Abstract
Short-spike canarygrass (Phalaris brachystachys (Link.)) from Iranian wheat fields has developed resistance to acetyl-coenzyme A carboxylase (ACCase) inhibitors due to a target-site mutation (Ile-1781-Thr). Target-site resistance mutations may confer pleiotropic effects in weeds. In this paper, the possible effect of the Ile-1781-Thr mutation on the ecological fitness during life cycles in P. brachystachys plants was investigated. ACCase genes of P. brachystachys populations resistant (R) and susceptible (S) to ACCase inhibitors were sequenced and the vegetative growth and reproductive characteristics of the plants were assessed. In the final growth stage (217 days after planting—DAP), R sub-population plants were 30 cm taller than the S plants. Additionally, the R sub-population produced up to 12 leaves and 2.8 tillers more per plant, and accumulated double the dry weight (2850 g m−2) compared to the S sub-population. The leaf area index (LAI) of the R sub-population was 1.1 times higher than that of the S sub-population. In addition, the net assimilation rate (NAR) and plant growth rate (PGR) between 114 and 182 DAP of the R sub-population were 0.11 and 13 g m−2 d−1 higher than the S sub-population, but the relative growth rate (RGR) was similar between R and S sub-populations. The number of spikes (6 vs. 3.8), the spike length (8.4 vs. 5.5), and number of seeds per plant (1276 vs. 751 seed plant−1) of the R sub-population were higher than the S ones, but the weight (3.25 g) and size (11.6 mm2) of 1000 seeds were similar between populations. The R sub-population of P. brachystachys exhibited higher plant growth and reproductive parameters than the S one, which may increase the frequency of resistance in the population in the absence of adequate weed-control methods.
1. Introduction
Weeds are the most important biotic factor affecting crop production by reducing yields globally [1]. Herbicide use for chemical weed control is the most effective tool to prevent reduction in crop yields due to competition [2]. However, the intensive and continuous use of herbicides has imposed a strong selective pressure that leads to the evolution of resistant weeds [3]. Herbicide resistance (HR) is a growing problem that threatens our ability to manage weeds that put agricultural production at risk [4,5]. Currently, there are 509 HR cases to nearly all known herbicide modes of action [3].
Herbicides that act on acetyl-coenzyme A carboxylase (ACCase) inhibit the synthesis of de novo fatty acids, which are essential components of cell membranes and secondary plant metabolites [6]. These herbicides are used to selectively control grasses in crop fields [7], due to their effectiveness and convenience for weed management in post-emergence [8]. Currently 50 weed species have been reported to be resistant to ACCase inhibitors (260 cases) worldwide [3]. Resistance to ACCase-inhibitors generally involves the detoxification of herbicides via enhanced metabolism (non-target site resistance, NTSR) or the modification of herbicide binding site on the ACCase via mutations (target-site resistance, TSR) [9]. These resistance mechanisms may confer varying degrees of resistance to different chemical classes of ACCase-inhibitors [10].
Herbicide target-site mutations conferring resistance are expected to be accompanied by some direct pleiotropic effects on plant growth [11] and in some cases on the reproductive capacities of the resistant population. One of the most important factors that influence the evolution and persistence of herbicide-resistant biotypes is the fitness, which describes the capacity of a plant to establish, survive, and reproduce in the environment. Plant fitness plays an important role in natural selection and adaptation [12]. The fitness cost associated with resistance alleles determines rate of herbicide-resistant evolution and the maintenance of genetic polymorphisms within populations [13]. Alleles that confer increased adaptive value in one environment may be detrimental to fitness in another environment [14]. The dynamics of HR in plants depend on fitness characteristics resulting from HR both in the presence and absence of herbicide selection [15].
Fitness costs depend on genetic variation, resistance mechanisms, and environmental conditions [16,17], i.e., the same mutation may affect vegetative growth and the allocation of resources in the vegetative and reproductive stage at different magnitudes in the same or different weed species [18]. For example, the Ile-2041-Asn mutation in the ACCase gene decreased vegetative biomass and seed production in Hordeum glaucum, while the Ile-1781-Leu/Val mutation did not represent any fitness cost in the same species [19]. However, the Ile-1781-Leu mutation conferred higher reproductive and germinative fitness in Setaria viridis [9]. In some cases, such as in Avena sterilis [20] and Alopecurus myosuroides [21], no fitness costs of resistance were reported. Therefore, accurately characterizing the impact of a given target-site mutation on fitness helps to understand the dynamics of HR evolution to implement appropriate resistant weed management strategies [15,22].
Changes in the ecological character of resistant individuals can change the competitive ability and dynamics of resistant populations, affecting resistance management and prevention [23,24,25]. Herbicide resistance strategies should aim to prevent or delay the evolution of herbicide resistance and whenever resistant weed biotypes appear, management strategies should aim to reduce the number and frequency of resistant plants within populations. It is necessary to evaluate fitness costs of each case of resistance individually to develop robust resistance management programs [26]. Short-spike canarygrass (Phalaris brachystachys (Link.)) is among the most important gramineous weeds in wheat and barley fields in West Asia [27]. To date, P. brachystachys was reported as being resistant to ACCase-inhibitors in Iran, Turkey, Italy, and Syria. In 2014, populations of P. brachystachys with cross-resistance to ACCase-inhibitors, carrying the Ile-1781-Thr mutation, were found in wheat fields from Golestan, Iran [28,29]. The objectives of this study were to evaluate plant growth characteristics of resistant and susceptible populations under non-competitive conditions.
2. Materials and Methods
2.1. Seed Source and Genetic Background Control
The resistant (R) population of P. brachystachys from the Golestan province in Iran (36°53′53.78″ N, 54°47′24.38″ E), carrying the Ile-1781-Thr mutation that confers cross-resistance to the ACCase-inhibiting herbicides diclofop-methyl, cycloxidim, and pinoxaden, was used in this study [29]. To minimize variability in genetic background, a single-population plant cloning technique was used to select for susceptible (S) and R plants within the population [15,22].
Seeds of 50 individuals of the R population were collected and then grown to the tillering stage. At the 2–3 tiller stage, plants were excavated from pots and 2 tillers were carefully excised (with intact roots) from each parent plant. One ‘clone’ from each individual was sprayed with the label recommended rate of diclofop-methyl (900 g ai ha−1) using a laboratory track sprayer equipped with a Tee Jet 8002E-VS flat-fan nozzle (Devries Manufacturing, Hollandale, MN, USA) delivering a spray volume of 300 L ha−1 at 200 kPa. Four weeks after herbicide treatment, R and S plants were assessed and the phenotype of the respective untreated clone was identified. Plants which appeared to be alive, but did not display vigorous new growth, were unclassified and dead plants were classified as S. The living plants and vigorous regrowth were classified as R. Twenty clones of each R and S population were grown separately, and a group of cloned plants were subjected to restricted cross-pollination to prevent gene flow between phenotypes. Upon maturity, seeds were collected separately for R and S sub-populations.
To confirm the resistance status of the R and S sub-populations selected within the mother population, 10 progeny individuals were randomly selected within each phenotype (R and S), and resistance and susceptibility to ACCase-inhibitors were verified by applying diclofop methyl (900 g ai ha−1). Selected lines were developed over two generations of group recurrent selection to obtain a sufficient number of seeds for the experiments. Additionally, the homozygosity of the progeny plants for the specific ACCase gene mutation in each purified population was confirmed using PCR-based marker analysis. F2 seeds of S and R sub-populations were collected at maturity and stored at 4 °C until fitness studies. Mean seed weight was calculated for homogeneity, without differences among populations (27 ± 0.15 mg, n = 100).
2.2. Evaluation of Growth
In order to evaluate growth traits and seed production of S and R P. brachystachys sub-populations, an experiment was conducted in a completely randomized design during November 2018 to June 2019. To prevent the spread of resistant seeds, the experiment was performed on plants growing in pots, rather than under field conditions. All pots were placed outdoors in an area covered with netting. To ensure uniformity, seeds of S and R sub-populations were pre-germinated (to break seed dormancy, seeds were immersed in sulfuric acid (98%) for 3 min) in Petri dishes containing filter paper moistened with 5 mL distilled water for 72 h in a refrigerator at 4 °C in the dark [28]. Then, 5 mL of distilled water was added to each Petri dish and placed in a 20 °C incubator.
The seedlings approximately 1 cm in root length were transplanted to square plastic pots (0.25 m2) containing peat and sandy loam soil (1:2, w/w). Plants of each sub-population were isolated. Each pot was one experimental unit composed of 8 rows (15 cm spaced) of 6 plants per row. Pots were irrigated as needed and weeding was performed to ensure that only P. brachystachys seeds that had been planted in pots grew throughout the experiment.
The plant height, the number of tillers per plant, leaf number, leaf area, and dry weight were measured at 49, 82, 114, 154, 182, and 217 days after planting (DAP). The first and last rows of each pot were considered margins and sampling of these plants was not performed to eliminate marginal effects. The leaf area of each harvested plant was measured using a digital leaf area meter (LiCor 3100; LiCor, Lincoln, NE, USA). Aboveground materials (shoot and leaves) were oven dried at 60 °C for 48 h and weighed. At maturity, the number of spikes per plant, spike length, 1000-seed weight, and seed size were recorded. This study was performed in three replications and each pot was used as a replication. At each sampling stage, 3 pots were sampled for each R and S sub-population.
2.3. Determination of Physiological Parameters of S and R Sub-Populations
Physiological parameters including leaf area index (LAI), leaf area ratio (LAR), net assimilation rate (NAR), relative growth rate (RGR), and plant growth rate (PGR) were estimated from measured plant traits using Equations (1)–(5), as follows [30]:
where LA is the total leaf area per plant (m2), GA is the ground surface area (m2), W is total dry weight per plant (g), t indicates the time, LnW is the natural logarithm of dry weight per plant, and LnL is the natural logarithm of leaf area per plant.
2.4. Determination of Physiological Parameters of S and R Populations
To evaluate the plant height, leaf number, tiller number, dry weight, LAI, and LAR of R and S sub-populations, a functional three-parameter sigmoid model Equation (6) was used [31]:
where y is the response variable, Amax is the maximum quantity of the trait assessed; t50 represents days that provide 50% of the response of the variable and b is the curve slope at t50.
The changes in NAR and PGR were described by fitting the beta model Equation (7) and RGR with the linear regression model Equation (8), as follows [30]:
where Amax is the maximum of NAR or PGR; tb NAR or PGR initiation; tp indicates the time at which PGR or NAR reach maximal values; and tc represents the time at which PGR or NAR end. In Equation (8), a is slope of the line, b the y-intercept, and x is the independent variable (it is plotted on the x-axis).
One thousand seeds from R and S of P. brachystachys sub-populations were weighed using an electronic balance (0.0001 g accuracy). Fifty seeds were randomly selected from each sub-population. For every individual seed, three main dimensions, which included length (L), width (W), and thickness (T), were measured using a digital caliper (0.001 mm accuracy). The geometric mean diameter (D) was calculated based on the three dimensions determined using Equation (9), and seed area (mm2) was calculated using Equation (10), as follows [32]:
The analysis of the traits was performed using PROC GLM in SAS v.10.2 (SAS Institute, Inc., Cary, NC, USA). Means were assessed using the least significant difference (LSD) test at a significance level of p = 0.05. All figures were prepared using SigmaPlot software v.12.5 (Systat Software, Inc., San Jose, CA, USA).
3. Results
3.1. Comparative Growth Analysis
The evaluated growth parameters, with the exception of LAR, differed between R and S P. brachystachys sub-populations (Table 1). The S plants had a shorter height than R plants from the vegetative and reproductive stages (49 to 217 DAP). R plants were up to 30 cm taller than the S plants at 217 DAP (Figure 1A). The number of leaves and tillers per plant, and dry weight, did not differ between R and S sub-populations at early growth stages. However, from 114 DAP, the R plants produced up to 12 leaves and 2.8 tillers more per plant than the S sub-population. At 217 DAP, the R plants had 36.6 leaves and 6.6 tillers per plant, while the S sub-population had 24.7 leaves and 3.8 tillers per plant (Figure 1B,C). From 154 DAP, the dry weights of the R sub-population were greater than those of the S sub-population, reaching 2850 g m−2 at 217 DAP, double the dry weight recorded for the S sub-population (Figure 1D). LAI values were generally higher for the R sub-population. Initially, the LAI differences between R and S plants were not very large, but from 114 DAP, the LAI of R increased more than that of the S sub-population, reaching 3.8. and 2.7, respectively, at 217 DAP (Figure 1E). Finally, LAR values decreased in similar proportions and there were no differences between populations throughout the experimental period (Figure 1F).

Table 1.
Parameters of the three-parameter sigmoid model for plant height, leaf number, number of tillers, dry weight, leaf area index (LAI), and leaf area ratio (LAR) in resistant (R) and susceptible (S) sub-populations of P. brachystachys to the ACCase-inhibiting herbicides.
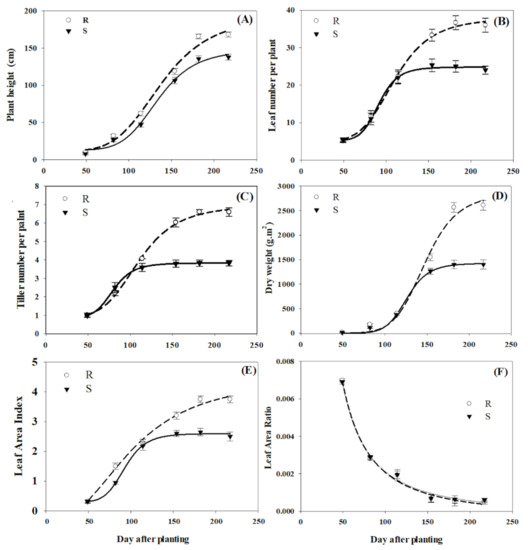
Figure 1.
Comparison of the growth characteristics of resistant (R) and susceptible (S) P. brachystachys sub-populations to the ACCase-inhibiting herbicides. (A) Plant height, (B) leaf number per plant, (C) tiller number per plant, (D) dry weight, (E) leaf area index, and (F) leaf area ratio.
3.2. Physiological Parameters
The estimated NAR and PGR for the R and S sub-populations of P. brachystachys were similar up to 82 DAP, but differed from 114 DAP (Figure 2, Table 2). The greatest increase in NAR and PGR occurred between 114 and 182 DAP. The maximum NAR values for R and S plants were 0.33 and 0.22 g m−2 d−1, respectively (Figure 2A), and of the PGR was recorded for the R sub-population (32.3 g m−2 d−1) (Figure 2B). On the other hand, RGR did not differ between sub-populations, and from 50 DAP and decreased in the same proportion for both sub-populations throughout the experimental period (Figure 2C).
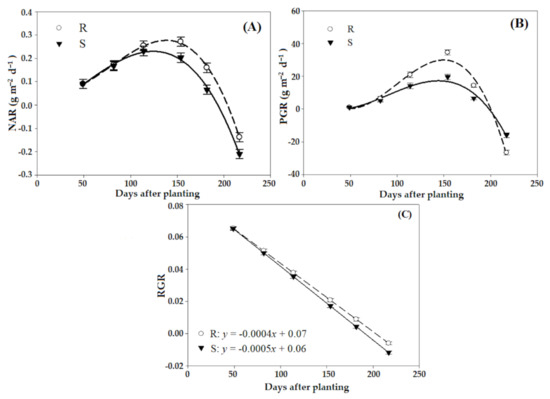
Figure 2.
Changes in growth characteristics of resistant (R) and susceptible (S) P. brachystachys sub-populations to the ACCase-inhibiting herbicides at different days after planting. (A) Net assimilation rates (NAR), (B) plant growth rates (PGR), and (C) relative growth rates (RGR) of populations.

Table 2.
Parameters of the beta model for net assimilation rate (NAR) and plant growth rate (PGR) of resistant (R) and sub-susceptible (S) P. brachystachys populations to the ACCase-inhibitors.
3.3. Seed Production
At the maturity stage, the number of spikes and seeds produced per plant and the length of the spikes were evaluated. The R sub-population produced 6 spikes per plant, 2.2 more spikes than the S sub-population. The spike length of the R sub-population (8.4 cm) was greater than that of the S sub-population (5.5 cm). R plants produced more than 500 seeds per plant than the S plants. The area (11.6 mm2) and weight (3.25 g) of 1000 seeds did not differ between the R and S sub-populations (Table 3).

Table 3.
Number of spikes, spike length, number of seeds, 1000-seeds weight, and seed area of resistant (R) and susceptible (S) P. brachystachys sub-populations to the ACCase-inhibiting herbicides.
4. Discussion
Considering that ACCase has a well-established role in the fatty acid synthesis [6], mutations conferring resistance to ACCase-inhibitors are expected to cause direct pleiotropic effects on the growth of resistant plants [11]. In this study we used a comprehensive approach to describe the ecological fitness of a R population of P. brachystachys carrying the Ile-1781-Thr mutation [29].
Plants of the R P. brachystachys sub-population were taller than those from the sub-population of S. Plant height is a good indicator of light interception, which directly influences the rate of photosynthesis and consequently the potential competition and reproduction [33]. In addition, R plants presented a greater number of leaves and tillers per plant in relation to S plants, which explains the greater dry weight accumulation of the R sub-population. The accumulation of dry weight depends on photosynthetic activity, and the plants that produce large dry weight amounts in the shortest time tend to be more competitive for resources (water, nutrients, light, space, etc.) than other plants, being able to suppress the growth of other plants in resource-limited environments [34]. The greater number of leaves contributed to the increase in LAI in R plants. Plants with a higher LAI are able to fix CO2 more efficiently than those with a lower LAI, due to a greater interception of light, i.e., plants with a high LAI are better adapted to ecological competition and crop management practices [35]. The drop in LAR was similar for the R and S sub-populations, i.e., the efficiency with which the R and S plants used their leaves to produce plant material was of the same magnitude, regardless of height, number of leaves, and/or the number of growers. Decreases in LAR occur when vegetative and reproductive structures with competing plant drains reduce leaf assimilates [36].
Phalaris brachystachys plants with the Ile-1781-Thr mutation exhibited higher NAR and PGR than S plants, indicating a positive association with the efficiency of R plants in CO2 capture and assimilation, as well as storage of photo assimilates [34]. Changes in NAR, PGR, and LAR modify the establishment of a plant by altering the use of resources and fitness [30]. The higher LAR of the R sub-population was associated with the greater number of leaves and leaf area compared to those of the S sub-population. In addition, the RGR of the R and S sub-populations were similar during the growth period (up to 88 DAP), which means that both sub-populations had the same growth behavior in the vegetative stage in the absence of stressful conditions. The RGR in the early growth stages is higher than at other times due to rapid plant growth and low initial plant weight [37].
The highest RGR peak recorded between 114 and 182 DAP reflects the relationship between dry weight accumulation and LAI, which was higher in the R sub-population. An increase in RGR contributes to the suppression of other plants [38], i.e., R P. brachystachys plants could suppress/limit the establishment and growth of S plants under field conditions. The decrease in RGR over time is due to mutual shading of the leaves and the increase in reproductive (photosynthetically inactive) tissues [39]. Growth differences between P. brachystachys populations suggest that the presence of a target site resistance trait (Ile-1781-Thr mutation) in the R population has a pleiotropic effect on growth. In annual weeds, changes in growth characteristics and plant size are positively correlated with altered reproductive traits [14].
In this study, the R sub-population of P. brachystachys showed greater reproductive capacities than the S sub-population, by producing more seeds per plant due to a greater number of reproductive tillers and spikes per plant, as well as a greater length of the spikes. Larger individual plants are capable of obtaining more resources than smaller plants; thus producing more seeds and biomass [20]. The production of viable seeds is an important reproductive trait that contributes to absolute fitness [19,40]. Previous studies of various weeds have shown that seed production is improved in R versus S populations [41,42]. For example, seed production of an ACCase-resistant biotype of S. viridis with the Ile-1781-Leu mutation was higher than that of the S biotype [9]. Studies found that the ACCase (Asp-2078-Gly) mutation in Lolium rigidum and Alpecurus myosuroides is also associated with fitness costs [40,43]. The homozygous Gly-2078 ACCase A. myosuroides showed a substantial fecundity and vegetative reduction [43]. In contrast, S populations of L. rigidum presented higher values of early vigor and grain weight compared to R populations (Ile-1781-Leu and Leu-2088-Arg) [44]. The seed production of an ACCase-TSR S. viridis phenotype carrying the Leu-1781 mutation was higher than the wild phenotype [9]. However, other ACCase mutations (Ile-1781-Leu) in L. rigidum do not show any reduction in the fitness traits which have a similar ACCase activity to the wild-type [45]. Additionally, many studies have found no fitness penalty associated with ACCase-inhibitor resistance in R biotypes [20,21,46]. In our study, there were no differences between sub-populations with respect to weight and size of 1000-seeds. In this context, differences in seed production between P. brachystachys sub-populations may determine their persistence and frequency within plant populations in the field in the absence of herbicide selection, which in this case, favor the R population.
In general, the fitness costs associated with resistance to ACCase inhibitors vary by species, resistance mechanism, the identity of the mutated allele that confers resistance, genetic background, experimental (greenhouse, field), competitive and non-competitive conditions, and environmental conditions [9,40,47]. In future studies, all aspects of fitness traits, including potential seed production and vegetative growth should be measured in resistant and susceptible plants in competitive and noncompetitive conditions. Environmental conditions can the affect fitness cost [43], i.e., our results cannot be generalized to other conditions. Additionally, there could be an interaction between the Ile-1781-Thr mutation and the genetic background. To reinforce these results, future research should focus on monitoring the frequency of the Ile-1781-Thr mutation for several years under field conditions, in the absence of ACCase herbicide selection, using R and S phenotypes.
5. Conclusions
The pleotropic effects on growth and reproductive traits derived from the presence or absence of the Ile-1781-Thr mutation may be important for the control of P. brachystachys in wheat fields from Golestan, Iran. The differences in plant traits between resistant and susceptible plants observed in this research can be used in management programs. These differences indicate that resistant biotypes are more productive in resource utilization and are likely to be more successful than sensitive biotypes in competitive conditions. In addition, resistant biotypes produced more seeds than susceptible biotypes, which could increase the population of environmentally resistant biotypes in their environment. This indicates the importance of controlling resistant biotypes and preventing their spread in the region. In a situation where ACCase-resistance is well established, a longer-term management based on crop rotation, lack of selection pressure from ACCase-inhibiting herbicides, and the use of any other available control tool should help to induce some change in the R/S plant ratio. The management practices such as competitive crops, sowing at high density, and planting competitive cultivars are suggested as management strategies to inhibit this weed. However, attention should be paid to how weed management measures are implemented, because the differences in vegetative and reproductive traits such as plant height, number of tillers, number of leaves, dry weight accumulation, and seed production between P. brachystachys sub-populations indicate that the R plants have more growth and reproduction characteristics than S plants. Therefore, based on the confirmed cross-resistance to ACCase inhibitors at target-site level in P. brachystachys, steps should be taken to delay the selection of new resistant populations, using integrated weed management techniques involving chemical and non-chemical approaches.
Author Contributions
Conceptualization, J.G. and R.D.P.; methodology, S.G., F.G.-F. and B.K.; software, S.G., F.G.-F. and B.K.; validation, S.G., J.G. and R.D.P.; formal analysis, S.G., M.D.O. and R.A.-d.l.C.; investigation, S.G.; resources, R.D.P. and J.G.; data curation, S.G. and M.D.O.; writing—original draft preparation, S.G., J.G. and R.A.-d.l.C.; writing—review and editing, J.G., R.D.P. and R.A.-d.l.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Spanish Government, through project AGL2017-83325-C4-2-R and the Asociación de Agroquímicos y Medioambiente.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank SCAI (Servicio Central de Apoyo a la Investigación) of the University of Cordoba, Spain for assistance with sequencing and the Gorgan University of Agricultural Science and Natural Resource (GUANR), Iran for providing the fitness experiments equipment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Colbach, N.; Chauvel, B.; Darmency, H.; Délye, C.; Le Corre, V. Choosing the best cropping systems to target pleiotropic effects when managing single-gene herbicide resistance in grass weeds. A blackgrass simulation study. Pest Manag. Sci. 2016, 72, 1910–1925. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Marjanovic, J.; Gornicki, P. Resistance to herbicides caused by single amino acid mutations in acetyl-CoA carboxylase in resistant populations of grassy weeds. New Phytol. 2013, 197, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. International Survey of Herbicide Resistant Weeds. 2022. Available online: http://www.weedscience.org (accessed on 11 April 2022).
- Owen, M.J.; Michael, P.J.; Renton, M.; Steadman, K.J.; Powles, S.B. Towards large-scale prediction of Lolium rigidum emergence. II. Correlation between dormancy and herbicide resistance levels suggests an impact of cropping systems. Weed Res. 2011, 51, 133–141. [Google Scholar] [CrossRef]
- Panozzo, S.; Scarabel, L.; Rosan, V.; Sattin, M. A new Ala-122-Asn amino acid change confers decreased fitness to ALS-resistant Echinochloa Crus-Gall. Front. Plant Sci. 2017, 8, 2042. [Google Scholar] [CrossRef]
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Weed resistance to acetyl coenzyme A carboxylase inhibitors: An update. Weed Sci. 2005, 53, 728–746. [Google Scholar] [CrossRef]
- Busi, R.; Vila-Aiub, M.M.; Beckie, H.J.; Gaines, T.A.; Goggin, D.E.; Kaundun, S.S.; Lacoste, M.; Neve, P.; Nissen, S.J.; Norsworthy, J.K.; et al. Herbicide resistant weeds: From research and knowledge to future needs. Evol. Appl. 2013, 6, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Picard, J.C.; Tian, X.; Darmency, H. A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity 2010, 105, 394–400. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Neve, P.; Powles, S. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium Rigidum. Theor. Appl. Genet. 2005, 110, 1154–1166. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, T.; Pan, L.; Wang, L.; Xu, H.; Dong, L. Germination requirements differ between fenoxaprop-P-ethyl resistant and susceptible Japanese foxtail (Alopecurus japonicus) biotypes. Weed Sci. 2016, 64, 653–663. [Google Scholar] [CrossRef]
- Frenkel, E.; Matzrafi, M.; Rubin, B.; Peleg, Z. Effects of environmental conditions on the fitness penalty in herbicide resistant Brachypodium Hybridum. Front. Plant Sci. 2017, 8, 94–102. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Gundel, P.E.; Preston, C. Experimental methods for estimation of plant fitness costs associated with herbicide-resistance genes. Weed Sci. 2015, 63, 203–216. [Google Scholar] [CrossRef]
- Yu, L.; Kim, Y.; Tong, L. Mechanism for the inhibition of the carboxyltransferase domain of acetyl-coenzyme A carboxylase by pinoxaden. Proc. Natl. Acad. Sci. USA 2010, 107, 22072–22077. [Google Scholar] [CrossRef]
- Fernandez-Moreno, P.; Alcántara-de la Cruz, R.; Smeda, R.J.; De Prado, R. Differential resistance mechanisms to Glyphosate result in fitness cost for Lolium perenne and L. multiflorum. Front. Plant Sci. 2017, 8, 1796. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Fitness costs associated with evolved herbicide resistance genes in plants. New Phytol. 2009, 184, 751–767. [Google Scholar] [CrossRef]
- Shergill, L.S.; Boutsalis, P.; Preston, C.; Gill, G.S. Fitness costs associated with 1781 and 2041 ACCase-mutant alleles conferring resistance to herbicides in Hordeum glaucum Steud. Crop Prot. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Travlos, I.S. Competition between ACCase-inhibitor resistant and susceptible sterile wild oat (Avena sterilis) biotypes. Weed Sci. 2013, 61, 26–31. [Google Scholar] [CrossRef]
- Keshtkar, E.; Mathiassen, S.K.; Kudsk, P. No vegetative and fecundity fitness cost associated with acetyl-coenzyme A carboxylase non-target-site resistance in a Black-grass (Alopecurus myosuroides Huds.) population. Front. Plant Sci. 2017, 8, 2011–2017. [Google Scholar] [CrossRef]
- Cousens, R.D.; Fournier-Level, A. Herbicide resistance costs: What are we actually measuring and why? Pest Manag. Sci. 2018, 74, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Darmency, H.; Menchari, Y.; Le Corre, V.; Délye, C. Fitness cost due to herbicide resistance may trigger genetic background evolution. Evolution 2015, 69, 271–278. [Google Scholar] [CrossRef]
- Li, M.; Yu, Q.; Han, H.; Vila-Aiub, M.; Powles, S.B. ALS herbicide resistance mutations in Raphanus raphanistrum: Evaluation of pleiotropic effects on vegetative growth and ALS activity. Pest Manag. Sci. 2013, 69, 689–695. [Google Scholar] [CrossRef]
- Kumar, V.; Jha, P. Differences in germination, growth, and fecundity characteristics of dicamba-fluroxypyr-resistant and susceptible Kochia Scoparia. PLoS ONE 2016, 11, e0161533. [Google Scholar] [CrossRef][Green Version]
- Keshtkar, E.; Abdolshahi, R.; Sasanfar, H.; Zand, E.; Beffa, R.; Dayan, F. Assessing fitness costs from a herbicide-resistance management perspective: A review and insight. Weed Sci. 2019, 67, 137–148. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Oveisi, M.; Zand, E.; De Prado, R. A review of herbicide resistance in Iran. Weed Sci. 2016, 64, 551–561. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna-Ruíz, M.D.; Kamkar, B.; Ghaderi-Far, F.; De Prado, R. The first case of short-spiked canarygrass (Phalaris brachystachys) with cross-resistance to ACCase-inhibiting herbicides in Iran. Agronomy 2019, 9, 377. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Rojano-Delgado, A.M.; Vázquez-García, J.G.; Romano, Y.; Osuna, M.D.; Gherekhloo, J.; De Prado, R. Cross-resistance mechanisms to ACCase-inhibiting herbicides in short-spike canarygrass (Phalaris brachystachys). Plant Physiol. Biochem. 2020, 15, 681–688. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harington, K.C. Fitness costs associated with multiple resistance to dicamba and atrazine in Chenopodium Album. Planta 2019, 249, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Goudriaan, J.; Lantinga, E.A.; Vos, J.; Spiertz, H.J. A flexible sigmoid function of determinate growth. Ann. Bot. 2003, 91, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, K.J.; Yiljep, Y.D.; Oyatoyan, O.B.; Bawa, G.S. Effect of moisture content on some physical properties of Lablab purpureus (L.) sweet seeds. Agric. Eng. Int. CIGR J. 2009, 5, 1279–1293. [Google Scholar]
- Niinemets, U. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167–178. [Google Scholar] [CrossRef]
- Henckes, J.R.; Chechin, J.; Schemitz, M.F.; Piasecki, C.; Vargas, L.; Agostinetto, D. Fitness cost and competitive ability of ryegrass susceptible and with multiple resistance to glyphosate, iodosulfuron-methyl, and pyroxsulam. Planta Daninha 2019, 37, e019197532. [Google Scholar] [CrossRef]
- Cechin, J.; Vargas, L.; Agostinetto, D.; Zimmer, V.; Pertile, M.; Dal Magro, T. Fitness costs of susceptible and resistant radish biotypes to ALS-inhibitor herbicides. Comun. Sci. 2017, 8, 281–286. [Google Scholar] [CrossRef]
- Zhang, P.; Hefting, M.M.; Soons, M.B.; Kowalchuk, G.A.; Rees, M.; Hector, A.; Turnbull, L.A.; Zhou, X.; Guo, Z.; Chu, C.; et al. Fast and furious: Early differences in growth rate drive short-term plant dominance and exclusion under eutrophication. Ecol. Evol. 2020, 10, 10116–10129. [Google Scholar] [CrossRef]
- Lowry, C.J.; Smith, R.G. Weed control through crop plant manipulations. In Non-Chemical Weed Control, 1st ed.; Jabran, K., Chauhan, B.S., Eds.; Academic Press: London, UK, 2018; pp. 73–96. [Google Scholar]
- Koelewijn, H.P. Rapid change in relative growth rate between the vegetative and reproductive stage of the life cycle in Plantago coronopus. New Phytol. 2004, 163, 67–76. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Steadman, K.J.; Powles, S.B. Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: Dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J. Appl. Ecol. 2005, 42, 288–298. [Google Scholar] [CrossRef]
- Sabet Zangeneh, H.; Mohammadust Chamanabad, H.R.; Zand, E.; Asghari, A.; Alamisaeid, K.; Travlos, I.S.; Alebrahim, M.T. Study of fitness cost in three rigid ryegrass populations susceptible and resistant to Acetyl-CoA Carboxylase Inhibiting herbicides. Front. Ecol. Evol. 2016, 4, 142. [Google Scholar] [CrossRef][Green Version]
- Park, K.W.; Mallory-Smith, C.A.; Ball, D.A.; Mueller-Warrant, G.W. Ecological fitness of acetolactate synthase inhibitor-resistant and -susceptible downy brome (Bromus tectorum) biotypes. Weed Sci. 2004, 52, 768–773. [Google Scholar] [CrossRef]
- Menchari, Y.; Chauvel, B.; Darmency, H.; D’elye, C. Fitness costs associated with three mutant acetylcoenzyme a carboxylase alleles endowing herbicide resistance in black-grass Alopecurus myosuroides. J. Appl. Ecol. 2008, 45, 939–947. [Google Scholar] [CrossRef]
- Matzarfi, M.; Gerson, O.; Rubin, B.; Peleg, Z. Different mutations endowing resistance to Acetyl-CoA Carboxylase inhibitors results in changes in ecological fitness of Lolium rigidum populations. Front. Plant Sci. 2017, 8, 1078–1083. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Yu, Q.; Han, H.; Powles, S.B. Effect of herbicide resistance endowing Ile-1781-Leu and Asp-2078-Gly ACCase gene mutations on ACCase kinetics and growth traits in Lolium rigidum. J. Exp. Bot. 2015, 66, 4711–4718. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour-Bourkheili, S.; Gherekhloo, J.; Kamkar, B.; Ramezanpour, S.S. Comparing fitness cost associated with haloxyfop-R-methyl ester resistance in winter wild oat biotypes. Planta Daninha 2020, 38, e020213759. [Google Scholar] [CrossRef]
- Du, L.; Qu, M.; Jiang, X.; Li, X.; Ju, Q.; Lu, X.; Wang, J. Fitness costs associated with acetyl-coenzyme A carboxylase mutations endowing herbicide resistance in American sloughgrass (Beckmannia syzigachne Steud.). Ecol. Evol. 2019, 9, 2220–2230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).