Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Conditions
2.2. Drought Treatment
2.3. Characterization of Iron Oxide Nanoparticles and Treatment Application
2.4. Analysis of Biomass Production (ABP)
2.5. Measurement of Total Chlorophyll Contents (MTCC)
2.6. Extraction of Anti-Oxidant Enzymes
2.6.1. Estimation of Superoxide Dismutase (SOD) Activity
2.6.2. Determination of Peroxidase (POD) Activity
2.6.3. Determination of Catalase (CAT) Activity
2.7. Malondialdehyde Contents
2.8. Hydrogen Peroxide Values
2.9. Agronomic Profile
2.10. Statistical Analysis
3. Results
3.1. Characterization
3.2. Biomass Production
3.3. Agronomic Profile
3.4. Osmotic Stress Indicators and Antioxidant Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somerville, C.; Briscoe, J. Genetic Engineering and Water. Science 2001, 292, 2217. [Google Scholar] [CrossRef]
- Cornic, G.; Massacci, A. Leaf Photosynthesis under Drought Stress. In Photosynthesis and the Environment; Springer: Dordrecht, The Netherlands, 1996; pp. 347–366. [Google Scholar]
- Yokota, J.; Takuma, D.; Hamada, A.; Onogawa, M.; Yoshioka, S.; Kusunose, M.; Miyamura, M.; Kyotani, S.; Nishioka, Y. Scavenging of Reactive Oxygen Species by Eriobotrya japonica Seed Extract. Biol. Pharm. Bull. 2006, 29, 467–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Oomah, B.D. Flaxseed as a functional food source. J. Sci. Food Agric. 2001, 81, 889–894. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar] [CrossRef] [Green Version]
- Qamar, H.; Ilyas, M.; Shabbir, G.; Irshad, G.; Nisar, F.; Abbas, S.M.; Ghias, M.; Arshad, A. 1. Flax: Ancient to modern food. Pure Appl. Biol. PAB 2019, 8, 2269–2276. [Google Scholar] [CrossRef]
- Mazhar, M.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Hayat Bhatti, K.; Azeem, M.; Thind, S.; Ajaib, M.; Maqbool, M.; Sardar, T.; et al. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar]
- Burke, D.J.; Zhu, S.; Pablico-Lansigan, M.P.; Hewins, C.R.; Samia, A.C.S. Titanium oxide nanoparticle effects on composition of soil microbial communities and plant performance. Biol. Fertil. Soils 2014, 50, 1169–1173. [Google Scholar] [CrossRef]
- Rui, Y.; Zhang, P.; Zhang, Y.; Ma, Y.; He, X.; Gui, X.; Li, Y.; Zhang, J.; Zheng, L.; Chu, S.; et al. Transformation of ceria nanoparticles in cucumber plants is influenced by phosphate. Environ. Pollut. 2015, 198, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nhan, L.V.; Ma, C.; Rui, Y.; Liu, S.; Li, X.; Xing, B.; Liu, L. Phytotoxic mechanism of nanoparticles: Destruction of chloroplasts and vascular bundles and alteration of nutrient absorption. Sci. Rep. 2015, 5, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rehman, Z.M.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Ali, L.; Hussain, A.; Chatha, S.A.S.; Imran, M. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 248, 358–367. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed Biofortification by Engineered Nanomaterials: A Pathway To Alleviate Malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conven-tional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Ye, L.; Li, L.; Wang, L.; Wang, S.; Li, S.; Du, J.; Zhang, S.; Shou, H. MPK3/MPK6 are involved in iron deficiency-induced ethylene production in Arabidopsis. Front. Plant Sci. 2015, 6, 953. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Xu, X.; Wu, F.; Li, J. Synthesis of cavity-containing iron oxide nanoparticles by hydrothermal treatment of colloidal dispersion. Mater. Lett. 2016, 164, 210–212. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Tuteja, S.K.; Kim, K.-H. Nanovehicles for plant modifications towards pest- and disease-resistance traits. Trends Plant Sci. 2020, 25, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irum, S.; Jabeen, N.; Ahmad, K.S.; Shafique, S.; Khan, T.F.; Gul, H.; Anwaar, S.; Shah, N.I.; Mehmood, A.; Hussain, S.Z. Biogenic iron oxide nanoparticles enhance callogenesis and regeneration pattern of recalcitrant Cicer arietinum L. PLoS ONE 2020, 15, e0242829. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, M.M.; Mohamed, M.H.; Sadak, M.S.; Thalooth, A.T. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull. Natl. Res. Cent. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Johnson, R.; Puthur, J.T. Seed priming as a cost effective technique for developing plants with cross tolerance to salinity stress. Plant Physiol. Biochem. 2021, 162, 247–257. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef] [Green Version]
- Ali, Q.; Mazhar, M.W.; Ishtiaq, M.; Hussain, A.I.; Bhatti, K.H.; Maqbool, M.; Hussain, T.; Khanum, H.; Sardar, T.; Mazhar, M. Efficacy of Zn-Aspartate in comparison with ZnSO4 and L-Aspartate in amelioration of drought stress in maize by modulating antiox-idant defence; osmolyte accumulation and photosynthetic attributes. PLoS ONE. 2021, 16, e0260662. [Google Scholar] [CrossRef]
- Capra, A.; Consoli, S.; Scicolone, B. Deficit irrigation: Theory and practice. In Agricultural Irrigation Research Progress; Nova Science Publisher: Hauppauge, NY, USA, 2008; pp. 53–82. [Google Scholar]
- Khan, I.; Awan, S.A.; Raza, M.A.; Rizwan, M.; Tariq, R.; Ali, S.; Huang, L. Silver nanoparticles improved the plant growth and reduced the sodium and chlorine accumulation in pearl millet: A life cycle study. Environ. Sci. Pollut. Res. 2021, 28, 13712–13724. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef]
- Chau, N.H.; Doan, Q.H.; Chu, T.H.; Nguyen, T.T.; Trong, H.D.; Ngo, Q.B. Effects of Different Nanoscale Microelement-Containing Formulations for Presowing Seed Treatment on Growth of Soybean Seedlings. J. Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- do Espirito Santo Pereira, A.; Caixeta Oliveira, H.; Fernandes Fraceto, L.; Santaella, C. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Guha, T.; Ravikumar, K.V.; Mukherjee, A.; Mukherjee, A.; Kundu, R. Nanopriming with zero valent iron (nZVI) enhances germi-nation and growth in aromatic rice cultivar (Oryza sativa cv. Gobindabhog L.). Plant Physiol. Biochem. 2018, 127, 403–413. [Google Scholar] [CrossRef]
- Li, J.; Chang, P.R.; Huang, J.; Wang, Y.; Yuan, H.; Ren, H. Physiological effects of magnetic iron oxide nanoparticles towards wa-termelon. J. Nanosci. Nanotechnol. 2013, 13, 5561–5567. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Mazhar, M.; Ali, Q.; Ishtiaq, M.; Ghani, A.; Maqbool, M.; Hussain, T.; Mushtaq, W. Zinc-aspartate-mediated drought amelioration in maize promises better growth and agronomic parameters than zinc sulfate and l-aspartate. SABRAO J. Breed. Genet. 2021, 53, 290–310. [Google Scholar]
- Das, C.K.; Jangir, H.; Kumar, J.; Verma, S.; Mahapatra, S.S.; Philip, D.; Srivastava, G.; Das, M. Nano-pyrite seed dressing: A sustainable design for NPK equivalent rice production. Nanotechnol. Environ. Eng. 2018, 3, 1–4. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environ-mental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Chouliaras, V.; Therios, I.; Molassiotis, A.; Diamantidis, G. Iron Chlorosis in Grafted Sweet Orange (Citrus sinensis L.) Plants: Physiological and Biochemical Responses. Biol. Plant. 2004, 48, 141–144. [Google Scholar] [CrossRef]
- Yasmeen, F.; Raja, N.I.; Razzaq, A.; Komatsu, S. Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochim. Biophys. Acta BBA—Proteins Proteom. 2017, 1865, 28–42. [Google Scholar] [CrossRef] [PubMed]
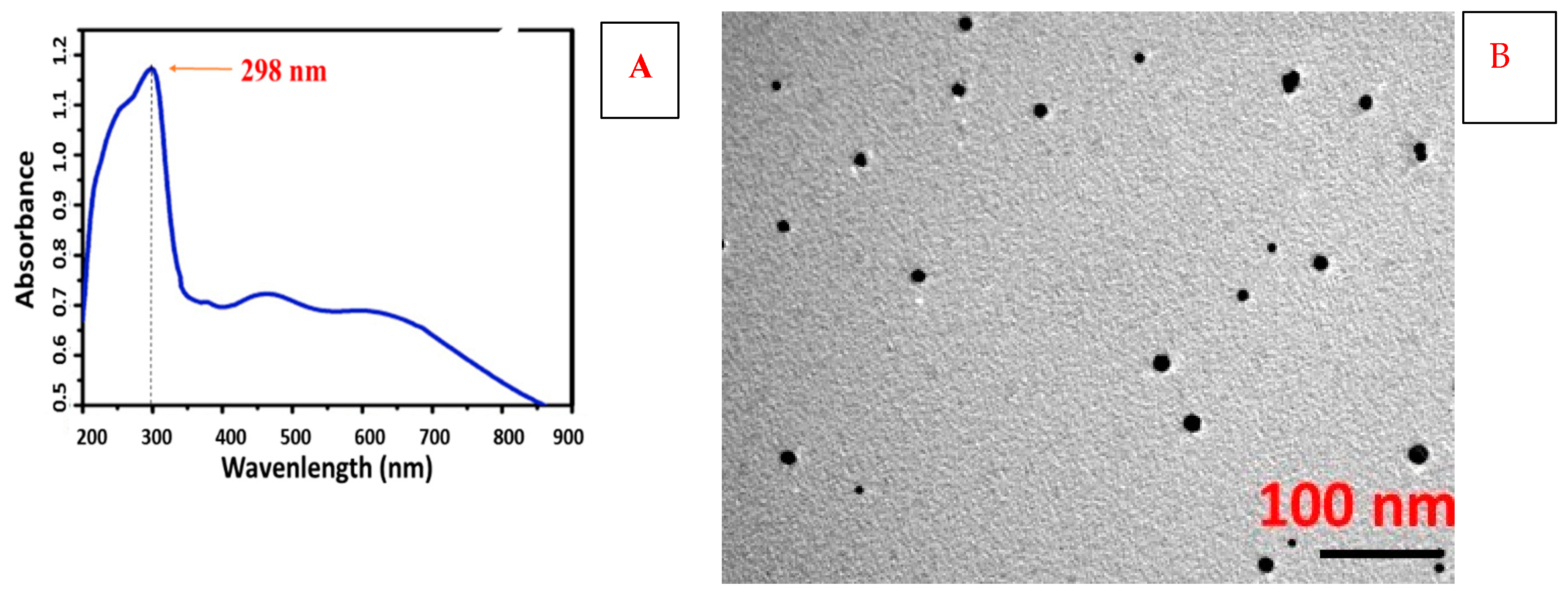
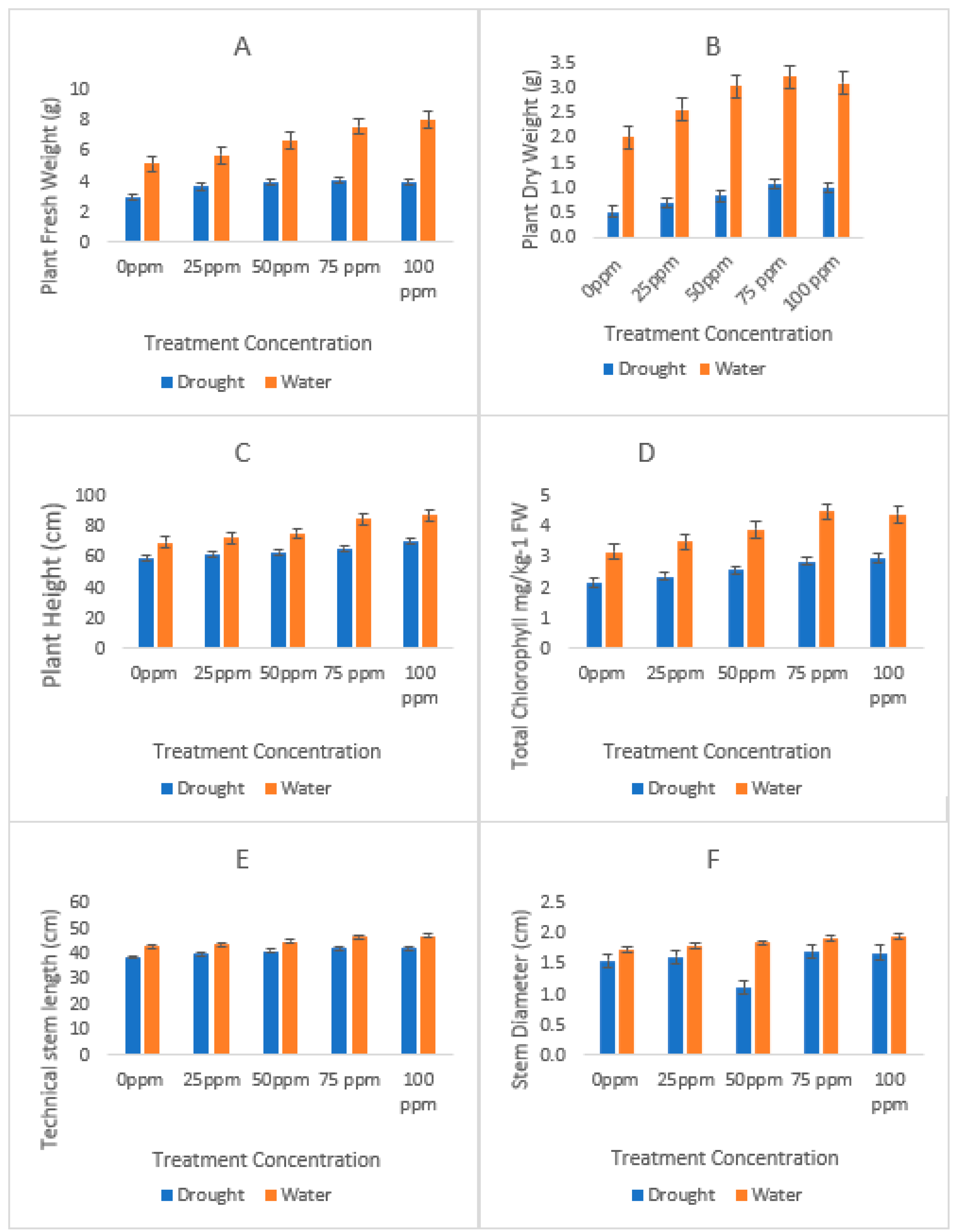
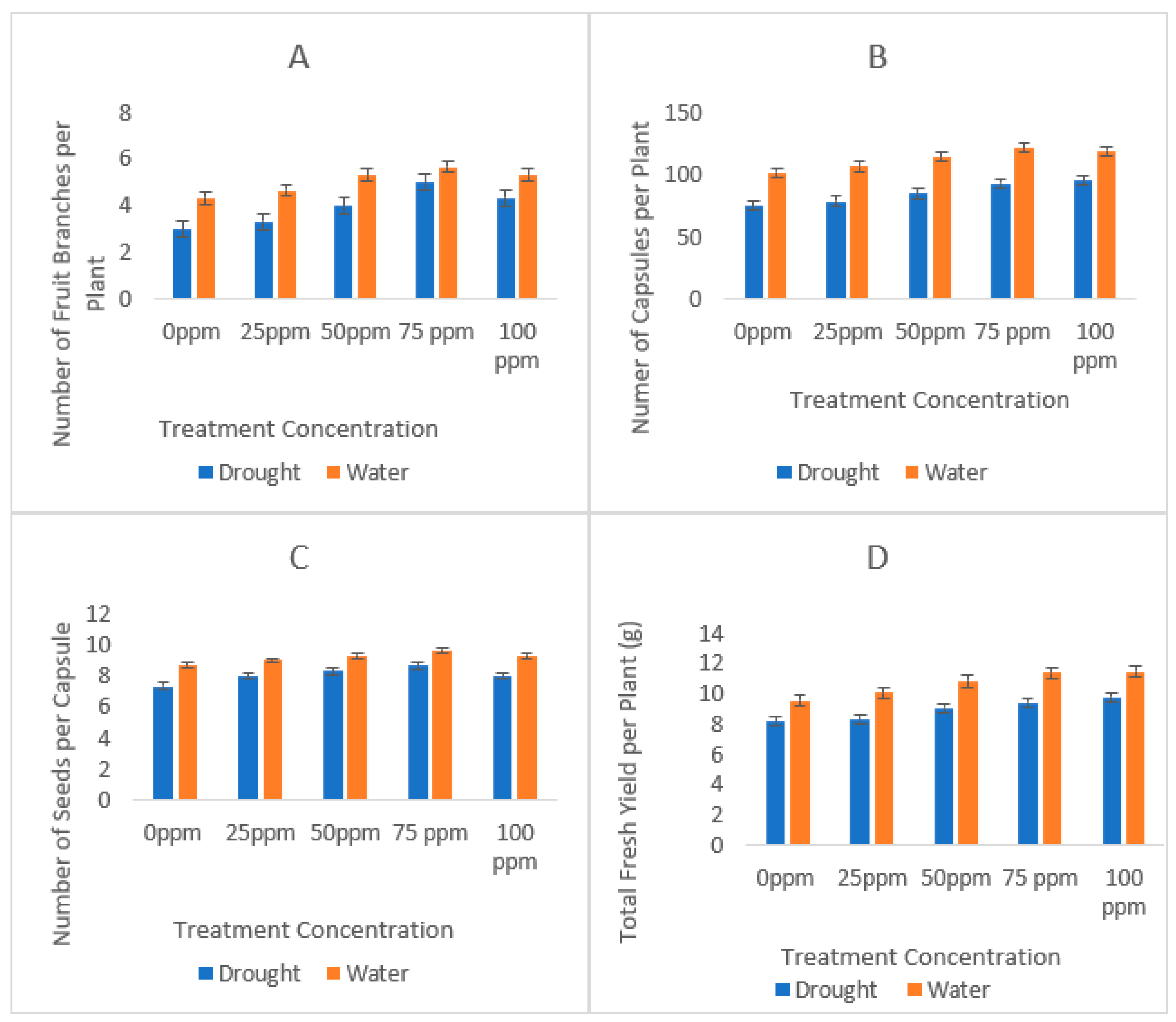

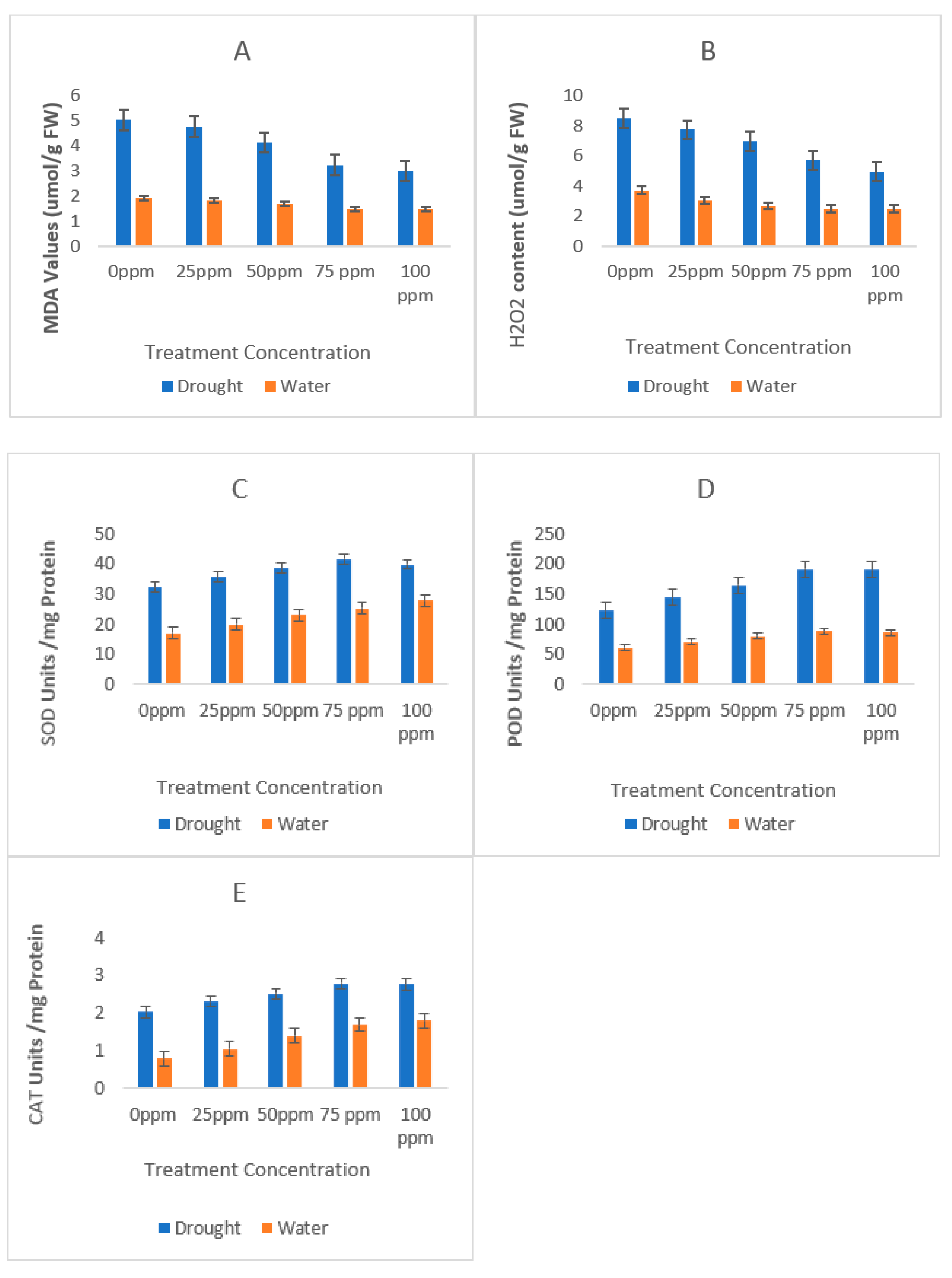
| Soil Character | Recorded Value |
|---|---|
| Soil Type | Clayey |
| EC | 1.99 dsM−1 |
| pH | 5.46 |
| Organic Matter | 1.99% |
| Sand | 28.01% |
| Silt | 23% |
| Clay | 47% |
| Treatment | Concentration mg/L or ppm |
|---|---|
| Control | One liter distilled water (D.W.) |
| 25 ppm | 25 mg of nanoparticles/L of D.W. |
| 50 ppm | 50 mg of nanoparticles/L of D.W. |
| 75 ppm | 75 mg of nanoparticles/L of D.W. |
| 100 ppm | 100 mg of nanoparticles/L of D.W. |
| Character | Value |
|---|---|
| Purity (%) | 98.7 |
| Surface Area | 179 m2/g |
| Density | 5.2 kg/L |
| Variation Source | a df | T.CHLO | TSL | SD | NFBP | H2O2 | MDA |
|---|---|---|---|---|---|---|---|
| Water Stress (WS) | 1 | 12.857 b,*** (0.000) | 132.720 *** (0.000) | 855.895 ns (0.338) | 9.633 *** (0.000) | 5.482 *** (0.000) | 41.418 *** (0.000) |
| Priming Treatment (PT) | 4 | 1.207 ***(0.000) | 16.463 *** (0.000) | 889.664 ns (0.430) | 2.666 *** (0.000) | 112.908 *** (0.000) | 1.782 *** (0.000) |
| WS X PT | 4 | 0.096 ***(0.000) | 0.452 ns (0.164) | 890.287 ns (0.430) | 0.133 ns (0.686) | 1.513 *** (0.000) | 0.765 *** (0.000) |
| Error | 20 | 0.012 | 0.248 | 0.898 | 0.233 | 0.083 | 0.015 |
| Variation Source | df | NCP | NSC | TFY | DSY | POD | CAT |
| Water Stress (WS) | 1 | 5548.8 ***(0.000) | 9.633 *** (0.000) | 22.188 *** (0.00) | 5.633 *** (0.000) | 54187.5 *** (0.000) | 9.633 *** (0.000) |
| Priming Treatment (PT) | 4 | 431.283 *** (0.000) | 1.116 ** (0.007) | 3.461 *** (0.000) | 1.089 *** (0.000) | 2553 *** (0.000) | 0.820 *** (0.000) |
| WS X PT | 4 | 6.716 ns (0.121) | 0.05 ns (0.9274) | 0.073 ns (0.1783) | 0.069 ns (0.077) | 510.166 *** (0.000) | 0.0203 ns (0.102) |
| Error | 20 | 3.233 | 0.233 | 0.041 | 0.028 | 38.5 | 0.009 |
| Variation Source | df | 1000 Wt | PH | PFW | PDW | SOD | |
| Water Stress (WS) | 1 | 7.510 ***(0.000) | 1477.008 *** (0.000) | 63.889 *** (0.000) | 29.323 *** (0.000) | 1675.371 *** (0.000) | |
| Priming Treatment (PT) | 4 | 0.529 *** (0.000) | 212.158 *** (0.000) | 3.947 *** (0.000) | 0.787 *** (0.000) | 92.352 *** (0.000) | |
| WS X PT | 4 | 0.030 * (0.023) | 24.0083 ** (0.002) | 1.129 **(0.002) | 0.134 ** (0.001) | 4.645 ** (0.009) | |
| Error | 20 | 0.008 | 4.058 | 0.188 | 0.019 | 1.033 |
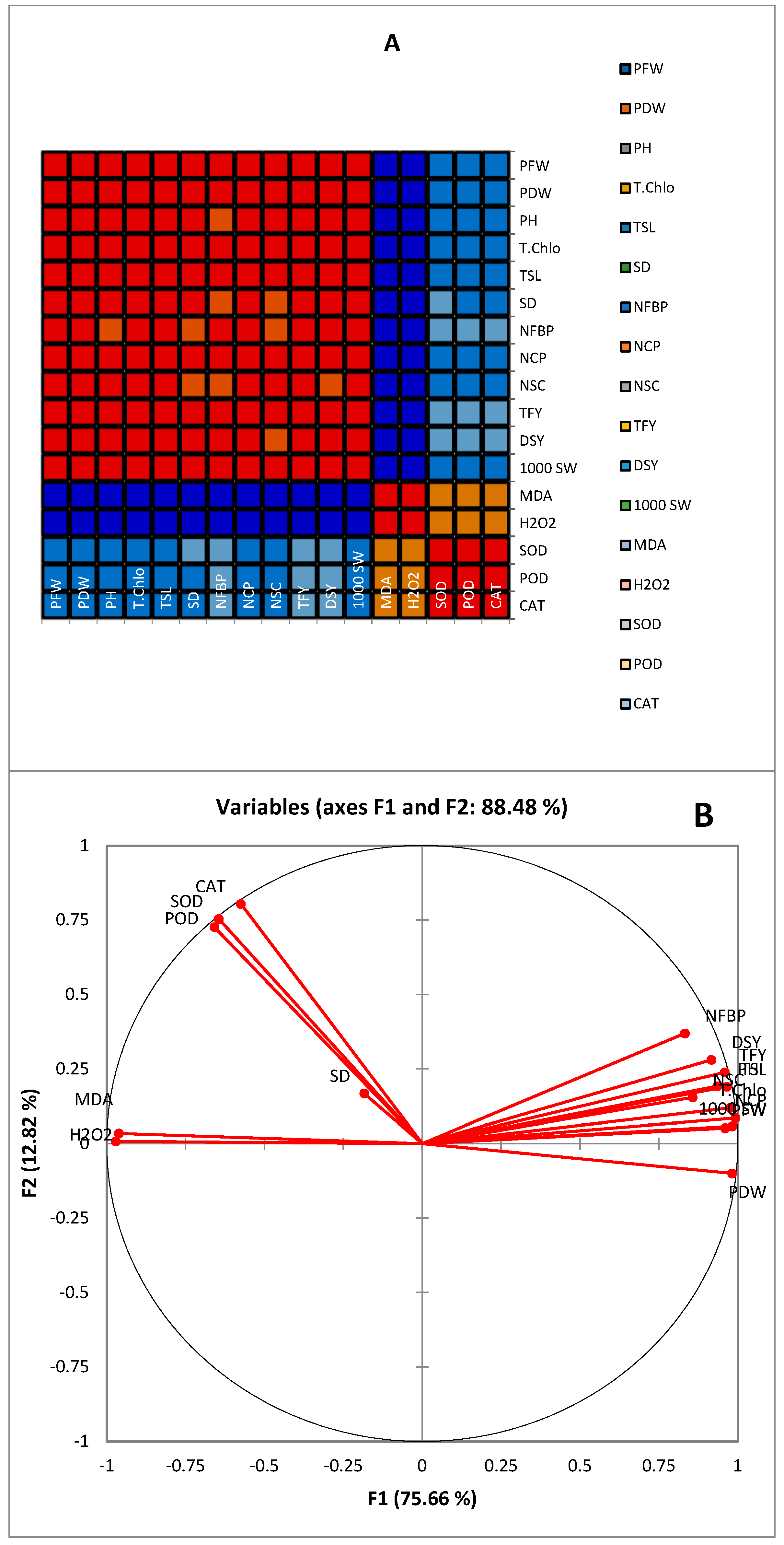
| Variables | PFW a | PDW | PH | T.Chlo | TSL | SD | NFBP | NCP | NSC | TFY | DSY | 1000 SW |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFW | 1 | |||||||||||
| PDW | 0.962 * | 1 | ||||||||||
| PH | 0.935 * | 0.950 * | 1 | |||||||||
| T.Chlo | 0.951 * | 0.973 * | 0.968 * | 1 | ||||||||
| TSL | 0.965 * | 0.975 * | 0.979 * | 0.987 * | 1 | |||||||
| SD | 0.875 * | 0.871 * | 0.861 * | 0.854 * | 0.863 * | 1 | ||||||
| NFBP | 0.817 * | 0.871 * | 0.774 * | 0.821 * | 0.828 * | 0.743 * | 1 | |||||
| NCP | 0.964 * | 0.976 * | 0.982 * | 0.989 * | 0.992 * | 0.863 * | 0.823 * | 1 | ||||
| NSC | 0.866 * | 0.867 * | 0.811 * | 0.829 * | 0.839 * | 0.775 * | 0.785 * | 0.846 * | 1 | |||
| TFY | 0.932 * | 0.944 * | 0.978 * | 0.970 * | 0.972 * | 0.838 * | 0.828 * | 0.972 * | 0.804 * | 1 | ||
| DSY | 0.880 * | 0.935 * | 0.913 * | 0.911 * | 0.904 * | 0.801 * | 0.859 * | 0.909 * | 0.796 * | 0.928 * | 1 | |
| 1000 SW | 0.953 * | 0.978 * | 0.972 * | 0.979 * | 0.979 * | 0.853 * | 0.817 * | 0.984 * | 0.822 * | 0.964 * | 0.918 * | 1 |
| MDA | −0.962 * | −0.966 * | −0.977 * | −0.983 * | −0.993 * | −0.858 * | −0.812 * | −0.992 * | −0.838 * | −0.963 * | −0.892 * | −0.976 * |
| H2O2 | −0.965 * | −0.981 * | −0.974 * | −0.981 * | −0.988 * | −0.863 * | −0.839 * | −0.988 * | −0.846 * | −0.963 * | −0.924 * | −0.983 * |
| SOD | −0.533 * | −0.527 * | −0.474 * | −0.522 * | −0.520 * | −0.397 * | −0.212 | −0.527 * | −0.439 * | −0.398 * | −0.338 | −0.531 * |
| POD | −0.550 * | −0.514 * | −0.471 * | −0.516 * | −0.530 * | −0.409 * | −0.208 | −0.527 * | −0.430 * | −0.394 * | −0.300 | −0.514 * |
| CAT | −0.544 * | −0.521 * | −0.460 * | −0.515 * | −0.515 * | −0.408 * | −0.219 | −0.524 * | −0.445 * | −0.383 * | −0.319 | −0.515 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waqas Mazhar, M.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy 2022, 12, 982. https://doi.org/10.3390/agronomy12050982
Waqas Mazhar M, Ishtiaq M, Maqbool M, Akram R, Shahid A, Shokralla S, Al-Ghobari H, Alataway A, Dewidar AZ, El-Sabrout AM, et al. Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy. 2022; 12(5):982. https://doi.org/10.3390/agronomy12050982
Chicago/Turabian StyleWaqas Mazhar, Muhammad, Muhammad Ishtiaq, Mehwish Maqbool, Raheel Akram, Adnan Shahid, Shadi Shokralla, Hussein Al-Ghobari, Abed Alataway, Ahmed Z. Dewidar, Ahmed M. El-Sabrout, and et al. 2022. "Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants" Agronomy 12, no. 5: 982. https://doi.org/10.3390/agronomy12050982
APA StyleWaqas Mazhar, M., Ishtiaq, M., Maqbool, M., Akram, R., Shahid, A., Shokralla, S., Al-Ghobari, H., Alataway, A., Dewidar, A. Z., El-Sabrout, A. M., & Elansary, H. O. (2022). Seed Priming with Iron Oxide Nanoparticles Raises Biomass Production and Agronomic Profile of Water-Stressed Flax Plants. Agronomy, 12(5), 982. https://doi.org/10.3390/agronomy12050982








