Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth and Sampling
2.2. Protein Extraction
2.2.1. Grinding
2.2.2. RIPA (Rapid Immunoprecipitation Assay) Lysis
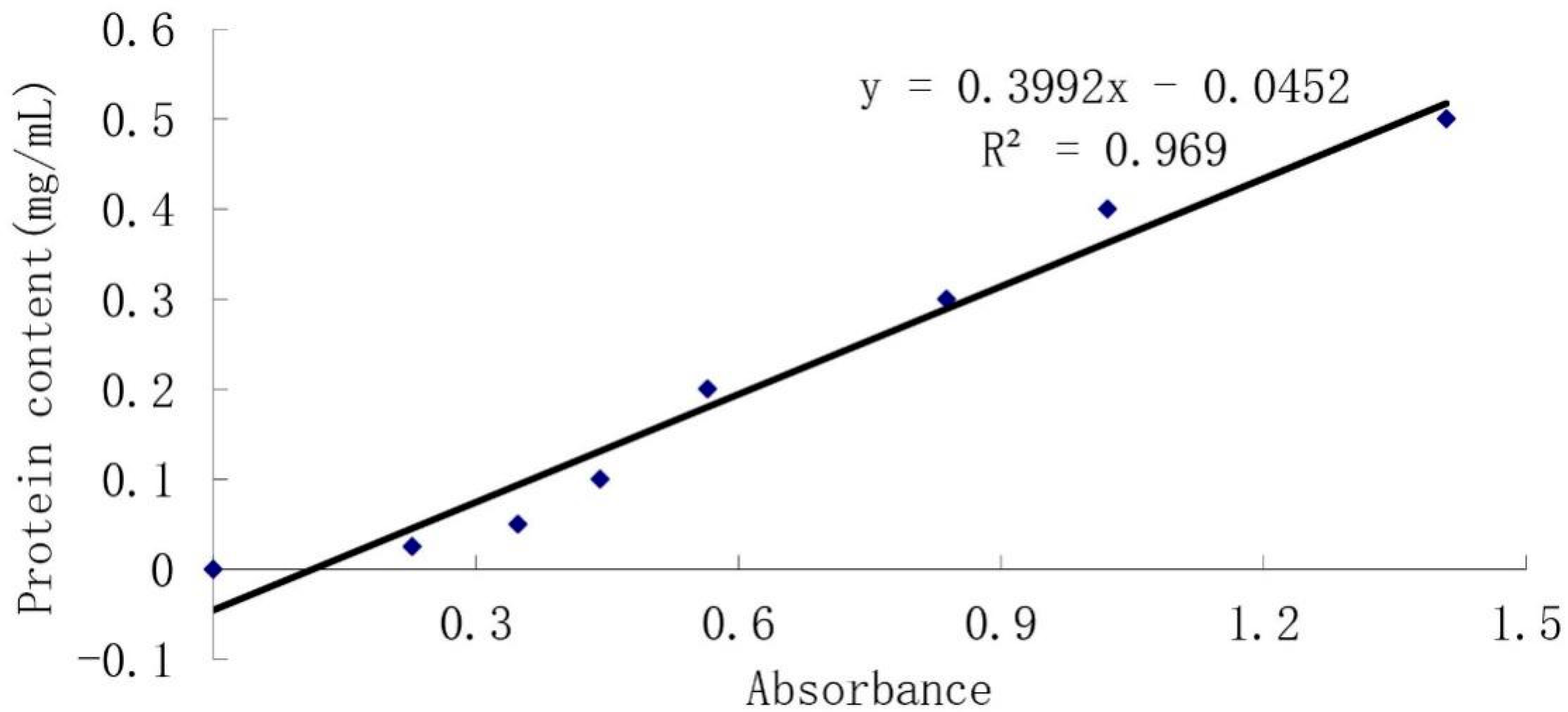
2.2.3. Protein Concentration Detection
2.2.4. TCA/Acetone Precipitation
2.3. Off-Line Strong Cation Exchange Chromatography
2.4. On-Line Nano-LC ESI QqTOF MS Analysis
2.5. Protein Identification and Quantification
3. Results
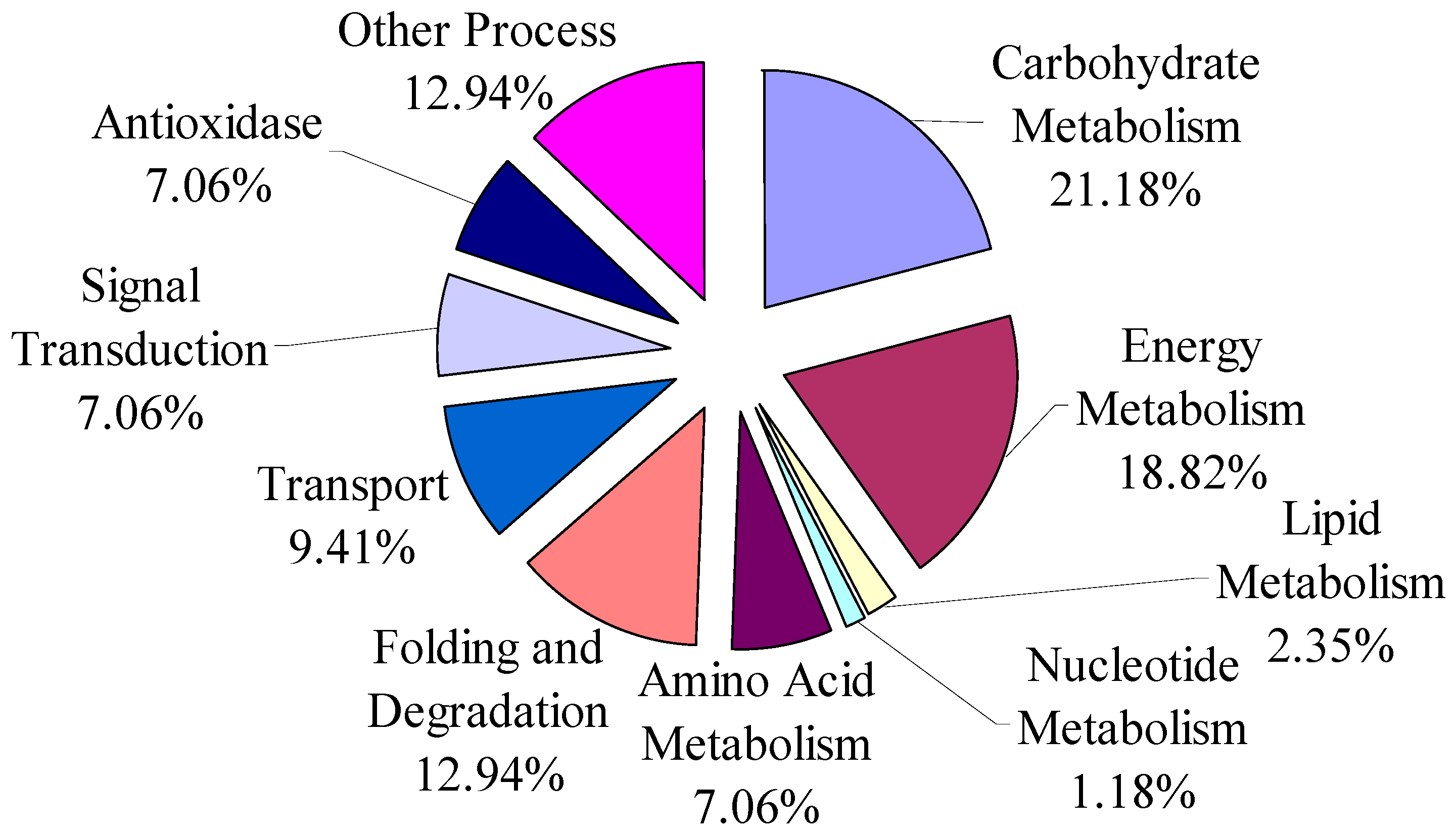
3.1. The Overview of Differential Proteins Based on Categories of Pathway
3.2. Induced Changes by Chilling Stress and Grafting in Cotton Proteins
3.3. Pathways of Differential Enzymes Referred and the Key Enzymes
3.4. Functional Classification of Cold-Responsive Proteins
3.4.1. Differential Proteins Related to Carbohydrate Metabolism
3.4.2. Differential Proteins Related to Energy Metabolism
3.4.3. Differential Proteins Related to Lipid Metabolism
3.4.4. Differential Proteins Related to Nucleotide Metabolism
3.4.5. Differential Proteins Related to Amino Acid Metabolism
3.4.6. Differential Proteins Related to Protein Folding and Degradation
3.4.7. Differential Proteins Related to Cellular Substance Transport
3.4.8. Differential Proteins Related to Signal Transduction
3.4.9. Differential Proteins Related to Antioxidation
3.4.10. Differential Proteins Related to Other Processes
4. Discussion
4.1. Induced Changes in Cotton Proteins and Biology Pathway by Chilling Stress and Grafting
4.2. Photosynthesis-Related Proteins by Chilling Stress and Grafting
4.3. Differential Proteins Related to Protein Folding and Degradation by Chilling Stress and Grafting
4.4. Differential Proteins Related to Cellular Material Transport by Chilling Stress and Grafting
4.5. Differential Proteins Related to Signal Transduction by Chilling Stress and Grafting
4.6. Differential Proteins Related to Antioxidation by Chilling Stress and Grafting
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, S.L. The Damage Study of Low-Temperature and Freezing Disaster in China. Master’s Thesis, Yunnan Universiry, Kunming, China, 2017. [Google Scholar]
- Xu, J.W.; Zhang, C.; Zeng, X.Y.; Zhang, X.J.; Lee, Z.B.; Wei, Y.N. Evaluation of seed germination of main-cultivated cotton under low temperature in northern Xinjiang in recent ten years. Xinjiang Agric. Sci. 2017, 54, 1569–1578. [Google Scholar]
- Cao, Z.Y.; Zhao, T.; Wang, L.Y.; Han, J.; Chen, J.W.; Hao, Y.P.; Guan, X.Y. The lincRNA XH123 is involved in cotton cold-stress regulation. Plant Mol. Biol. 2021, 106, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Xie, L.; Lee, Z.B.; Wei, Y.N.; Lin, H.R. Effects of low temperature stress to germination of cotton seeds and evaluation of their cold resistance in northern Xinjiang. Seed 2014, 33, 74–77. [Google Scholar]
- Wang, J.J.; Wang, D.L.; Yin, Z.J.; Wang, S.; Fan, W.L.; Lu, X.K.; Mu, M.; Guo, L.X.; Ye, W.W.; Yu, S.X. Identification of the chilling resistance from germination stage to seedling stage in upland cotton. Sci. Agric. Sin. 2016, 49, 3332–3346. [Google Scholar]
- Huang, J.F.; Wang, X.Z. Study on cotton phenology and climate in Xinjiang. J. Arid. Land Resour. Environ. 1999, 13, 91–96. [Google Scholar]
- Zhao, L.H.; Feng, H.J.; Feng, Z.L.; Wei, F.; Zhang, Y.L.; Zhu, H.Q. Effects of biological cold induction treatment of cotton seeds on stress resistance and yield. China Cotton 2021, 48, 25–29. [Google Scholar]
- Sha, X.M.; Lee, J.; Abulizi, P.; Mijiti, A.; Wang, R.Q. Effects of low temperature stress on seed germination of cotton. Seed 2021, 40, 80–85. [Google Scholar]
- Yang, Y.F.; Ge, C.F.; Shen, Q.; Zhang, S.P.; Liu, S.D.; Ma, H.J.; Chen, J.; Liu, R.H.; Lee, S.C.; Zhao, X.H.; et al. Cloning and functional analysis of low temperature response gene, GhZAT10, in upland cotton at seedling stage. China Cotton 2020, 32, 305–315. [Google Scholar]
- Leila, H.; Reza, M.A. What happens in plant molecular responses to cold stress? Acta Physiol. Plant 2010, 32, 419–431. [Google Scholar]
- Wang, J.J.; Wang, S.; Yin, Z.J.; Wang, D.L.; Fan, W.L.; Mu, M.; Guo, L.X.; Ye, W.W.; Yu, S.X. The effect of low temperature stress on the growth of upland cotton seedlings and a preliminary study of cold-resistance mecha-nisms. China Cotton 2017, 29, 147–156. [Google Scholar]
- Zhang, L.Y.; Chen, G.M.; Wei, H.L.; Wang, H.T.; Lu, J.H.; Ma, Z.Y.; Yu, S.X. Chilling tolerance identification and response to cold stress of Gossypium hirsutum Varieties (Lines) during germination stage. Sci. Agric. Sin. 2021, 54, 19–33. [Google Scholar]
- Yan, S.P.; Zhang, Q.Y.; Tang, Z.C.; Su, W.A.; Sun, W.N. Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol. Cell. Proteomics. 2006, 5, 484–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.B.; Shi, Q.W.; Xiong, L.D.; Xin, G.; Xiao, Y.Q.; Huang, W.X. Transcriptome profiling of cells exposed to par-ticular and intense electromagnetic radiation emitted by the "SG-III" prototype laser facility. Sci. Rep.-Uk 2021, 11, 2017. [Google Scholar] [CrossRef] [PubMed]
- Sarhadi, E.; Mahfoozi, S.; Hosseini, S.A.; Salekdeh, G.H. Cold acclimation proteome analysis reveals close link be-tween the up-regulation of low-temperature associated proteins and vernalization fulfillment. J. Proteome Res. 2010, 9, 5658–5667. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Zolla, L.; Rinalducci, S. Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 2015, 117, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Magwanga, R.; Jin, D.D.S.; Cai, X.Y.; Hou, Y.Q.; Stephen, G.; Zhou, B.L.; Wang, K.B.; Zhou, Z.L. Comparative transcriptome analysis reveals evolutionary divergence and shared network of cold and salt stress response in dip-loid d-genome cotton current status: Under review subject areas plant molecular biology and genetics. BMC Plant Biol. 2018, 20, 1–17. [Google Scholar]
- Wang, X.N. Isolation and Functional Characterization of Chilling Responsive Genes and Quantitative Proteomic Analysis Responding to Chilling Stress in Maize. Ph.D. Thesis, Jilin University, Changchun, China, 2016. [Google Scholar]
- Wang, Q.; Wang, B.K.; Liu, H.F.; Han, H.W.; Zhuang, H.M.; Wang, J.; Yang, T.; Wang, H.; Qin, Y. Advances in tomato proteomics research under abiotic stress. Xinjiang Agric. Sci. 2021, 58, 1829–1837. [Google Scholar]
- Balbuena, T.S.; Salas, J.J.; Marti Nez-Force, E.; Garce, S.R.; Thelen, J.J. Proteome analysis of cold acclimation in sunflower. J. Proteome Res. 2011, 10, 2330–2346. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. J. Plant Physiol. 2016, 193, 47–56. [Google Scholar] [CrossRef]
- Bian, Y.W.; Lv, D.W.; Cheng, Z.W.; Gu, A.Q.; Cao, H.; Yan, Y.M. Integrative proteome analysis of Brachypodium distachyon roots and leaves reveals a synergetic responsive network under H2O2 stress. J. Proteomics 2015, 128, 388–402. [Google Scholar] [CrossRef]
- Zhang, X.; Khan, A.; Zhou, R.Y.; Liu, Y.S.; Zhang, B.H.; Wang, Q.L.; Zhang, Z.Y. Grafting in cotton: A mechanistic approach for stress tolerance and sustainable development. Ind. Crop. Prod. 2022, 175, 114227. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, Z.J.; Lv, G.H. Effect of low temperature stress on chilling tolerance and protective system against active oxygen of grafted watermelon. Chin. J. Appl. Ecol. 2004, 15, 659–662. [Google Scholar]
- Zhou, K.Q.; Shang, S.; Tian, L.B.; Zhu, G.P.; Zhou, M.M.; Pan, Q.Y.; Zeng, L.P. Effects of low temperature stress on osmotic solutes of grafted bitter gourd seedlings. Chin. J. Trop. Crops 2018, 39, 1533–1539. [Google Scholar]
- Wu, B. Mechanism of Improving Chilling Adaptability of Tomato Seedlings by Grafting onto Cold-Tolerant Rootstock. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Zhang, X.; Li, C.Q.; Wang, X.Y.; Chen, G.P.; Zhang, J.B.; Zhou, R.Y. Genetic Analysis of Cryotolerance in Cotton During the Overwintering Period Using Mixed Model of Major Gene and Polygene. J. Integr. Agric. 2012, 11, 537–544. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.Y.; Wang, Q.L.; Chen, P.; Chen, G.; Zhou, R.Y. Effects of Rootstocks on Cryotolerance and Overwintering Survivorship of Genic Male Sterile Lines in Upland Cotton (Gossypium hirsutum L.). PLoS ONE 2013, 8, e63534. [Google Scholar] [CrossRef]
- Xiong, E.; Zhang, C.; Ye, C.; Jiang, Y.; Zhang, Y.; Chen, F.; Dong, G.; Zeng, D.; Yu, Y.; Wu, L. iTRAQ-based proteo-mic analysis provides insights into the molecular mechanisms of rice formyl tetrahydrofolate deformylase in salt response. Planta 2021, 254, 76. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Fan, H.; Wang, F. iTRAQ-based comparative prote-omic analysis of two coconut varieties reveals aromatic coconut cold-sensitive in response to low temperature. J. Proteomics 2020, 220, 103766. [Google Scholar] [CrossRef]
- Luo, J.L.; Ning, T.T.; Sun, Y.F.; Zhu, J.H.; Zhu, Y.G.; Lin, Q.S.; Yang, D.C. Proteomic analysis of rice endosperm cells in response to expression of hGM-CSF. J. Proteome Res. 2009, 8, 829–837. [Google Scholar] [CrossRef]
- Ding, D.W.; Ding, Y.R.; Cai, Y.J.; Chen, S.W.; Xu, W.B. Application of Pathway Hunter Tool in metabolic pathway analysising. Comput. Appliend Chem. 2008, 25, 81–84. [Google Scholar]
- Jin, D.S. Myotype-specific enolase RIA and clinical significance. J. Radioimmunol. 1995, 8, 64–65. [Google Scholar]
- Danshina, P.V.; Schmalhausen, E.V.; Avetisyan, A.V.; Muronetz, V.I. Mildly oxidized glyceraldehyde-3-phosphate dehydrogenase as a possible regulator of Glycolysis. Iubmb Life 2001, 51, 309–314. [Google Scholar] [PubMed]
- Hannah, M.A.; Wiese, D.; Freund, S.; Fiehn, O.; Heyer, A.G.; Hincha, D.K. Natural genetic variation of freezing tolerance in Arabidopsis. Plant Physiol. 2006, 142, 98–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Wang, B.; Hou, S.T.; Zhu, G.P. Structure and function of malate dehydrogenases. J. Biol. 2009, 26, 69–72. [Google Scholar]
- Lv, Y.B.; Zhou, X.H.; Chen, W.; Tan, X.R. Advances in the development of S-adenosine-L-homocysteine hydrolase and its inhibitors. Guangdong Chem. Ind. 2021, 48, 74–78. [Google Scholar]
- Greco, C.M.; Cervantes, M.; Fustin, J.; Ito, K.; Ceglia, N.; Samad, M.; Shi, J.; Koronowski, K.B.; Forne, I.; Ranjit, S.; et al. S-adenosyl-l-homocysteine hydrolase links methionine metabolism to the circadian clock and chromatin remodeling. Sci. Adv. 2020, 6, 5629–5645. [Google Scholar] [CrossRef]
- Renaut, J.; Hausman, J.F.; Bassett, C.; Artlip, T.; Cauchie, H.M.; Witters, E.; Wisniewski, M. Quantitative proteomic analysis of short photoperiod and low-temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genet Genomes 2008, 4, 589–600. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Poole, R.J.; Dhindsa, R.S. Changes in Protein Patterns and Translatable Messenger RNA Populations during Cold Acclimation of Alfalfa. Plant Physiol. 1987, 84, 1172–1176. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Yang, P.F.; Zhang, X.F.; Xu, Y.N.; Kuang, T.Y.; Shen, S.H.; He, Y.K. Proteomic analysis of the cold stress response in the moss, Physcomitrella patens. Proteomics 2009, 9, 4529–4538. [Google Scholar] [CrossRef]
- Li, X.G.; Bi, Y.P.; Zhao, S.J.; Meng, Q.W.; He, Q. Effects of short-term chilling stress on the photosystems and chlo-roplast ultrastructure in sweet peppe. Sci. Agric. Sin. 2005, 38, 1226–1231. [Google Scholar]
- Janmohammadi, M.; Enayati, V.; Sabaghnia, N. Impact of cold acclimation, de-acclimation and re-acclimation on carbohydrate content and antioxidant enzyme activities in spring and winter wheat. Icel. Agric. Sci. 2012, 25, 3–11. [Google Scholar]
- Pino, M.T.; Skinner, J.S.; Jeknic, Z.; Park, E.J.; Chen, T. Ectopic overexpression of AtCBF1 in potato enhances freezing tolerance. Plant Biotechnol. J. 2006, 31, 393–406. [Google Scholar]
- Wu, H.M.; Dorse, S.; Bhave, M. In silico identification and analysis of the protein disulphide isomerases in wheat and rice. Biologia 2012, 67, 48–60. [Google Scholar] [CrossRef]
- Sable, A.; Rai, K.M.; Choudhary, A.; Yadav, V.K.; Agarwal, S.K.; Sawant, S.V. Inhibition of Heat Shock proteins HSP90 and HSP70 induce oxidative stress, suppressing cotton fiber development. Sci. Rep. 2018, 8, 3620. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Ci, Z.L. Advances in study of heat shock protein. Shanxi For. Sci. Technol. 2008, 41, 27–32. [Google Scholar]
- He, Y.J.; Guo, W.Z.; Zhang, T.Z. Cloning, expression, and mapping of six low-molecular-weight heat-shock protein genes in cotton. Acta Agronmica Sinica 2008, 34, 1574–1580. [Google Scholar] [CrossRef]
- Zhu, S.J.; Ji, Z.L. Heat-shock proteins as related to the chilling tolerance of horticultural plants. Acta Hortic. Sin. 2002, 41, 607–612. [Google Scholar]
- Cabané, M.; Calvet, P.; Vincens, P.; Boudet, A.M. Characterization of chilling-acclimation-related proteins in soy-bean and identification of one as a member of the heat shock protein (HSP 70) family. Planta 1993, 190, 346–353. [Google Scholar] [CrossRef]
- Cui, N.; Li, T.L.; Li, Y. The main action of 14- 3–3 proteins in plants. Biotechnology 2007, 17, 86–89. [Google Scholar]
- Yaffe, M.B. How do 14-3-3 proteins work?-- Gatekeeper phosphorylation and the molecular anvil hypothesis. Febs. Lett. 2002, 513, 53–57. [Google Scholar] [CrossRef]
- Wang, Y.N.; Zhao, B. Mechanisms of Ubiquitin Chain Formation. Prog. Biochem. Biophys. 2021, 48, 515–528. [Google Scholar]
- Zhang, Y. Cloning and Structure-Function Study of a Novel Member of Annexin Family. Ph.D. Thesis, Naval Medical University, Shanghai, China, 2004. [Google Scholar]
- Wang, C.; Xiao, Q.; Li, Y.W.; Zhao, C.; Li, R.L.; Cao, S.S.; Cui, J.; Wang, L.; Wu, Y. Regulatory mecha-nisms of annexin-induced chemotherapy resistance in cisplatin resistant lung adenocarcinoma. Asian Pac. J. Cancer Prev. Apjcp 2014, 15, 3191–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, H.T.; Zhen, D.F.; He, N.; Li, W.; Wang, M.L.; Wang, S.Y. Research progress on the physiological response of plants to low temperature and the amelioration effcectiveness of exogenous ABA. Acta Prataculturae Sin. 2021, 30, 208–219. [Google Scholar]
- Lee, S.; Lee, E.J.; Yang, E.J.; Ji, E.L.; Park, O.K. Proteomic Identification of Annexins, Calcium-Dependent Membrane Binding Proteins That Mediate Osmotic Stress and Abscisic Acid Signal Transduction in Arabidopsis. Plant Cell 2004, 16, 1378–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makoto, H.; Mahmoud, T.; Keiko, M.; Yukimoto, I.; Setsuko, K. Proteome Analysis of Rice Root Plasma Membrane and Detection of Cold Stress Responsive Proteins. Protein Pept. Lett. 2009, 16, 685–697. [Google Scholar]
- Andrawis, A.; Solomon, M.; Delmer, D.P. Cotton fiber annexins: A potential role in the regulation of callose synthase. Plant J. Cell Mol. Biol. 1993, 3, 763–772. [Google Scholar] [CrossRef]
- Zhang, S.J.; Song, G.Q.; Li, Y.L.; Gao, J.; Wang, J.; Chen, G.J.; Li, H.S.; Li, G.Y.; Zhao, Z.D. Comparative proteomic analysis of cold responsive proteins in two wheat cultivars with different tolerance to spring radiation frost. Front. Agric. Sci. Eng. 2014, 1, 37–41. [Google Scholar]
- Forde, B.G.; Cutler, S.R.; Zaman, N.; Krysan, P.J. Glutamate signalling via a MEKK1 kinase-dependent pathway in-duces changes in Arabidopsis root architecture. Plant J. Cell Mol. Biol. 2013, 75, 1–10. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Zhang, R.X.; Zhang, Z.L.; Zhang, Z.J.; Huang, R.F. Signal perception, transduction and transcriptional regulation dur-ing cold stress in plant. J. Agric. Sci. Tech.-Iran 2009, 11, 5–11. [Google Scholar]
- Ge, C.W.; Wang, L.; Yang, Y.F.; Liu, R.H.; Liu, S.D.; Chen, J.; Shen, Q.; Ma, H.J.; Yang, L.; Zhang, S.P.; et al. Ge-nome-wide association study identifies variants of GhSAD1 conferring cold tolerance in cotton. J. Exp. Bot. 2021, 72, b555. [Google Scholar]
- Kargiotidou, A.; Deli, D.; Galanopoulou, D.; Tsaftaris, A.; Farmaki, T. Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum). J. Exp. Bot. 2008, 59, 2043–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.Y.; Lu, C.; Zhang, Y.S.; Tong, W.J.; Du, L.H.; Liu, F. Proteomic analysis of Aspergillus flavus reveals the anti-fungal action of Perilla frutescens essential oil by interfering with energy metabolism and defense function. LWT 2022, 154, 112660. [Google Scholar] [CrossRef]
- Azcón, R.; Perálvarez, M.D.C.; Roldán, A.; Barea, J.M. Arbuscular Mycorrhizal Fungi, Bacillus cereus, and Candida parapsilosis from a Multicontaminated Soil Alleviate Metal Toxicity in Plants. Microb. Ecol. 2010, 59, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Shah, T.; Cheng, Y.; LÜ, Y.; Zhang, X.K.; Zou, X.L. Physiological and molecular responses to cold stress in rapeseed (Brassica napus L.). J. Integr. Agric. 2019, 18, 2043–2056. [Google Scholar] [CrossRef]
- Li, L.J.; Lu, X.C.; Ma, H.Y.; Lyu, D.G. Comparative proteomic analysis reveals the roots response to low root-zone temperature in Malus baccata. J. Plant Res. 2018, 131, 865–878. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, Y.T.; Liu, X.Y.; Xu, J.W.; Li, Z.B. Effect of exogenous ascorbic acid on chilling tolerance of cotton seed germination. Seed 2019, 38, 77–80. [Google Scholar]

| Stem Bark | Scion of Non-Grafted Seedling | Rootstock of Non-Grafted Seedling | Scion of Grafted Plant | Rootstock of Grafted Plant |
|---|---|---|---|---|
| Chilling stress | S1 | S2 | S3 | S4 |
| Control | S1′ | S2′ | S3′ | S4′ |
| Unused (Conf) Cutoff | Proteins Detected | Proteins Before Grouping | Distinct Peptides | Spectra Identified | % Total Spectra |
|---|---|---|---|---|---|
| >2.0 (99%) | 50 | 6207 | 562 | 3885 | 8.1 |
| >1.3 (95%) | 89 | 11,242 | 748 | 4659 | 9.7 |
| >0.47 (66%) | 143 | 22,092 | 951 | 5419 | 11.3 |
| Cutoff Applied: >0.05 (10%) | 768 | 37,568 | 2140 | 8006 | 16.7 |
| Unused (Conf) Cutoff | Proteins Detected | Proteins before Grouping | Distinct Peptides | Spectra Identified | % Total Spectra |
|---|---|---|---|---|---|
| >2.0 (99%) | 23 | 288 | 429 | 2458 | 5.1 |
| >1.3 (95%) | 30 | 326 | 490 | 2670 | 5.6 |
| >0.47 (66%) | 41 | 607 | 616 | 3091 | 6.4 |
| Cutoff Applied: >0.05 (10%) | 54 | 650 | 736 | 3393 | 7.1 |
| Biology Pathway | ID | Sum | |
|---|---|---|---|
| Carbohydrate Metabolism | Glycolysis/Gluconeogenesis | ABW21688; BAD08850; ABK95670; ABK25148; BAA02729 | 18 |
| Citrate cycle (TCA cycle) | XP_002285356; XP_002263337 | ||
| Pentose phosphate pathway | CAB87149; XP_002271514; BAA02729 | ||
| Pentose and glucuronate interconversions | XP_002526594; XP_001759864; O04886 | ||
| Fructose and mannose metabolism | ABK95670; BAA02729; Q9SID0 | ||
| Galactose metabolism | XP_002526594; XP_001759864 | ||
| Starch and sucrose metabolism | O04886; Q9SID0; XP_002526594; XP_001759864 | ||
| Amino sugar and nucleotide sugar metabolism | ABK94952; XP_002526594; XP_001767949; XP_001759864; Q9SID0; NP_187685 | ||
| Pyruvate metabolism | CAO21229; XP_002285356 | ||
| Glyoxylate and dicarboxylate metabolism | XP_002285356; XP_002263337; ABE26954 | ||
| Inositol phosphate metabolism | ABK95670 | ||
| Energy Metabolism | Oxidative phosphorylation | Q01859 | 16 |
| Photosynthesis | AAS59949; ABG74856; Q6ENJ7; ABK95197; ACG24344; Q8MAV7; NP_683825; CAN64486; ACJ85810; Q01859; CAO47122 | ||
| Carbon fixation in photosynthetic organisms | ABK95670; BAA02729; XP_002285356; ABE26954 | ||
| Methane metabolism | P85433 | ||
| Lipid Metabolism | Glycerolipid metabolism | AAT01325 | 2 |
| Glycerophospholipid metabolism | ACG70839 | ||
| Nucleotide Metabolism | Purine metabolism | Q96559 | 1 |
| Pyrimidine metabolism | Q96559 | ||
| Amino Acid Metabolism | Aspartate metabolism | CAB81805 | 6 |
| Threonine metabolism | ABV27483 | ||
| Cysteine and methionine metabolism | Q42699; BAF28208 | ||
| Glutathione metabolism | ABO41849 | ||
| Phenylalanine metabolism | P85433 | ||
| Selenoamino acid metabolism | BAF28208 | ||
| Folding and Degradation | Chaperonin and isomerase | CBC02991; ACG33102; ACB70177; ABY65001; ABX76300; O49886; ABK95670; EAZ30819; CAZ91440 | 11 |
| Ubiquitin mediated proteolysis | ABR16377; NP_568552; | ||
| Transport | Vesicular transport | XP_001780193; ABK92828; Q01111; BAB90396 | 8 |
| Annexin | 1N00_A; CBC30882 | ||
| Phosphotransferase system | ABK92937 | ||
| Transfer protein | ACI26701 | ||
| Signal Transduction | MAPK signaling pathway | ABW34390 | 6 |
| Calcium signaling pathway | 1N00_A; ACG33102; ABY65001;Q7Y052; Q9ZSW9 | ||
| Antioxidase | Superoxide dismutase | ACC93637 | 6 |
| Peroxidase | CBC66557; P85433 | ||
| Oxidoreductase | ABK25148; XP_002303520; CAO47501 | ||
| Other Process | Cell cycle | XP_002446509 | 12 |
| Porphyrin metabolism | NP_171847 | ||
| Spliceosome | XP_002461966 | ||
| Chromosome scaffold | XP_001690796 | ||
| Ribosome | P41099 | ||
| Biosynthesis of alkaloids | ABW21688; BAD08850; BAA02729; XP_002285356; XP_002263337 | ||
| Biosynthesis of phenylpropanoids | ABW21688; BAD08850; BAA02729; XP_002285356; XP_002263337; P85433 | ||
| Biosynthesis of plant hormones | ABW21688; BAD08850; BAA02729; XP_002285356; XP_002263337; Q42699 | ||
| Biosynthesis of terpenoids and steroids | ABW21688; BAD08850; BAA02729; XP_002285356; XP_002263337 | ||
| Group | Sample | Material | Protein Concent (mg/mL) | p < 5% | p < 1% |
|---|---|---|---|---|---|
| Control | S1′ | Scion of non-grafted seedling | 0.28 ± 0.02 | d | D |
| S2′ | Rootstock of non-grafted seedling | 0.41 ± 0.03 | c | CD | |
| S3′ | Scion of grafted plant | 0.29 ± 0.03 | d | D | |
| S4′ | Rootstock of grafted plant | 0.51 ± 0.03 | b | BC | |
| Chilling | S1 | Scion of non-grafted seedling | 0.55 ± 0.01 | b | B |
| S2 | Rootstock of non-grafted seedling | 0.93 ± 0.00 | a | A | |
| S3 | Scion of grafted plant | 0.57 ± 0.06 | b | B | |
| S4 | Rootstock of grafted plant | 0.89 ± 0.11 | a | A |
| Protein ID | Enzyme ID | Enzyme Name | Incoming Degree SP | Outgoing Degree SP |
|---|---|---|---|---|
| BAA02729 | 4.1.2.13 | fructose-bisphosphate aldolase | 57 | 57 |
| Q96559 | 2.7.4.6 | nucleoside-diphosphate kinase | 31 | 31 |
| BAD08850 | 1.2.1.12 | glyceraldehyde-3-phosphate dehydrogenase | 18 | 18 |
| Q9SID0 | 2.7.1.4 | Fructokinase | 15 | 15 |
| ACC93637 | 1.15.1.1 | superoxide dismutase | 8 | 8 |
| XP_002526594 | 2.7.7.9 | UDP glucose pyrophosphorylase | 8 | 8 |
| P85433 | 1.11.1.7 | Peroxidase | 7 | 7 |
| BAF28208 | 3.3.1.1 | Adenosylhomocysteinase | 6 | 6 |
| ABW21688 | 4.2.1.11 | enolase, phosphopyruvate hydratase | 6 | 6 |
| XP_002263337 | 4.2.1.3 | aconitate hydratase | 6 | 6 |
| Protein ID | Name | Molecular Function | Chilling Treat | Grafting | ||||
|---|---|---|---|---|---|---|---|---|
| S1/S1′ | S2/S2′ | S3/S3′ | S4/S4′ | S3/S1 | S3′/S1′ | |||
| Carbohydrate metabolism | ||||||||
| ABW21688 | Enolase | magnesium ion binding, phosphopyruvate hydratase activity | 1.47 | 1.30 | 1.16 | 1.13 | 1.16 | 0.91 |
| BAD08850 | Glyceraldehyde-3-phosphate dehydrogenase | NAD or NADH binding, glyceraldehyde-3-phosphate dehydrogenase activity | 1.33 | 1.47 | 1.43 | 1.33 | 0.79 | 0.85 |
| ABK25148 | Putative uncharacterized protein | pyruvate dehydrogenase (acetyl-transferring) activity | 0.74 | 0.89 | 0.62 | 1.15 | 1.21 | 1.01 |
| BAA02729 | Fructose-bisphosphate aldolase | fructose-bisphosphate aldolase activity | 2.52 | 1.57 | 1.22 | 2.54 | 1.52 | 0.73 |
| XP_002285356 | Malate dehydrogenase | L-malate dehydrogenase activity; binding | 0.34 | 2.61 | 1.53 | 5.95 | 0.72 | 3.24 |
| XP_002263337 | Aconitate hydratase 1 | 4 iron, 4 sulfur cluster binding; aconitate hydratase activity | 1.28 | 1.14 | 0.76 | 1.40 | 0.97 | 0.58 |
| ABK94952 | Putative uncharacterized protein | ribokinase activity | 1.47 | 1.48 | 1.74 | 1.36 | 0.98 | 1.16 |
| CAB87149 | Transaldolase-like protein | transaldolase activity | NK | 1.10 | NK | 1.11 | NK | NK |
| XP_002271514 | Chromosome chr1 scaffold_166 | transaldolase activity | 1.63 | 1.06 | 0.34 | 1.22 | 2.95 | 0.61 |
| XP_001759864 | Predicted protein | nucleotidyltransferase activity | 1.97 | 1.68 | 1.75 | 1.37 | 1.14 | 1.01 |
| XP_002526594 | UTP-glucose-1-phosphate uridylyltransferase | UTP: glucose-1-phosphate uridylyltransferase activity | 0.00 | 0.00 | 0.00 | 0.00 | 0.51 | 0.00 |
| CAO21229 | Chromosome chr10 scaffold_297 | lactoylglutathione lyase activity; metal ion binding | 1.15 | 1.37 | 0.95 | 0.88 | 1.05 | 0.87 |
| Q9SID0 | Probable fructokinase-1 | ATP binding; fructokinase activity; ribokinase activity | 1.03 | 1.19 | 1.66 | 0.90 | 0.79 | 1.27 |
| O04886 | Pectinesterase 1 | aspartyl esterase activity; enzyme inhibitor activity; pectinesterase activity | 0.86 | 1.29 | 1.01 | 2.00 | 0.97 | 1.14 |
| ABE26954 | Ribulose bisphosphate carboxylase large chain | monooxygenase activity; ribulose-bisphosphate carboxylase activity | 1.43 | NK | NK | NK | 1.01 | NK |
| Amino acid metabolism | ||||||||
| CAB81805 | Nucleoid DNA-binding-like protein | DNA binding; aspartic-type endopeptidase activity | 0.71 | 1.02 | 0.76 | 1.56 | 0.51 | 0.55 |
| ABV27483 | Fasciclin-like arabinogalactan protein 12 | cell adhesion | 1.36 | 0.91 | 1.00 | 0.97 | 1.14 | 0.84 |
| Q42699 | Homocysteine methyltransferase | homocysteine S-methyltransferase activity; zinc ion binding | 1.65 | 1.54 | 1.57 | 1.04 | 1.22 | 1.16 |
| BAF28208 | Adenosyl-homocysteinase | Hydrolase | 1.18 | 1.12 | 1.07 | 1.11 | 1.21 | 1.09 |
| ABO41849 | Alcohol dehydrogenase A | oxidoreductase activity; zinc ion binding | 0.77 | 1.07 | 1.27 | 0.75 | 0.89 | 1.47 |
| Lipid metabolism | ||||||||
| AAT01325 | Os05g0518300 protein | hydrolase activity, acting on ester bonds | 0.68 | 0.68 | 1.04 | 1.62 | 1.01 | 1.54 |
| ACG70839 | Phospholipase D alpha | calcium ion binding; phospholipase D activity | 0.80 | 1.07 | 0.93 | 1.48 | 0.82 | 0.95 |
| Nucleotide metabolism | ||||||||
| Q96559 | Nucleoside diphosphate kinase | ATP binding; magnesium ion binding; nucleoside diphosphate kinase activity | 2.47 | 1.53 | 1.23 | 1.56 | 1.88 | 0.94 |
| Protein ID | Name | Molecular Function | Chilling Treat | Grafting | ||||
|---|---|---|---|---|---|---|---|---|
| S1/S1’ | S2/S2’ | S3/S3’ | S4/S4’ | S3/S1 | S3’/S1’ | |||
| AAS59949 | Photosystem II CP47 chlorophyll apoprotein | chlorophyll binding | 1.00 | 0.52 | 0.71 | 0.57 | 1.75 | 1.24 |
| ABG74856 | Cytochrome b559 subunit alpha | heme binding | NK | NK | 2.62 | 8.76 | 4.82 | NK |
| Q6ENJ7 | Photosystem Q(B) protein; 32 kDa thylakoid membrane protein; hotosystem II protein D1 | electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity; iron ion binding | 0.56 | 0.47 | 0.80 | 0.89 | 0.60 | 0.86 |
| ABK95197 | Light-harvesting complex II protein Lhcb6 | chlorophyll binding; metal ion binding | 1.00 | 0.80 | 1.00 | 0.49 | 0.99 | 0.99 |
| ACG24344 | Photosystem I reaction center subunit XI | NK | 0.66 | 0.54 | 0.71 | 0.72 | 0.87 | 0.94 |
| NP_683825 | Photosystem II D2 protein (Photosystem Q(A) protein) | electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity; iron ion binding | NK | NK | 0.59 | 0.90 | 1.05 | NK |
| CAN64486 | CAAD domain-containing protein | NK | 0.89 | 0.88 | 0.13 | 0.62 | 2.75 | 0.39 |
| Q01859 | ATP synthase subunit beta, mitochondrial | ATP binding Ref.1; ydrogen ion transporting ATP synthase activity, rotational mechanism; ydrogen-exporting ATPase activity, phosphorylative mechanism; roton-transporting ATPase activity, rotational mechanism | 0.75 | 0.73 | 0.94 | 0.75 | 0.81 | 1.01 |
| P85433 | Peroxidase 7 | calcium ion binding; heme binding; peroxidase activity | 0.67 | 0.97 | 0.81 | 1.14 | 0.86 | 1.04 |
| Family | Function |
|---|---|
| HSP10 | HSP60 of auxiliary chaperonin, Helping the substrate of HSP60 to fold to bind to HSP60 |
| sHSP | Including a variety of proteins, depend on ATP for their function binding to unnatural state proteins |
| HSP40 | Auxiliary chaperonin, regulating the activity of HSP70, Some of them can bind to non-natural state proteins |
| HSP60 | Helping 15–30% of cellular proteins to fold through ATP |
| HSP70 | Preventing the adhesion and aggregation of unfolded polypeptide chains, Depolymerizes multifolded proteins, participating in protein transport, and regulating heat shock response |
| HSP90 | Acting together with some kinases and steroid receptors in signal transduction pathways, they may also play the role of some “typical” molecular chaperones |
| HSP100 | Depolymerization of protein polyplexes and aggregates |
| HSP110 | Highly homologous to HSP70, function unknown |
| Protein ID | Name | Molecular Function | Chilling Treat | Grafting | ||||
|---|---|---|---|---|---|---|---|---|
| S1/S1′ | S2/S2′ | S3/S3′ | S4/S4′ | S3/S1 | S3′/S1′ | |||
| Protein folding and degradation | ||||||||
| CBC02991 | Unnamed protein product | ATP binding | NK | NK | NK | NK | NK | NK |
| ACG33102 | 14-3-3-like protein | protein domain specific binding | 1.99 | 1.22 | 1.21 | 1.16 | 1.57 | 0.95 |
| ACB70177 | 70 kDa heat shock protein | ATP binding | 1.19 | 1.51 | 1.52 | 1.37 | 0.75 | 0.96 |
| ABY65001 | 14-3-3b protein | protein domain specific binding | 1.45 | 1.16 | 1.12 | 0.91 | 0.92 | 0.71 |
| ABX76300 | Heat shock protein 60 | ATP binding;protein binding | 0.37 | 0.55 | 0.86 | 1.00 | 0.90 | 2.08 |
| EAZ30819 | Putative uncharacterized protein | isomerase activity | 1.79 | 0.84 | 0.80 | 1.12 | 2.46 | 1.11 |
| O49886 | Peptidyl-prolyl cis-trans isomerase | peptidyl-prolyl cis-trans isomerase activity | 0.92 | 1.02 | 0.93 | 0.86 | 0.90 | 0.91 |
| CAZ91440 | Calreticulin | unfolded protein binding | 0.97 | 1.00 | 0.82 | 1.19 | 1.42 | 1.21 |
| ABR16377 | Uncharacterized protein (Ubiquitin) | mRNA binding | 1.34 | 1.19 | 1.06 | 1.00 | 1.09 | 0.86 |
| NP_568552 | Polyubiquitin | mRNA binding; ubiquitin protein ligase binding | 1.68 | 1.32 | 1.39 | 1.28 | 1.33 | 1.10 |
| Substance transport | ||||||||
| ABK92828 | Predicted protein | GTP binding | 0.70 | 0.66 | 0.72 | 0.87 | 0.88 | 0.90 |
| 1N00_A | Annexin | calcium ion binding; calcium-dependent phospholipid binding | 0.70 | 1.84 | 3.44 | 1.36 | 0.27 | 1.33 |
| CBC30882 | Anx1 | calcium ion binding; calcium-dependent phospholipid binding | 0.53 | 0.96 | 0.95 | 1.40 | 0.65 | 1.17 |
| ABK92937 | Predicted protein | adenosine kinase activity | 0.03 | 0.00 | 0.00 | 0.00 | 1.11 | 0.00 |
| ACI26701 | Non-specific lipid-transfer protein | lipid binding | 0.65 | 0.86 | 1.09 | 0.80 | 0.70 | 1.17 |
| Signal transduction | ||||||||
| ABW34390 | Glutathione S-transferase 5 | transferase activity | NK | NK | NK | 0.98 | NK | NK |
| Q7Y052 | Calmodulin (CaM) | calcium ion binding | 1.09 | 0.96 | 0.82 | 0.96 | 1.17 | 0.88 |
| Q9ZSW9 | tumor protein homolog (TCTP) | calcium ion binding | 1.55 | 1.22 | 0.93 | 1.18 | 1.41 | 0.85 |
| Antioxidation | ||||||||
| ACC93637 | Superoxide dismutase | copper ion binding; zinc ion binding | 1.20 | 1.28 | 0.88 | 1.79 | 1.32 | 0.97 |
| CBC66557 | Class III peroxidase | calcium ion binding; electron carrier activity; heme binding | 1.10 | 1.98 | 2.55 | 2.29 | 0.56 | 1.30 |
| XP_002303520 | Predicted protein | copper ion binding; oxidoreductase activity | 0.91 | NK | NK | NK | NK | NK |
| CAO47501 | Chromosome chr3 scaffold_8 | copper ion binding; oxidoreductase activity | 0.77 | 0.98 | 0.92 | 0.87 | 0.87 | 1.04 |
| Other processes | ||||||||
| XP_002446509 | ultraviolet-B receptor UVR8 | ultraviolet-B receptor | 0.52 | 0.45 | 0.27 | 0.58 | 1.70 | 0.88 |
| XP_002461966 | 28 kDa ribonucleo protein | nucleic acid binding; nucleotide binding | 0.84 | 0.80 | 0.80 | 0.77 | 1.15 | 1.10 |
| XP_001690796 | Predicted protein | snRNA processing | 0.23 | 1.35 | 0.43 | 3.52 | 0.46 | 0.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Feng, Y.; Khan, A.; Ullah, N.; Li, Z.; Zaheer, S.; Zhou, R.; Zhang, Z. Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings. Agronomy 2022, 12, 1152. https://doi.org/10.3390/agronomy12051152
Zhang X, Feng Y, Khan A, Ullah N, Li Z, Zaheer S, Zhou R, Zhang Z. Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings. Agronomy. 2022; 12(5):1152. https://doi.org/10.3390/agronomy12051152
Chicago/Turabian StyleZhang, Xin, Yan Feng, Aziz Khan, Najeeb Ullah, Zengqiang Li, Saira Zaheer, Ruiyang Zhou, and Zhiyong Zhang. 2022. "Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings" Agronomy 12, no. 5: 1152. https://doi.org/10.3390/agronomy12051152
APA StyleZhang, X., Feng, Y., Khan, A., Ullah, N., Li, Z., Zaheer, S., Zhou, R., & Zhang, Z. (2022). Quantitative Proteomics-Based Analysis Reveals Molecular Mechanisms of Chilling Tolerance in Grafted Cotton Seedlings. Agronomy, 12(5), 1152. https://doi.org/10.3390/agronomy12051152






