Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection and Seedling Growth
2.2. Treatment and Experimental Design
2.3. Growth Measurements
2.4. Assessing the 88Sr, 133Cs, and 88Sr + 133Cs Accumulation
2.5. Phytoextraction Potential
2.6. Assessing the Physiological and Biochemical Indexes
2.7. Statistical Analysis
3. Results
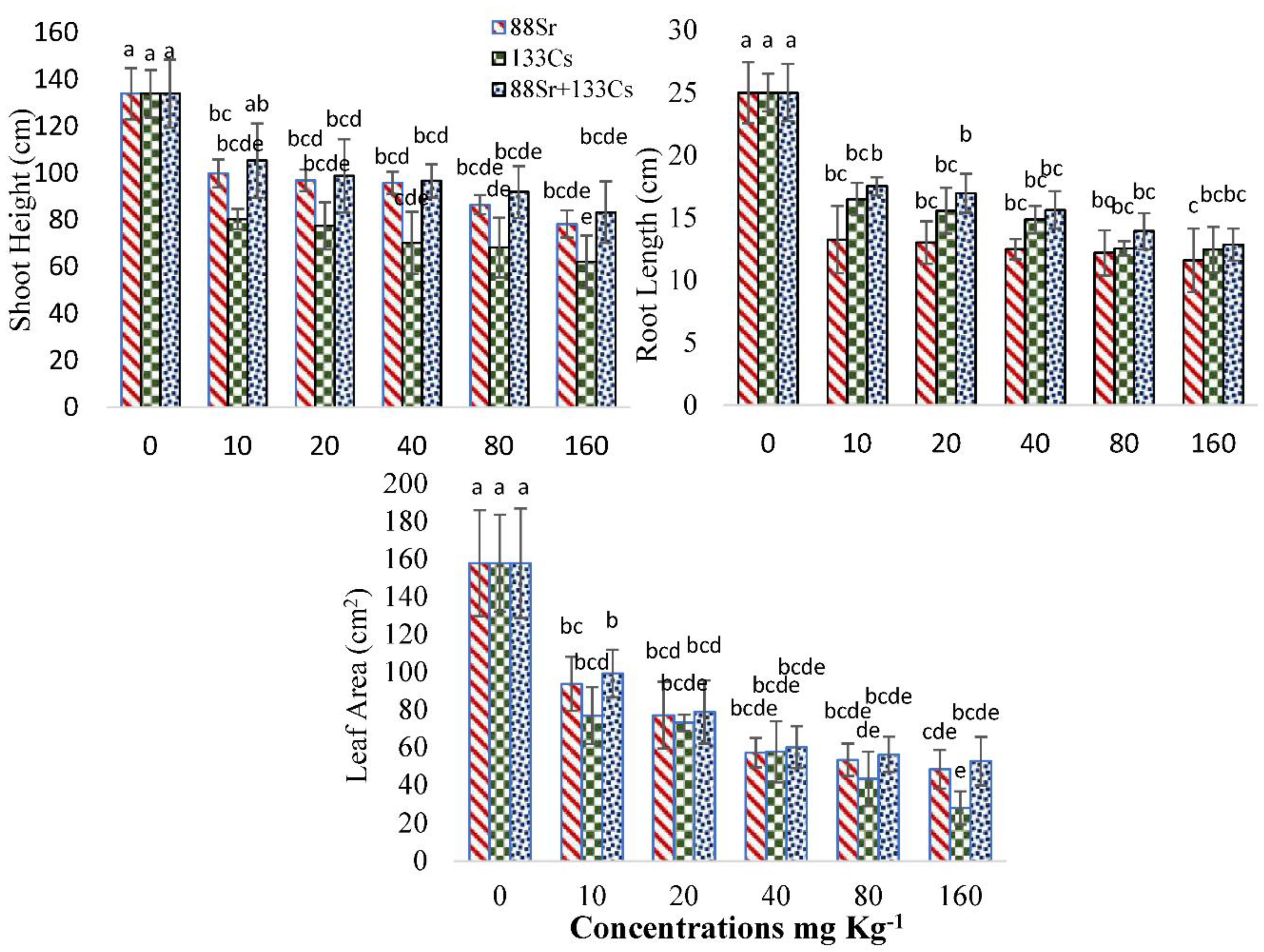
3.1. Growth Characteristics of Cucumber under 88Sr, 133Cs, and 88Sr + 133Cs Stress
3.2. Effect of 88Sr, 133Cs, and 88Sr + 133Cs on the Biomass Distribution of Cucumber
3.3. 88Sr, 133Cs, and 88Sr + 133Cs Bio-Accumulation in Plant and Phytoextraction Potential
3.4. The Effect of 88Sr, 133Cs, and 88Sr + 133Cs Extraction Efficiency on Cucumber
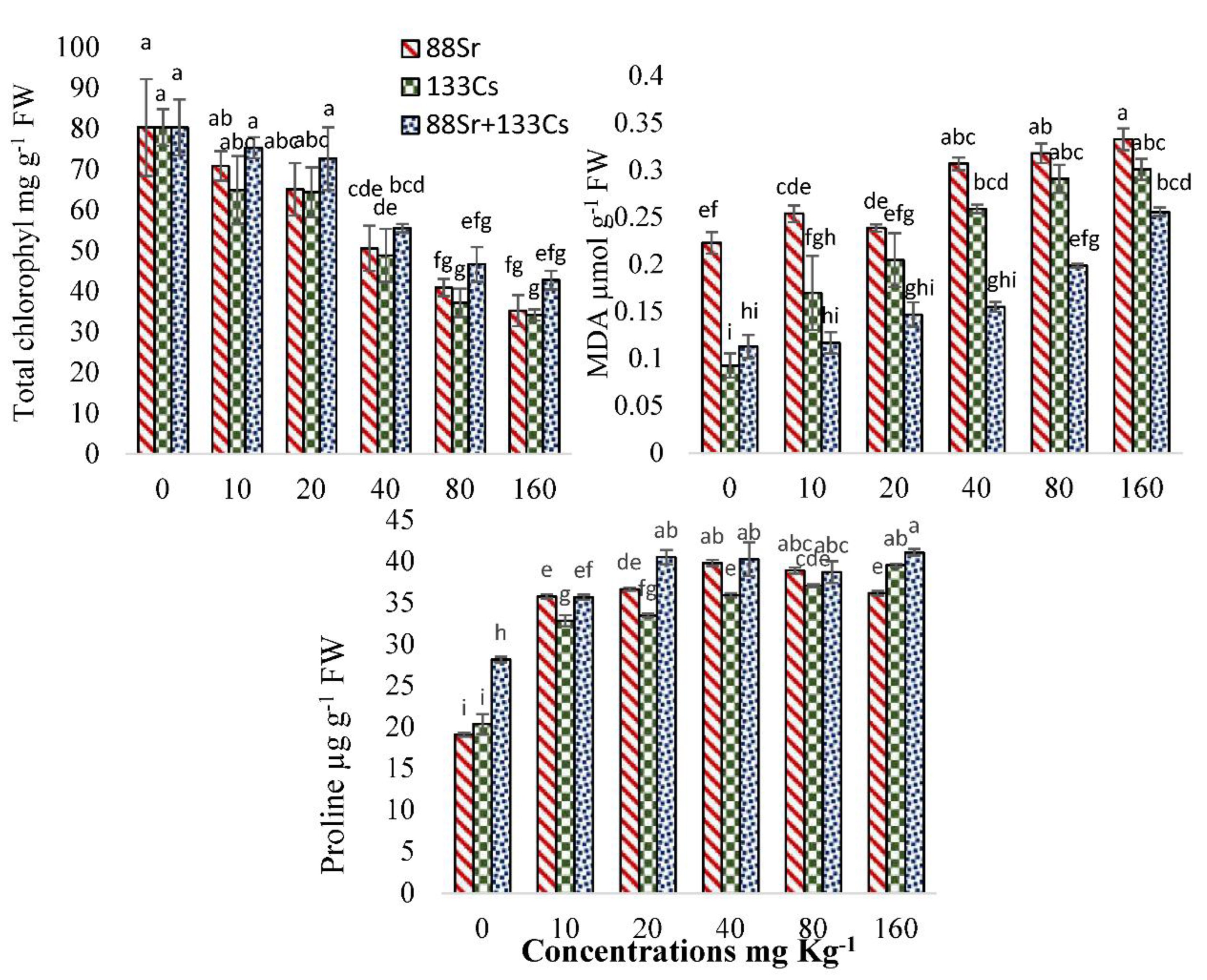
3.5. Physiological and Biochemical Response of Cucumber to 88Sr, 133Cs, and 88Sr + 133Cs Stress
4. Discussion
4.1. Growth Characteristics of Cucumber under 88Sr, 133Cs, and 88Sr + 133Cs Stress
4.2. Effect of 88Sr, 133Cs, and 88Sr + 133Cs on the Biomass Distribution of Cucumber
4.3. Sr, 133Cs, and 88Sr + 133Cs Bio-Accumulation in Plant and Phytoextraction Potential
4.4. Physiological and Biochemical Response of Cucumber to 88Sr, 133Cs, and 88Sr + 133Cs Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baba, T.; Makino, Y.; Yamada, M.; Fujiyama, H.J.E.-E. Evaluation of the Cs-and Sr-absorption ability of plant species for phytoremediation. Eco-Engineering 2016, 28, 112368. [Google Scholar]
- Aung, H.P.; Mensah, A.D.; Aye, Y.S.; Djedidi, S.; Oikawa, Y.; Yokoyama, T.; Suzuki, S.; Bellingrath-Kimura, S.D. Transfer of radiocesium from rhizosphere soil to four cruciferous vegetables in association with a Bacillus pumilus strain and root exudation. J. Environ. Radioact. 2016, 164, 209–219. [Google Scholar] [CrossRef]
- Eapen, S.; Suseelan, K.N.; Tivarekar, S.; Kotwal, S.A.; Mitra, R. Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ. Res. 2003, 91, 127–133. [Google Scholar] [CrossRef]
- Malek, M.A.; Hinton, T.G.; Webb, S.B. A comparison of 90Sr and 137Cs uptake in plants via three pathways at two Chernobyl-contaminated sites. J. Environ. Radioact. 2002, 58, 129–141. [Google Scholar] [CrossRef]
- Burger, A.; Weidinger, M.; Adlassnig, W.; Puschenreiter, M.; Lichtscheidl, I. Response of Plantago major to cesium and strontium in hydroponics: Absorption and effects on morphology, physiology and photosynthesis. Environ. Pollut. 2019, 254, 113084. [Google Scholar] [CrossRef]
- Moyen, C.; Roblin, G. Uptake and translocation of strontium in hydroponically grown maize plants, and subsequent effects on tissue ion content, growth and chlorophyll a/b ratio: Comparison with Ca effects. Environ. Exp. Bot. 2010, 68, 247–257. [Google Scholar] [CrossRef]
- Margon, A.; Mondini, C.; Valentini, M.; Ritota, M.; Leita, L. Bioavailability. Soil microbial biomass influence on strontium availability in mine soil. Chem. Speciat. Bioavailab. 2013, 25, 119–124. [Google Scholar] [CrossRef] [Green Version]
- El-Shazly, A.A.; Farid, I.M.; Rezk, M.A.; Abbas, M.H.H.; Abdel-Sabour, M.; Mousa, E.; Mostafa, M.A.Z.; Lotfy, S. Effect of calcium levels on strontium uptake by canola plants grown on different texture soils. J. Nucl. Technol. Appl. Sci. 2016, 4, 1–10. [Google Scholar]
- Bystrzejewska-Piotrowska, G.Y.; Urban, P.Ł. Accumulation of cesium in leaves of Lepidium sativum. Acta Biol. Crac. Ser. Bot. 2003, 45, 131–137. [Google Scholar]
- Dasch, A.A.; Blum, J.D.; Eagar, C.; Fahey, T.J.; Driscoll, C.T.; Siccama, T.G. The relative uptake of Ca and Sr into tree foliage using a whole-watershed calcium addition. Biogeochemistry 2006, 80, 21–41. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M.; Telias, A.; Hoover, E.E. Foliar-and fruit-applied strontium as a tracer for calcium transport in apple trees. HortScience 2006, 41, 220–224. [Google Scholar] [CrossRef]
- Chou, F.I.; Chung, H.P.; Teng, S.P.; Sheu, S.T. Screening plant species native to Taiwan for remediation of 137Cs-contaminated soil and the effects of K addition and soil amendment on the transfer of 137Cs from soil to plants. J. Environ. Radioact. 2005, 80, 175–181. [Google Scholar] [CrossRef]
- Tsukada, H.; Takeda, A.; Takahashi, T.; Hasegawa, H.; Hisamatsu, S.I.; Inaba, J. Uptake and distribution of 90Sr and stable Sr in rice plants. J. Environ. Radioact. 2005, 81, 221–231. [Google Scholar] [CrossRef]
- Soudek, P.; Valenová, Š.; Vavříková, Z.; Vaněk, T. 137Cs and 90Sr uptake by sunflower cultivated under hydroponic conditions. J. Environ. Radioact. 2006, 88, 236–250. [Google Scholar] [CrossRef]
- Moogouei, R.; Borghei, M.; Arjmandi, R. Phytoremediation of stable Cs from solutions by Calendula alata, Amaranthus chlorostachys and Chenopodium album. Ecotoxicol. Environ. Saf. 2011, 74, 2036–2039. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.L.; Tao, Z.Y.; Han, N.; Wu, G. Characterizations of bio-accumulations, subcellular distribution and chemical forms of cesium in Brassica juncea, and Vicia faba. J. Environ. Radioact. 2016, 154, 52–59. [Google Scholar] [CrossRef]
- Singh, S.; Eapen, S.; Thorat, V.; Kaushik, C.P.; Raj, K.; D’souza, S.F. Phytoremediation of 137cesium and 90strontium from solutions and low-level nuclear waste by Vetiveria zizanoides. Ecotoxicol. Environ. Saf. 2008, 69, 306–311. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Lasat, M.M.; Ebbs, S.D.; Kochian, L.V.; Cornish, J. Uptake of cesium-137 and strontium-90 from contaminated soil by three plant species; application to phytoremediation. J. Environ. Qual. 2002, 31, 904–909. [Google Scholar] [CrossRef]
- Vandenhove, H.; Van Hees, M. Phytoextraction for clean-up of low-level uranium contaminated soil evaluated. J. Environ. Radioact. 2004, 72, 41–45. [Google Scholar] [CrossRef]
- Ehlken, S.; Kirchner, G. Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: A review. J. Environ. Radioact. 2002, 58, 97–112. [Google Scholar] [CrossRef]
- Sami, M.; Reyhani, H. Environmental assessment of cucumber farming using energy and greenhouse gas emission indexes. J. Inst. Integr. Omics Appl. Biotechnol. 2015, 6, 15–21. [Google Scholar]
- Akca, M.S.; Agus, E.; Altınbaş, M. ITU Sustainable Bioenergy Production Plant: Preliminary Results from Container-type Digester Utilizing Food Waste Produced at ITU Campus. In Proceedings of the 5th Euro Asia Waste Management Symposium, Online, 26–28 October 2020. [Google Scholar]
- Oleszek, M.; Tys, J.; Wiącek, D.; Król, A.; Kuna, J. The possibility of meeting greenhouse energy and CO2 demands through utilisation of cucumber and tomato residues. BioEnergy Res. 2016, 9, 624–632. [Google Scholar] [CrossRef]
- Diwan, H.; Ahmad, A.; Iqbal, M. Chromium-induced alterations in photosynthesis and associated attributes in Indian mustard. J. Environ. Biol. 2012, 33, 239. [Google Scholar]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Abbas, F.; Bukhari, S.A.H.; Saeed, R.; Wu, L.J.E.; Safety, E. Citric acid assisted phytoextraction of chromium by sunflower; morpho-physiological and biochemical alterations in plants. Ecotoxicol. Environ. Saf. 2017, 145, 90–102. [Google Scholar] [CrossRef]
- Amin, H.; Ahmed Arain, B.; Abbasi, M.S.; Amin, F.; Jahangir, T.M.; Soomro, N.U.A. Evaluation of chromium phyto-toxicity, phyto-tolerance, and phyto-accumulation using biofuel plants for effective phytoremediation. Int. J. Phytoremediat. 2019, 21, 352–363. [Google Scholar] [CrossRef]
- Hs, L.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Experimental Principle and Technique for Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Tansley Review No. 113 Mechanisms of caesium uptake by plants. New Phytol. 2000, 147, 241–256. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, C.; Wang, J. Cs phytoremediation by Sorghum bicolor cultivated in soil and in hydroponic system. Int. J. Phytormediat. 2017, 19, 402–412. [Google Scholar] [CrossRef]
- Heidenreich, B.; Mayer, K.; Sandermann, H., Jr.; Ernst, D. Environment. Mercury-induced genes in Arabidopsis thaliana: Identification of induced genes upon long-term mercuric ion exposure. Plant Cell Environ. 2001, 24, 1227–1234. [Google Scholar] [CrossRef]
- Sahr, T.; Voigt, G.; Schimmack, W.; Paretzke, H.G.; Ernst, D. Low-level radiocaesium exposure alters gene expression in roots of Arabidopsis. New Phytol. 2005, 168, 141–148. [Google Scholar] [CrossRef]
- Tagami, K.; Tsukada, H.; Uchida, S.; Howard, B.J. Changes in the soil to brown rice concentration ratio of radiocaesium before and after the Fukushima Daiichi Nuclear Power Plant accident in 2011. Environ. Sci. Technol. 2018, 52, 8339–8345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, T.; Okada, K. Interactive effects of Al, Ca and other cations on root elongation of rice cultivars under low pH. Ann. Bot. 2005, 95, 379–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Maruthi Sridhar, B.B.; Han, F.X.; Diehl, S.V.; Monts, D.L. Effect of bioaccumulation of Cs and Sr natural isotopes on foliar structure and plant spectral reflectance of Indian mustard (Brassica juncea). Water Air Soil Pollut. 2007, 180, 65–74. [Google Scholar] [CrossRef]
- Kanter, U.; Hauser, A.; Michalke, B.; Dräxl, S.; Schäffner, A.R. Caesium and strontium accumulation in shoots of Arabidopsis thaliana: Genetic and physiological aspects. J. Exp. Bot. 2010, 61, 3995–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozgen, S.; Busse, J.S.; Palta, J.P. Influence of root zone calcium on shoot tip necrosis and apical dominance of potato shoot: Simulation of this disorder by ethylene glycol tetra acetic acid and prevention by strontium. HortScience 2011, 46, 1358–1362. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Chen, C.; Wang, J. Response of Amaranthus Tricolor to Cesium Stress in Hydroponic System: Growth, Photosynthesis and Cesium Accumulation. Available online: https://ssrn.com/abstract=4085206 (accessed on 25 May 2022).
- Lasat, M.M.; Fuhrmann, M.; Ebbs, S.D.; Cornish, J.E.; Kochian, L.V. Phytoremediation of a Radiocesium-Contaminated Soil: Evaluation of Cesium-137 Bioaccumulation in the Shoots of Three Plant Species; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1998; Volume 27, pp. 165–169. [Google Scholar]
- Schulze, E.-D.; Beck, E.; Buchmann, N.; Clemens, S.; Müller-Hohenstein, K.; Scherer-Lorenzen, M. Plant Ecology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2019; p. 926. [Google Scholar]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kováčik, J.; Blicharski, T. Effect of long-term strontium exposure on the content of phytoestrogens and allantoin in soybean. Int. J. Mol. Sci. 2018, 19, 3864. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wen, F.; Xu, C.; Tang, Y.; Luo, X. The uptake of Cs and Sr from soil to radish (Raphanus sativus L.)-potential for phytoextraction and remediation of contaminated soils. J. Environ. Radioact. 2012, 110, 78–83. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Saito, T.; Suzuki, H.; Ishii, Y. Uptake and translocation of cesium-133 in napiergrass (Pennisetum purpureum Schum.) under hydroponic conditions. Ecotoxicol. Environ. Saf. 2012, 82, 122–126. [Google Scholar] [CrossRef]
- Komínková, D.; Berchová-Bímová, K.; Součková, L. Influence of potassium concentration gradient on stable caesium uptake by Calla palustris. Ecotoxicol. Environ. Saf. 2018, 165, 582–588. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Luo, X.; Tang, Y. Phytoextraction ability of Amaranthus mangostanus L. from contaminated soils with Cs or Sr. Bioremediat. Biodegrad. 2015, 6, 277. [Google Scholar]
- Chen, M.; Tang, Y.L.; Ao, J.; Wang, D. Effects of strontium on photosynthetic characteristics of oilseed rape seedlings. Russ. J. Plant Physiol. 2012, 59, 772–780. [Google Scholar] [CrossRef]
- Chamba-Eras, I.; Griffith, D.M.; Kalinhoff, C.; Ramírez, J.; Gázquez, M.J. Native Hyperaccumulator Plants with Differential Phytoremediation Potential in an Artisanal Gold Mine of the Ecuadorian Amazon. Plants 2022, 11, 1186. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.; Pratas, J.; Varun, M.; D’Souza, R.; Paul, M.S. Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flore. In Envormental Risk Assessment of Soil Contamination’nın Içinde; Hernandez-Soriano, M.C., Ed.; Intechopen: London, UK, 2014. [Google Scholar]
- Zhang, Y.; Liu, G.J. Uptake, accumulation and phytoextraction efficiency of cesium in Gypsophila paniculata. Int. J. Phytoremediation 2019, 21, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Ghnaya, A.B.; Charles, G.; Hourmant, A.; Hamida, J.B.; Branchard, M. Physiological behaviour of four rapeseed cultivar (Brassica napus L.) submitted to metal stress. Comptes Rendus Biol. 2009, 332, 363–370. [Google Scholar] [CrossRef]
- Kalingan, M.; Rajagopal, S.; Venkatachalam, R. Effect of metal stress due to strontium and the mechanisms of tolerating it by Amaranthus caudatus Linn. Biochem. Physiol. 2016, 5, 2. [Google Scholar]
- Atapaththu, K.S.S.; Rashid, M.H.; Asaeda, T. Growth and oxidative stress of Brittlewort (Nitella pseudoflabellata) in response to cesium exposure. Bull. Environ. Contam. Toxicol. 2016, 96, 347–353. [Google Scholar] [CrossRef]
- Jing, Z.; Ke, C.; Yuan, Z.; Ye, Y. Accumulation and physio-biochemical responses of Salix paraplesia to caesium stress. Chin. J. Environ. Eng. 2016, 10, 1515–1520. [Google Scholar]
- Jing, X.U.; Yunlai, T.A.N.G.; Jianbao, W.A.N.G.; Dan, W.A.N.G. Study on the effects of cesium on photosynthesis of spinach. J. Nucl. Agric. Sci. 2015, 29, 986. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Fodor, F. Physiological responses of vascular plants to heavy metals. In Physiology and Biochemistry of Metal Toxicity and Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2002; pp. 149–177. [Google Scholar]
- Dixit, V.; Pandey, V.; Shyam, R. Differential antioxidative responses to cadmium in roots and leaves of pea (Pisum sativum L. cv. Azad). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Eapen, S.; D’souza, S. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, Y.; He, Z.; Ma, M. Cadmium accumulation and oxidative burst in garlic (Allium sativum). J. Plant Physiol. 2005, 162, 977–984. [Google Scholar] [CrossRef] [PubMed]

| Treatment (mg/kg−1) | Treatment | |||||
|---|---|---|---|---|---|---|
| 0 (ck) | 1 | 2 | 3 | 4 | 5 | |
| 88Sr | 0 | 10 | 20 | 40 | 80 | 160 |
| 133Cs | 0 | 10 | 20 | 40 | 80 | 160 |
| 88Sr + 133Cs (1:1) | 0 | 10 + 10 | 20 + 20 | 40 + 40 | 80 + 80 | 160 + 160 |
| Concentration (mg kg−1) | Plant Biomass (g dw Plant−1) | |||
|---|---|---|---|---|
| Shoot | Root | Total | ||
| 88Sr | 0 | 9.443a | 2.362a | 11.80a |
| 10 | 4.848bc | 1.133cd | 5.981bcd | |
| 20 | 4.431bc | 1.040de | 5.472bcd | |
| 40 | 4.321bc | 0.995de | 5.316bcd | |
| 80 | 4.193bc | 0.966de | 5.160bcd | |
| 160 | 3.501bc | 0.815ef | 4.317bcd | |
| Treatment mean for 88Sr | 5.122AB | 1.218AB | 6.341AB | |
| 133Cs | 0 | 9.443a | 2.362a | 11.80a |
| 10 | 3.373bc | 0.815ef | 4.188bcd | |
| 20 | 3.266bc | 0.787ef | 4.054bcd | |
| 40 | 3.217bc | 0.610fg | 3.828cd | |
| 80 | 2.838bc | 0.592fg | 3.431cd | |
| 160 | 2.054c | 0.470g | 2.525d | |
| Treatment mean for 88Sr | 4.031B | 0.939B | 4.971B | |
| 88Sr + 133Cs | 0 | 9.443a | 2.362a | 11.80a |
| 10 | 6.297ab | 1.499b | 7.797b | |
| 20 | 5.709bc | 1.378bc | 7.087bc | |
| 40 | 5.083bc | 1.209bcd | 6.293bcd | |
| 80 | 4.392bc | 1.068de | 5.460bcd | |
| 160 | 3.836bc | 0.925de | 4.762bc | |
| Treatment mean for 88Sr 133Cs | 5.793A | 1.406A | 7.199A | |
| Treatment × concentration interaction | NS | NS | NS | |
| Accumulation (µg. g dw−1) | TF | BCF | BCF | ||||
|---|---|---|---|---|---|---|---|
| Treatments | Concentrations | Shoot | Root | Total Plant | Shoot | Shoot | Total Plant |
| 88Sr | 0 | - | - | - | - | - | - |
| 10 | 156.23fg | 198.05f | 354.28 | 0.79e | 15.62bcd | 35.42ab | |
| 20 | 226.95fg | 341.52cde | 568.46de | 0.67de | 11.34cd | 28.42bc | |
| 40 | 448.64efg | 457.91abc | 906.55cd | 0.98d | 11.21cd | 22.66bcd | |
| 80 | 962.44bcd | 579.07ab | 1541.51bc | 1.65c | 12.03bcd | 19.26cde | |
| 160 | 1196.84abc | 621.38a | 1818.22 | 1.97b | 7.48d | 11.36ed | |
| Treatment mean for 88Sr | 598.22B | 439.58A | 1037.80AB | 1.212B | 11.53 | 23.42AB | |
| 133Cs | 0 | - | - | - | - | - | - |
| 10 | 125.79g | 143.12f | 268.90f | 0.88e | 12.57bcd | 26.89bcd | |
| 20 | 204.27fg | 217.48ef | 421.74ef | 0.94d | 10.21cd | 21.08bcd | |
| 40 | 449.02efg | 240.05def | 689.06d | 1.87c | 11.22cd | 17.22cde | |
| 80 | 838.6cde | 426.96abc | 1265.5c | 1.97b | 10.48cd | 15.81def | |
| 160 | 1132.27abc | 606.16a | 1738.4b | 1.93b | 7.07d | 10.86ef | |
| Treatment mean for 133Cs | 549.99B | 326.75B | 876.72B | 1.518B | 18.37B | ||
| 88Sr + 133Cs | 0 | - | - | - | - | - | - |
| 10 | 257.65fg | 158.72f | 416.37ef | 1.61c | 25.76a | 41.63a | |
| 20 | 422.69efg | 241.39def | 664.08de | 1.73b | 21.13ab | 33.20ab | |
| 40 | 648.1def | 401.38bcd | 1049.4cd | 1.59c | 16.20bcd | 26.23cde | |
| 80 | 1365.01ab | 488.59abc | 1853.5ab | 2.78a | 17.06bc | 23.16cde | |
| 160 | 1514.45a | 614.13a | 2128.5a | 2.51ab | 9.46cd | 13.30ef | |
| Treatment mean for 88Sr + 133Cs | 841.58A | 380.84AB | 1222.37A | 2.044A | 22.45A | 27.505A | |
| Treatment × concentration interaction | NS | NS | NS | NS | NS | NS | |
| 88Sr Treatment | |||||
|---|---|---|---|---|---|
| Concentration in Soil (mg/kg) | Concentration in Plant (mg kg−1 DW) | Biomass of Total Plant (g /Plant) | Contents in Plant (mg/Plant) | Contents in Pot (mg/Pot) | Ratio b/w Contents in the plant\Contents in a Pot (%) |
| 0 | - | 11.80 | - | - | |
| 10 | 177.14 | 5.98 | 1.059 | 45 | 7.06 |
| 20 | 284.23 | 5.47 | 1.554 | 90 | 5.18 |
| 40 | 453.27 | 5.31 | 2.406 | 180 | 4.01 |
| 80 | 770.75 | 5.16 | 3.977 | 360 | 3.31 |
| 160 | 909.10 | 4.31 | 3.918 | 720 | 1.63 |
| 133Cs treatment | |||||
| 0 | - | 11.80 | - | - | |
| 10 | 134.45 | 4.18 | 0.562001 | 45 | 3.74 |
| 20 | 210.87 | 4.05 | 0.854024 | 90 | 2.84 |
| 40 | 344.53 | 3.82 | 1.316105 | 180 | 2.19 |
| 80 | 632.77 | 3.43 | 2.170401 | 360 | 1.80 |
| 160 | 869.21 | 2.52 | 2.190409 | 720 | 0.91 |
| 88Sr + 133Cs treatment | |||||
| 0 | - | 11.80 | - | - | |
| 10 | 208.18 | 7.79 | 1.621 | 45 | 10.80 |
| 20 | 332.04 | 7.08 | 2.350 | 90 | 7.83 |
| 40 | 524.74 | 6.29 | 3.300 | 180 | 5.5 |
| 80 | 926.79 | 5.46 | 5.060 | 360 | 4.21 |
| 160 | 1064.2 | 4.76 | 5.065 | 720 | 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.; Wang, D.; Kaleri, A.R.; Baloch, S.B.; Brtnicky, M.; Kucerik, J.; Mustafa, A. Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils. Agronomy 2022, 12, 1311. https://doi.org/10.3390/agronomy12061311
Ali S, Wang D, Kaleri AR, Baloch SB, Brtnicky M, Kucerik J, Mustafa A. Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils. Agronomy. 2022; 12(6):1311. https://doi.org/10.3390/agronomy12061311
Chicago/Turabian StyleAli, Shahzaib, Dan Wang, Abdul Rasheed Kaleri, Sadia Babar Baloch, Martin Brtnicky, Jiri Kucerik, and Adnan Mustafa. 2022. "Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils" Agronomy 12, no. 6: 1311. https://doi.org/10.3390/agronomy12061311
APA StyleAli, S., Wang, D., Kaleri, A. R., Baloch, S. B., Brtnicky, M., Kucerik, J., & Mustafa, A. (2022). Physiological Responses and Phytoremediation Abilities of Cucumber (Cucumis sativus L.) under Cesium and Strontium Contaminated Soils. Agronomy, 12(6), 1311. https://doi.org/10.3390/agronomy12061311







