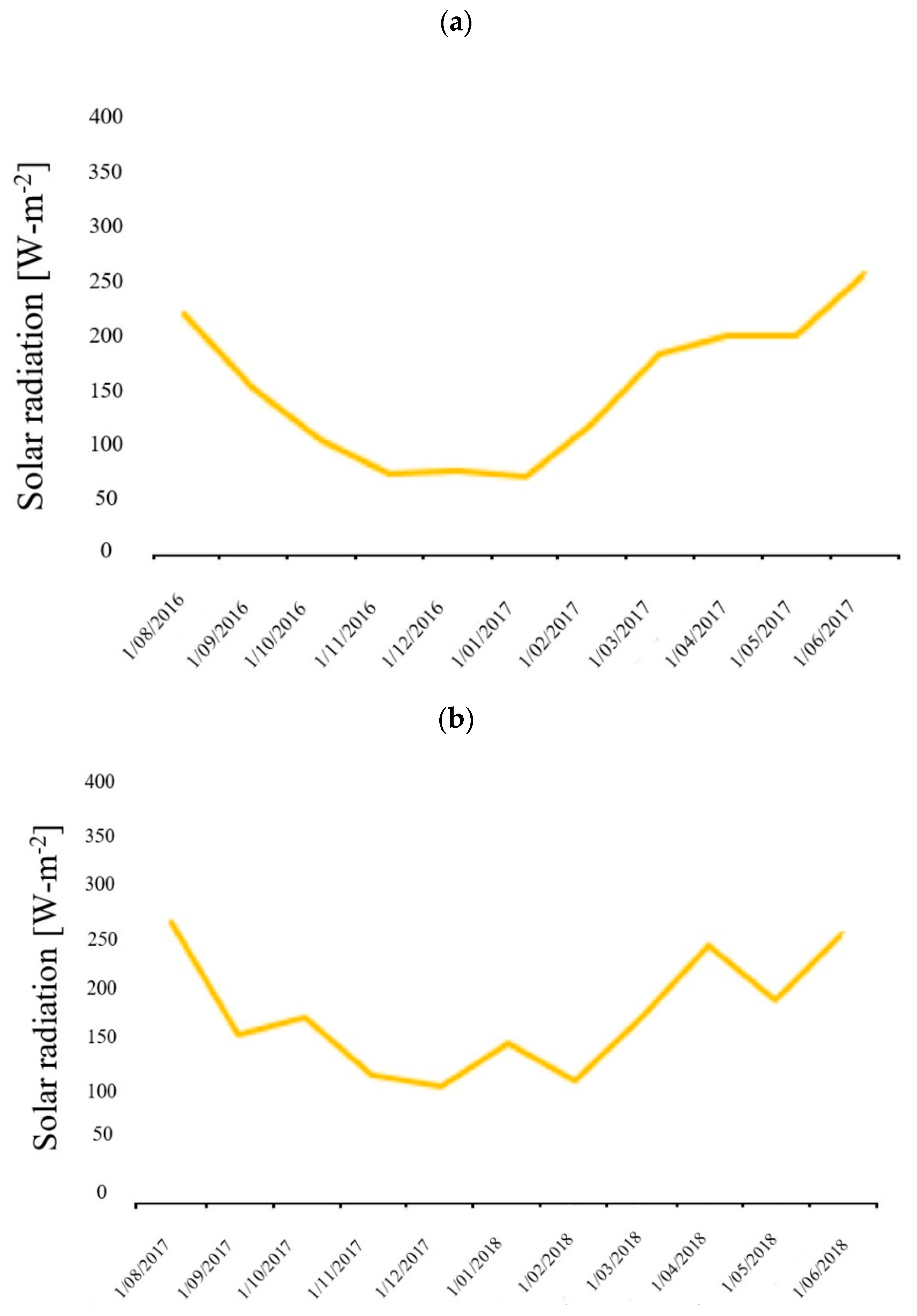
2.3. Meteorological Data
2.4. Analyzed Parameters
2.4.1. Growth Parameters
2.4.2. Productive Parameters
2.4.3. Qualitative Parameters
2.5. Statistical Analyses
3. Results and Discussion
3.1. Growth Parameters of Single-Cropping Cultivars with Reduced Water Supply
3.2. Productive Parameters of Single-Cropping Cultivars with Reduced Water Supply
3.3. Qualitative Parameters of Single-Cropping Cultivars with Reduced Water Supply
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, K.; Li, X.; Liu, X.; Seto, K.C. Projecting global urban land expansion and heat island intensification through 2050. Environ. Res. Lett. 2019, 14, 114037. [Google Scholar] [CrossRef]
- O’Neill, D.W.; Fanning, A.L.; Lamb, W.F.; Steinberger, J.K. A good life for all within planetary boundaries. Nat. Sustain. 2018, 1, 88–95. [Google Scholar] [CrossRef]
- Hickel, J.; Kallis, G. Is Green Growth Possible? New Political Econ. 2020, 25, 469–486. [Google Scholar] [CrossRef]
- Tefera, T.; Tysnes, K.R.; Utaaker, K.S.; Robertson, L.J. Parasite contamination of berries: Risk, occurrence, and approaches for mitigation. Food Waterborne Parasitol. 2018, 10, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Battino, M.; Mezzetti, B. Update on fruit antioxidant capacity: A key tool for Mediterranean diet. Public Health Nutr. 2006, 9, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Takkouche, B.; Caamaño-Isorna, F. The role of tomato products and lycopene in the prevention of prostate cancer: A meta-analysis of observational studies. Cancer Epidemiol. Biomark. Prev. 2004, 13, 340–345. [Google Scholar]
- Capocasa, F.; Scalzo, J.; Mezzetti, B.; Battino, M. Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chem. 2008, 111, 872–878. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, C.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Battino, M. Impact of strawberries on human health: Insight into marginally discussed bioactive compounds for the Mediterranean diet. Public Health Nutr. 2009, 12, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Alvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence-based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Provenzano, A.E.; Perisico, G.; Mezzetti, B. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Macori, G.; Gilardi, G.; Bellio, A.; Bianchi, D.M.; Gallina, S.; Vitale, N.; Gullino, M.L.; Decastelli, L. Microbiological parameters in the primary production of berries: A pilot study. Foods 2018, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.M.; Helmy, Y.I.; Karas, A.N.; Abou-Hadid, A.F. Influences of different water-stress treatments on growth, development and production of snap bean (Phaseolus vulgaris. L.). Acta Hortic. 2002, 614. [Google Scholar] [CrossRef]
- Davies, F.S.; Albrigo, L.G. Water relations of small fruits. In Additional Woody Crop Plants; Kozlowski, T.T., Ed.; Saunders College: Rochester, NY, USA; Harcourt Brace: San Diego, CA, USA, 1983; Volume 7, pp. 89–129. [Google Scholar]
- Gehrmann, H. Growth, yield and fruit quality of strawberries as affected by water supply. Acta Hortic. 1984, 171, 463–469. [Google Scholar] [CrossRef]
- Yuan, B.Z.; Sun, J.; Nishiyama, S. Effect of drip irrigation on strawberry growth and yield inside a plastic greenhouse. Biosyst. Eng. 2004, 87, 237–245. [Google Scholar] [CrossRef]
- Johnson, A.W.; Grant, O.M.; Davies, M.J.; James, C.M.; Passey, A.J.; Simpson, D.W. Investigating the response of strawberry to water deficit conditions. In Workshop on Berry Production in Changing Climate Conditions and Cultivation Systems. COST-Action 863: Euroberry Research: From 838; ISHS: Leuven, Belgium, 2008; pp. 169–174. [Google Scholar]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Modise, D.M.; Wright, C.J.; Atherton, J.G. Changes in strawberry aroma in response to water stress. Botsw. J. Agric. Appl. Sci. 2006, 2, 50–60. [Google Scholar]
- Capocasa, F.; Balducci, F.; Di Vittori, L.; Mazzoni, L.; Stewart, D.; Williams, S.; Hargreves, R.; Bernardini, D.; Danesi, L.; Zhong, C.F.; et al. Romina and cristina: Two new strawberry cultivars with high sensorial and nutritional values. Int. J. Fruit Sci. 2016, 16 (Suppl. S1), 207–219. [Google Scholar] [CrossRef]
- Serrano, L.; Carbonell, X.; Save, R.; Marfà, O.; Peñuelas, J. Effects of irrigation regimes on the yield and water use of strawberry. Irrig. Sci. 1992, 13, 45–48. [Google Scholar] [CrossRef]
- Pires, R.C.D.M.; Folegatti, M.V.; Passos, F.A.; Arruda, F.B.; Sakai, E. Vegetative growth and yield of strawberry under irrigation and soil mulches for different cultivation environments. Sci. Agric. 2006, 63, 417–425. [Google Scholar] [CrossRef][Green Version]
- Ançay, A.; Michel, V.; Baroffio, C.A. Comparison of manual and automatic irrigation systems in strawberries. Acta Hortic. 2017, 1156, 263–268. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria × ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J. Fruit Ornam. Plant Res. 2008, 16, 179–188. [Google Scholar]
- Kumar, R.; Bakshi, M.; Singh, B.D. Influence of plant growth regulators on growth, yield and quality of strawberry (Fragaria × ananassa Duch.) Under U.P. Subtropics. Asian J. Hortic. 2012, 7, 434–436. [Google Scholar]
- Grant, O.M.; Johnson, A.W.; Davies, M.J.; James, C.M.; Simpson, D.W. Physiological and morphological diversity of cultivated strawberry (Fragaria × ananassa) in response to water deficit. Environ. Exp. Bot. 2010, 68, 264–272. [Google Scholar] [CrossRef]
- Krüger, E.; Schmidt, G.; Brückner, U. Scheduling strawberry irrigation based upon tensiometer measurement and a climatic water balance model. Sci. Hortic. 1999, 81, 409–424. [Google Scholar] [CrossRef]
- Adak, N.; Gubbuk, H.; Tetik, N. Yield, quality and biochemical properties of various strawberry cultivars under water stress. J. Sci. Food Agric. 1968, 98, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Mi, L.; Chen, B.; Li, J.; Huo, H.; Xu, J.; Chen, X. Effects of nitrogen during nursery stage on flower bud differentiation and early harvest after transplanting in strawberry. Rev. Bras. Bot. 2018, 41, 1–10. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits-making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.T.; Miranda, L.; Gómez-Mora, J.A.; Medina, J.J.; Lozano, D.; Gavilán, P.; Soria, C.; Martínez-Ferri, E. Yield and Fruit Quality of Strawberry Cultivars under Different Irrigation Regimes. Agronomy 2021, 11, 261. [Google Scholar] [CrossRef]
- Terry, L.A.; Chope, G.A.; Bordonaba, J.G. Effect of water deficit irrigation on strawberry (Fragaria × ananassa) fruit quality. In VI International Strawberry Symposium 842; ISHS: Leuven, Belgium, 2008; pp. 839–842. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Soria, C.; Ariza, M.T.; Medina, J.J.; Miranda, L.; Domíguez, P.; Muriel, J.L. Water relations, growth and physiological response of seven strawberry cultivars (Fragaria × ananassa Duch.) to different water availability. Agric. Water Manag. 2016, 164, 73–82. [Google Scholar] [CrossRef]
- Mezzetti, B.; Galvez, A.C.; Cera, T.; Marcellini, M.; Capocasa, F. Evaluation of strawberry genotypes response to reduced water irrigation trial in southern Spain. Acta Hortic. 2021, 1309, 585–590. [Google Scholar] [CrossRef]
- Tunc, T.; Sahin, U.; Evren, S.; Dasci, E.; Guney, E.; Aslantas, R. The deficit irrigation productivity and economy in strawberry in the different drip irrigation practices in a high plain with semi-arid climate. Sci. Hortic. 2019, 245, 47–56. [Google Scholar] [CrossRef]
- Davik, J.; Kjersti Bakken, A.; Holte, K.; Blomhoff, R. Effects of genotype and environment on total anti-oxidant capacity and the content of sugars and acids in strawberries (Fragaria × ananassa Duch.). J. Hortic. Sci. Biotechnol. 2006, 81, 1057–1063. [Google Scholar] [CrossRef]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. ‘Elsanta’ and ‘Korona’. Sci. Hortic. 2004, 103, 289–303. [Google Scholar] [CrossRef]

| Trial Field | U.M. | Results | Method |
|---|---|---|---|
| pH | 8.14 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 III.1 | |
| Sand | g Kg−1 | 304 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 II.5 |
| Silt | g Kg−1 | 399 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 II.5 |
| Clay | g Kg−1 | 297 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 II.5 |
| Active Limestone | g Kg−1 | 61 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 V.2 |
| Total Limestone | g Kg−1 | 174 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 V.1 |
| Assimilable P | g Kg−1 | 3.7 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 XV.3 |
| Exchangeable Na | g Kg−1 | 15 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 III.2, XIII.2.6 |
| Exchangeable Ca | g Kg−1 | 4597 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 III.2, XIII.2.6 |
| Cation exchange capacity | meq/100 g | 21.9 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 III.2 |
| Assimilable iron | g Kg−1 | 9.7 | D.M. 11/05/92 GU n.121 del 25/05/1992 Method n.37 |
| Assimilable Mn | g Kg−1 | 4.1 | D.M. 11/05/92 GU n.121 del 25/05/1992 Method n.37 |
| Assimilable Zn | g Kg−1 | 0.52 | D.M. 13/09/99 GU n.248 del 21/10/1999 XII.1 |
| Assimilable Cu | g Kg−1 | 2.7 | D.M. 13/09/99 GU n.248 del 21/10/1999 XII.1 |
| Boron soluble | 0.1 | D.M. 13/09/99 GU n.248 del 21/10/1999 XII.1 | |
| C/N | 7.7 | ||
| Organic Matter | g Kg−1 | 11.9 | D.M. 13/09/99 GU SO n.248 del 21/10/1999-VII.3. VII.3.6 |
| Total N | g Kg−1 | 0.90 | D.M. 13/09/99 GU SO n.248 del 21/10/1999-XIV.2 + XIV.3 mod D.M. 25/03/2002 GU n.84 del 10704/2002 |
| Mg/K | 2.7 | ||
| Exchangeable Mn | mg Kg−1 | 155 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 XIII.2, XIII.2.6 |
| Exchangeable K | mg Kg−1 | 410 | D.M. 13/09/99 GU SO n.248 del 21/10/1999 XIII.2, XIII.2.6 |
| Irrigation Treatment | First Season (m3 ha−1) | Second Season (m3 ha−1) |
|---|---|---|
| W100 | 1104 | 1135 |
| W80 | 899 | 883 |
| W60 | 700 | 631 |
| Year | Month | Medium Temperature (°C) | Maximum Temperature (°C) | Minimum Temperature (°C) |
|---|---|---|---|---|
| 2017 | March | 12.2 | 27.6 | 0.2 |
| April | 14.6 | 31.4 | 0.1 | |
| May | 19.8 | 36.1 | 4.1 | |
| June | 25.6 | 42 | 10.9 | |
| 2018 | March | 8.5 | 20.1 | 0 |
| April | 15.9 | 26.6 | 7 | |
| May | 18.6 | 29.6 | 9.9 | |
| June | 22 | 31 | 14 |
| Year | Month (Harvest Time) | Date |
|---|---|---|
| Season 2016/2017 | April | 18–21–26–28 |
| May | 2–4–8–10–11–12–15–17–19–22–25–26–29 | |
| June | 1–6 | |
| Season 2017/2018 | April | 30 |
| May | 3–7–9–11–14–16–18–21–23–25–28–30 | |
| June | 1–4–6–8–12 |
| Parameter | Number of Crowns | Number of Inflorescences | Plant Height | Number of Leaves | Leaf Length | Leaf Width |
|---|---|---|---|---|---|---|
| Year (a) | ** | NS | ** | ** | NS | ** |
| Cultivar (b) | ** | ** | ** | NS | ** | ** |
| Treatment (c) | NS | NS | ** | ** | ** | ** |
| Year × Treatment (a) × (c) | NS | NS | NS | * | NS | NS |
| Cultivar × Treatment (b) × (c) | * | NS | NS | NS | NS | NS |
| Year × Cv × Treatment (a) × (b) × (c) | NS | NS | * | ** | NS | ** |
| Cultivar | Plant Height | Number of Leaves | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 32.6 ± 4.4 d | 31.4 ± 4.3 d | 28.1 ± 3.6 e | 25.5 ± 7.2 ab | 24.6 ± 5.3 abc | 23.5 ± 7.1 bc |
| ‘Romina’ | 39.1 ± 3.1 a | 39 ± 3.0 a | 37 ± 3.1 bc | 25.1 ± 5.9 ab | 25.4 ± 5.3 ab | 22.5 ± 4.8 c |
| ‘Sibilla’ | 39.1 ± 5.1 a | 38.2 ± 6.3 ab | 36.2 ± 6.5 c | 26.4 ± 5.0 a | 24.7 ± 6.1 abc | 24.7 ± 5.4 abc |
| Cultivar | Leaf Length (cm) | Leaf Width (cm) | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 8.9 ± 0.7 d | 9 ± 0.9 de | 8.4 ± 0.9 e | 7.9 ± 0.9 c | 7.8 ± 0.9 c | 7.2 ± 1.0 d |
| ‘Romina’ | 9.5 ± 1.1 abc | 9.2 ± 1.0 bcde | 9.2 ± 1.1 cde | 9.4 ± 1.0 a | 9.4 ± 1.1 a | 9.2 ± 1.2 a |
| ‘Sibilla’ | 9.6 ± 1.1 ab | 9.8 ± 1.2 a | 9.3 ± 1.0 bcd | 8.4 ± 0.9 b | 8.1 ± 1.2 bc | 8.0 ± 0.8 c |
| Cultivar | Number of Crowns | Number of Inflorescences | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 4.0 ± 1.6 bc | 4.3 ± 1.4 abc | 3.9 ± 1.3 c | 12.9 ± 3.7 ab | 13.3 ± 3.3 a | 12.0 ± 4.3 abc |
| ‘Romina’ | 4.9 ± 2.3 a | 4.8 ± 2.0 ab | 5.0 ± 2.3 a | 12.0 ± 3.5 abc | 12.6 ± 2.9 abc | 12.0 ± 3.7 abc |
| ‘Sibilla’ | 4.9 ± 2.0 a | 4.2 ± 1.9 abc | 4.4 ± 1.9 abc | 11.4 ± 4 bc | 11.4 ± 4.1 c | 11.2 ± 3.7 c |
| Parameter | Precocity Index | Average Fruit Weight | Marketable Production | Total Yield |
|---|---|---|---|---|
| Year (a) | ** | ** | ** | ** |
| Cv (b) | ** | ** | ** | ** |
| Treatment (c) | ** | * | ** | ** |
| Year × Cv (a) × (b) | ** | ** | * | ** |
| Year × Treatment (a) × (c) | NS | NS | NS | NS |
| Cv × Treatment (b) × (c) | NS | NS | NS | NS |
| Year × Cv × Treatment (a) × (b) × (c) | NS | ** | NS | NS |
| Cultivar | Precocity Index (Days) | Average Fruit Weight (g) | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 146.3 ± 2.6 a | 145.6 ± 2.8 a | 145.2 ± 2.7 a | 30.2 ± 2.0 a | 30.2 ± 1.7 a | 29.8 ± 3.4 a |
| ‘Romina’ | 129.6 ± 3.1 cde | 128.1 ± 3.4 e | 128.2 ± 3 de | 17.9 ± 1.7cd | 16.6 ± 1.5 d | 16.5 ± 0.8 d |
| ‘Sibilla’ | 134.0 ± 3.6 b | 132.5 ± 4.4 bc | 132.1 ± 4.2 bcd | 20.0 ± 1.3 b | 20.1 ± 0.5 b | 19.0 ± 1.4 bc |
| Cultivar | Marketable Production (g plant−1) | Total Yield (g plant−1) | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 755.1 ± 110.1 a | 694.0 ± 143 a | 573.8 ± 151.8 b | 856.2 ± 121.2 a | 809.9 ± 138.6 ab | 689.6 ± 162.9 cd |
| ‘Romina’ | 505.6 ± 53.3bcd | 494.3 ± 66.6 bcd | 409.8 ± 51.1 d | 695 ± 51.4 c | 712.0 ± 75.9 bc | 578.6 ± 45 d |
| ‘Sibilla’ | 555.6 ± 54.9 bc | 465.5 ± 69.8 cd | 461.1 ± 36 cd | 809.8 ± 76.9 ab | 707.4 ± 78.1 bc | 703.9 ± 57.5 bc |
| Parameter | Sugar Content | Titratable Acidity | Firmness | L* | a* | b* | Chroma |
|---|---|---|---|---|---|---|---|
| Year (a) | ** | ** | ** | ** | ** | ** | NS |
| Cv (b) | ** | ** | ** | ** | ** | ** | ** |
| Treatment (c) | ** | * | NS | * | * | ** | ** |
| Year × Cv (a) × (b) | ** | ** | ** | ** | ** | ** | ** |
| Year × Treatment (a) × (c) | NS | NS | NS | NS | NS | NS | NS |
| Cv × Treatment (b) × (c) | NS | NS | NS | NS | NS | NS | NS |
| Year × Cv × Treatment (a) × (b) × (c) | ** | NS | NS | NS | NS | NS | NS |
| Cultivar | Sugar Content (Brix°) | Titratable Acidity (meqNaOH 100 g−1 Fruit Weight) | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 6.6 ± 0.4 f | 6.9 ± 0.6 f | 7.1 ± 0.8 ef | 10.4 ± 1.1 d | 10.9 ± 1.1 bcd | 11.3 ± 1.6 abc |
| ‘Romina’ | 7.6 ± 0.8 de | 7.7 ± 1 cd | 8.1 ± 1.1 bcd | 10.7 ± 0.7 cd | 10.7 ± 1.1 cd | 11.1 ± 1.0 abc |
| ‘Sibilla’ | 8.2 ± 0.9abc | 8.6 ± 1.2ab | 8.8 ± 1.3 a | 11.5 ± 1.0 ab | 11.7 ± 1.1 a | 11.7 ± 1.1 a |
| Cultivar | Firmness (g/cm2) | Chroma | ||||
|---|---|---|---|---|---|---|
| Treatment | W100 | W100 | W80 | W60 | W80 | W60 |
| ‘Cristina’ | 271.7 ± 42.9 c | 45.1 ± 3.4 e | 44.7 ± 3.3 e | 44.2 ± 2.9 e | 286.1 ± 42.8 c | 275.5 ± 31.7 c |
| ‘Romina’ | 342.8 ± 38.6 b | 49.8 ± 1.4 cd | 48.5 ± 1.4 d | 49.5 ± 1.7 d | 355.2 ± 24.1 b | 367.2 ± 32.4 b |
| ‘Sibilla’ | 410.6 ± 84.0 a | 53.3 ± 1.5 a | 51.4 ± 2.2 b | 51 ± 2.0 bc | 409.3 ± 94.1 a | 415.5 ± 90.0 a |
| Cultivar | L* | a* | b* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | W100 | W80 | W60 | W100 | W80 | W60 | W100 | W80 | W60 |
| ‘Cristina’ | 38.1 ± 2.5 cde | 37.8 ± 2.2 de | 37.6 ± 2.2 e | 38.6 ± 2.2 d | 38.3 ± 2.1 d | 38.1 ± 1.9 d | 23.1 ± 3.1 d | 22.9 ± 3.2 d | 22.1 ± 2.9 d |
| ‘Romina’ | 39.5 ± 2.4 bc | 38.3 ± 2.4 cde | 39.2 ± 2.4 bcd | 41.2 ± 0.7 c | 40.5 ± 0.7 c | 41 ± 1.1 c | 27.9 ± 2.2 bc | 26.5 ± 2.1 c | 27.6 ± 1.9 bc |
| ‘Sibilla’ | 41.7 ± 2.6 a | 40.4 ± 2.5 ab | 40.0 ± 2.6 b | 43.6 ± 0.6 a | 42.6 ± 1.0 b | 42.5 ± 1.0 b | 30.6 ± 2.3 a | 28.6 ± 2.8 b | 28.2 ± 2.4 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcellini, M.; Mazzoni, L.; Raffaelli, D.; Pergolotti, V.; Balducci, F.; Capocasa, F.; Mezzetti, B. Evaluation of Single-Cropping under Reduced Water Supply in Strawberry Cultivation. Agronomy 2022, 12, 1396. https://doi.org/10.3390/agronomy12061396
Marcellini M, Mazzoni L, Raffaelli D, Pergolotti V, Balducci F, Capocasa F, Mezzetti B. Evaluation of Single-Cropping under Reduced Water Supply in Strawberry Cultivation. Agronomy. 2022; 12(6):1396. https://doi.org/10.3390/agronomy12061396
Chicago/Turabian StyleMarcellini, Micol, Luca Mazzoni, Davide Raffaelli, Valeria Pergolotti, Francesca Balducci, Franco Capocasa, and Bruno Mezzetti. 2022. "Evaluation of Single-Cropping under Reduced Water Supply in Strawberry Cultivation" Agronomy 12, no. 6: 1396. https://doi.org/10.3390/agronomy12061396
APA StyleMarcellini, M., Mazzoni, L., Raffaelli, D., Pergolotti, V., Balducci, F., Capocasa, F., & Mezzetti, B. (2022). Evaluation of Single-Cropping under Reduced Water Supply in Strawberry Cultivation. Agronomy, 12(6), 1396. https://doi.org/10.3390/agronomy12061396










