Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review
Abstract
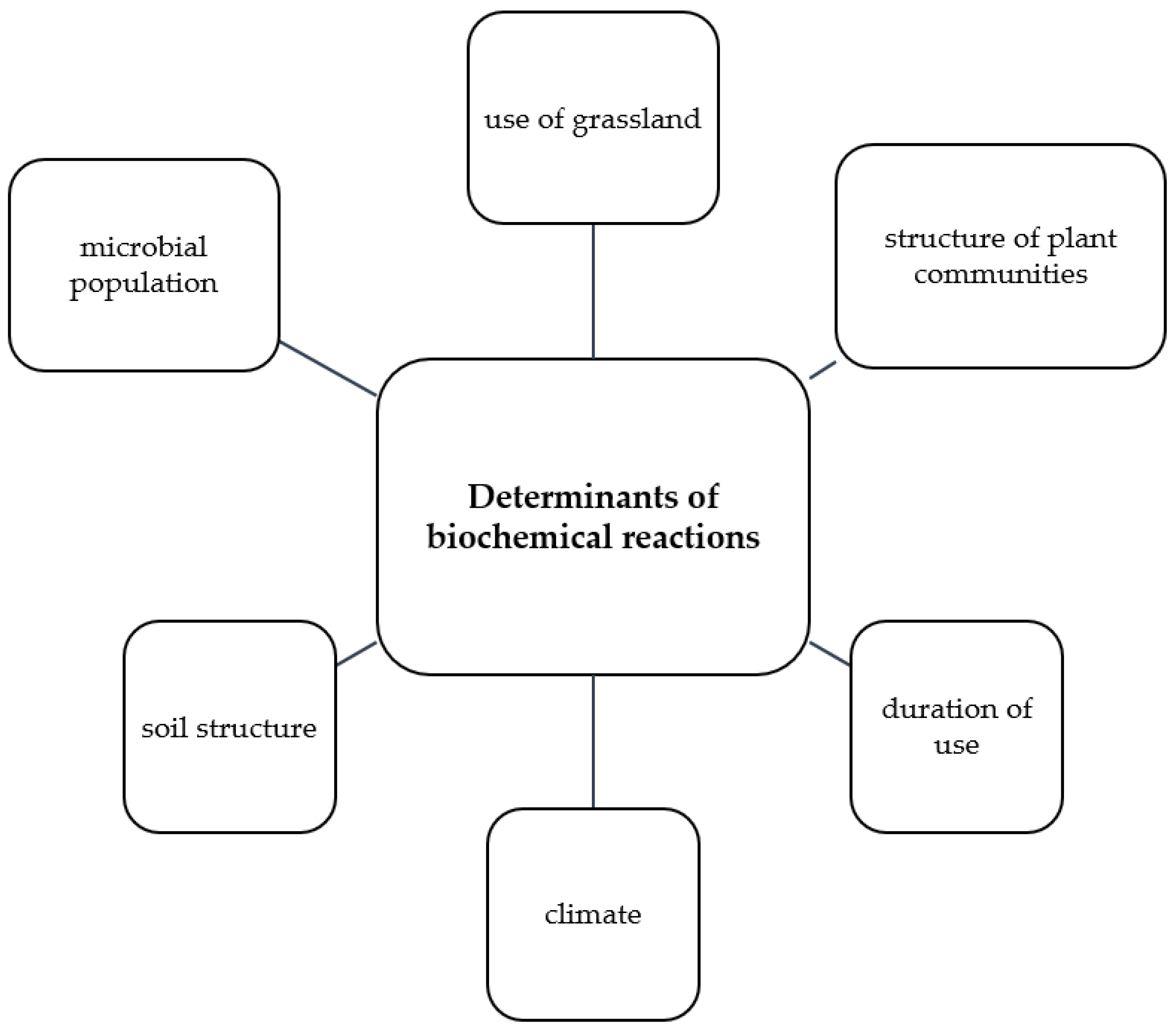
1. Introduction
2. Types of Grassland Use
2.1. Use of Grasslands by Mowing
2.2. Use of Grasslands by Grazing
2.3. Alternate Use of Grassland by Mowing and Grazing
3. The Occurrence of Microorganisms in Grassland Soils
3.1. Bacteria
3.2. Fungi
4. Enzyme Activity in Grassland Soils
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Are Grasslands under Threat? Brief Analysis of FAO Statistical Data on Pasture and Fodder Crops. 2008. Available online: http://www.Fao.Org/Uploads/Media/Grass_stats_1.Pdf (accessed on 1 February 2022).
- Wróbel, B.; Świechowska, I.; Krupa, A. The Production-Related and Natural Aspects of the Use of Meadows and Pastures in 507 Organic Farms; Agricultural Advisory Centre in Brwinów: Poznań, Poland, 2021.
- Khalil, M.I.; Cordovil, C.M.D.S.; Francaviglia, R.; Beverley, H.; Klumpp, K.; Koncz, P.; Llorente, M.; Madari, B.E.; Muñoz-Rojas, M.; Nerger, R. Grasslands. In Recarbonizing Global Soils: A Technical Manual of Recommended Sustainable Soil Management; FAO: Italy, Rome, 2021; Volume 3, ISBN 978-92-5-134893-2. [Google Scholar]
- Klarzyńska, A.A.; Kryszak, A. Floristic Diversity of Extensively Used Fresh Meadows (6510) in the Wielki Łęg Obrzański Complex. Acta Agrobot. 2015, 68, 115–123. [Google Scholar] [CrossRef][Green Version]
- Szuleta, M.; Kitczak, T.; Łazar, E.; Kirkiewicz, A. Floristic characteristics and some chemical properties of soil and sward of meadows located in the Natura 2000 area in the valley of river Parsęta in Sulikowo. Grassl. Sci. Pol. 2017, 20, 183–197. [Google Scholar]
- Wróbel, B.; Terlikowski, J.; Weso, P.; Barszczewski, J. Rational Use of Lowland Meadows. ITP Falenty 2015, 24. [Google Scholar]
- Barszczewski, J.; Wasilewski, Z.; Mendra, M. Rational Use of Lowland Pastures. ITP Falenty 2015, 24. [Google Scholar]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. Mowing: A Meta-Analysis of Biodiversity Benefits for Grassland Management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Burczyk, P.; Gamrat, R.; Gałczyńska, M.; Saran, E. The role of grasslands in providing ecological sustainability of the natural environment. Water-Environ.-Rural Areas 2018, 18, 21–37. [Google Scholar]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef]
- Laidlaw, A.S.; Šebek, L.B.J. Grassland for Sustainable Animal Production. Grassl. Sci. Eur. 2012, 17, 47–58. [Google Scholar]
- Grzegorczyk, S. The role of grassland ecosystems in environmental management. Zesz. Probl. Postępów Nauk Rol. 2016, 586, 19–32. [Google Scholar]
- Grzelak, M.; Bocian, T. Geobotanical diversity of semi-natural communities of the “Bystra” Noteć valley and their role in the landscape. Ann. UMCS 2006, 61, 257–266. [Google Scholar]
- Marinari, S.; Mancinelli, R.; Campiglia, E.; Grego, S. Chemical and Biological Indicators of Soil Quality in Organic and Conventional Farming Systems in Central Italy. Ecol. Indic. 2006, 6, 701–711. [Google Scholar] [CrossRef]
- Kucharski, J.; Barabasz, W.; Bielinska, E.J. The Biological and Biochemical Properties of Soil. In Gleboznawstwo; Mocek, A., Ed.; PWN: Warszawa, Poland, 2015; pp. 232–280. [Google Scholar]
- Kozieł, M.; Gałązka, A.; Martyniuk, S. Free-Living Atmospheric Nitrogen-Fixing Bacteria of the Azotobacter Genus—534 Occurrence, Abundance, and Significance. Stud. Rep. IUNG-PIB 2018, 56, 57–70. [Google Scholar] [CrossRef]
- Martyniuk, S. Is Conventional (Intensive) Agriculture Deterimental to Soil Microorganisms? Pol. J. Agron. 2014, 17, 25–29. [Google Scholar]
- Łyszcz, M.; Gałązka, A. The Biological Fixation of Atmospheric Nitrogen. Stud. Rep. IUNG-PIB 2016, 49, 59–70. [Google Scholar] [CrossRef]
- Musiał, M.; Kryszak, J.; Grzebisz, W.; Wolna-Maruwka, A.; Łukowiak, R. Effect of Pasture Management System Change on In-Season Inorganic Nitrogen Pools and Heterotrophic Microbial Communities. Agronomy 2020, 10, 724. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Ramasamy, M. Role of Fungi in Agriculture. In Biostimulants in Plant Science; Mirmajlessi, S.M., Radhakrishnan, R., Eds.; IntechOpen: London, UK, 2020; p. 12. [Google Scholar] [CrossRef]
- Lenart-Boroń, A.; Banach, T. Actinobacteria Streptomyces Spp in the Heavy Metal-Contaminated Environment. Kosmos. Probl. Nauk Biol. 2014, 63, 87–93. [Google Scholar]
- Ukalska-Jaruga, A.; Smreczak, B.; Strzelecka, J. Effect of Organic Matter on Soil Quality Used for Agricultural Purposes. Stud. Rep. IUNG-PIB 2017, 54, 25–39. [Google Scholar] [CrossRef]
- Podlesny, J.; Kowalska, B.; Niewiadomska, A.; Barabasz, W. Instytut Uprawy Nawożenia i Gleboznawstwa—Państwowy Instytut Badawczy. In Protection of Soil Biodiversity for the Health of Present and Future Generations; Institute of Soil Science and Plant Cultivation State Research Institute: Pulawy, Poland, 2019; ISBN 978-83-7562-318-5. [Google Scholar]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes Benefaction Role in Soil and Plant Health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Russel, S.; Wyczółkowski, A.J. Methods of Determining the Activity of Soil Enzymes; Acta Agrophysica: Lublin, Poland, 2005; Volume 120. [Google Scholar]
- Bielinska, E.J.; Futa, B.; Mocek-Płóciniak, A. Soil Enzymes As Bioindicators of Soil Quality and Health; Libropolis Scientific 550 Publishers Society: Lublin, Poland, 2014; ISBN 978-83-63761-25-7. [Google Scholar]
- Andrés, P.; Moore, J.C.; Cotrufo, F.; Denef, K.; Haddix, M.L.; Molowny-Horas, R.; Riba, M.; Wall, D.H. Grazing and Edaphic Properties Mediate Soil Biotic Response to Altered Precipitation Patterns in a Semiarid Prairie. Soil Biol. Biochem. 2017, 113, 263–274. [Google Scholar] [CrossRef]
- Jing, Z.; Cheng, J.; Su, J.; Bai, Y.; Jin, J. Changes in Plant Community Composition and Soil Properties under 3-Decade Grazing Exclusion in Semiarid Grassland. Ecol. Eng. 2014, 64, 171–178. [Google Scholar] [CrossRef]
- McSherry, M.E.; Ritchie, M.E. Effects of Grazing on Grassland Soil Carbon: A Global Review. Glob. Chang. Biol. 2013, 19, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Du, W.; Yuan, X.; Yang, Z.; Liu, D.; Wang, D.; Yu, L. Impacts of Grazing Intensity and Plant Community Composition on Soil Bacterial Community Diversity in a Steppe Grassland. PLoS ONE 2016, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Schuman, G.E.; Derner, J.D. Carbon Sequestration and Rangelands: A Synthesis of Land Management and Precipitation Effects. J. Soil Water Conserv. 2007, 62, 77–85. [Google Scholar]
- Stavi, I.; Ungar, E.D.; Lavee, H.; Sarah, P. Grazing-Induced Spatial Variability of Soil Bulk Density and Content of Moisture, Organic Carbon and Calcium Carbonate in a Semi-Arid Rangeland. CATENA 2008, 75, 288–296. [Google Scholar] [CrossRef]
- Steffens, M.; Kölbl, A.; Totsche, K.U.; Kögel-Knabner, I. Grazing Effects on Soil Chemical and Physical Properties in a Semiarid Steppe of Inner Mongolia (P.R. China). Geoderma 2008, 143, 63–72. [Google Scholar] [CrossRef]
- Valls Fox, H.; Bonnet, O.; Cromsigt, J.P.G.M.; Fritz, H.; Shrader, A.M. Legacy Effects of Different Land-Use Histories Interact with Current Grazing Patterns to Determine Grazing Lawn Soil Properties. Ecosystems 2015, 18, 720–733. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Shi, L.; Zhou, Y.; Yang, M.; Cao, J.; Wu, S.; Lei, G. Effects of Different Grazing Intensities on Soil C, N, and P in an Alpine Meadow on the Qinghai—Tibetan Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 2584. [Google Scholar] [CrossRef]
- Mayel, S.; Jarrah, M.; Kuka, K. How Does Grassland Management Affect Physical and Biochemical Properties of Temperate Grassland Soils? A Review Study. Grass Forage Sci. 2021, 76, 215–244. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, L.; Laanbroek, H.J.; Guo, D.; Wang, Q. Effects of Mowing Heights on N2O Emissionfrom Temperate Grasslands in InnerMongolia, Northern China. AJCC 2015, 4, 397–407. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.-Y.; Zhao, Y.; Gao, Z.; Zhao, S.-J. Changes in Soil Microbial Community during Shrub Encroachment Process in the Inner Mongolia Grassland of Northern China. CATENA 2021, 202, 10. [Google Scholar] [CrossRef]
- Li, W.; Huang, H.Z.; Zhang, Z.N.; Wu, G.L. Effects of Grazing on the Soil Properties and C and N Storage in Relation to Biomass Allocation in an Alpine Meadow. J. Soil Sci. Plant Nutr. 2011, 11, 27–39. [Google Scholar] [CrossRef]
- Stroh, P.A.; Bragg, J.; Carey, P.; Laidlaw, C.; Lester, M.; Mountford, J.O.; Smith, G.; Sparks, T.H.; Warrington, S.; Hughes, F.M.R. The Effects of Extensive Grazing on The Vegetation of a Landscape-Scale Restoration Site. Eur. J. Ecol. 2021, 7, 88–104. [Google Scholar] [CrossRef]
- Eriksson, O.; Bolmgren, K.; Westin, A.; Lennartsson, T. Historic Hay Cutting Dates from Sweden 1873–1951 and Their Implications for Conservation Management of Species-Rich Meadows. Biol. Conserv. 2015, 184, 100–107. [Google Scholar] [CrossRef]
- Tälle, M. Conservation of Semi-Natural Grasslands: Effects of Different Management Methods on Biodiversity. Ph.D. Thesis, Linköping University, Linköping, Sweden, 2018. [Google Scholar]
- Jankowska-Huflejt, H.; Domański, P.J. Present and possible directions of utilising permanent grasslands in Poland. Water Environ. Rural Areas 2008, 8, 31–49. [Google Scholar]
- Nieróbca, A.; Kozyra, J.; Mizak, K.; Wróblewska, E. Changes in the Length of the Growing Season in Poland. Water Environ. Rural Areas 2013, 13, 81–94. [Google Scholar]
- Dubeux, J.C.B.; Sollenberger, L.E.; Gaston, L.A.; Vendramini, J.M.B.; Interrante, S.M.; Stewart, R.L. Animal Behavior and Soil Nutrient Redistribution in Continuously Stocked Pensacola Bahiagrass Pastures Managed at Different Intensities. Crop Sci. 2009, 49, 1503–1510. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, L.; Lin, Q.; Yuan, M.; Xu, D.; Yu, H.; Hu, Y.; Duan, J.; Li, X.; He, Z.; et al. Responses of the Functional Structure of Soil Microbial Community to Livestock Grazing in the Tibetan Alpine Grassland. Glob. Chang. Biol. 2013, 19, 637–648. [Google Scholar] [CrossRef]
- Twardy, S.; Mikołajczyk-Rusin, K. Mountain pastures use as a way for sustainable management of permanent grasslands in Carpathian areas. Water-Environ.-Rural Areas 2018, 18, 93–108. [Google Scholar]
- Franzluebbers, A.J.; Paine, L.K.; Winsten, J.R.; Krome, M.; Sanderson, M.A.; Ogles, K.; Thompson, D. Well-Managed Grazing Systems: A Forgotten Hero of Conservation. J. Soil Water Conserv. 2012, 67, 100A–104A. [Google Scholar] [CrossRef]
- Behnke, R.H. Grazing Into the Anthropocene or Back to the Future? Front. Sustain. Food Syst. 2021, 5, 638806. [Google Scholar] [CrossRef]
- Metera, E.; Sakowski, T.; Słoniewski, K.; Romanowicz, B. Grazing as a Tool to Maintain Biodiversity of Grassland—A Review. Anim. Sci. Pap. Rep. 2010, 28, 315–334. [Google Scholar]
- Radkowska, I. Use of pastures in organic dairy farming. Wiadomości. Zootech. 2013, 51, 43–54. [Google Scholar]
- Stachowicz, T. Rational Use of Grassland on an Organic Farm; Agricultural Advisory Centre in Brwinów Branch in Radom: Radom, Poland, 2010; ISBN 978-83-60185-69-8.
- Tälle, M.; Fogelfors, H.; Westerberg, L.; Milberg, P. The Conservation Benefit of Mowing vs Grazing for Management of Species-Rich Grasslands: A Multi-Site, Multi-Year Field Experiment. Nord. J. Bot. 2015, 33, 761–768. [Google Scholar] [CrossRef]
- Kamiński, J.; Chrzanowski, S. The effect of mowing and grazing on physical soil properties and species composition of plant communities on reclaimed peatland. Water-Environ.-Rural Areas 2007, 7, 75–86. [Google Scholar]
- Frąc, M.; Jezierska-Tys, S. Microbial diversity of soil environment. Postępy Mikrobiol. Adv. Microbiol. 2010, 49, 47–58. [Google Scholar]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. EJSS 2017, 68, 12–26. [Google Scholar] [CrossRef]
- Nazir, N.; Kamili, A.N.; Zargar, M.Y.; Khan, I.; Shah, D.; Tyub, S. Effect of Root Exudates on Rhizosphere Soil Microbial Communities. J. Res. Dev. 2016, 16, 9. [Google Scholar]
- Ingram, L.J.; Stahl, P.D.; Schuman, G.E.; Buyer, J.S.; Vance, G.F.; Ganjegunte, G.K.; Welker, J.M.; Derner, J.D. Grazing Impacts on Soil Carbon and Microbial Communities in a Mixed-Grass Ecosystem. SSSA J. 2008, 72, 939–948. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, Y.; Chang, S.; Kan, H.; Lin, L. Impact of Grazing on Soil Carbon and Microbial Biomass in Typical Steppe and Desert Steppe of Inner Mongolia. PLoS ONE 2012, 7, 9. [Google Scholar] [CrossRef]
- Zhao, F.; Ren, C.; Shelton, S.; Wang, Z.; Pang, G.; Chen, J.; Wang, J. Grazing Intensity Influence Soil Microbial Communities and Their Implications for Soil Respiration. Agric. Ecosyst. Environ. 2017, 249, 50–56. [Google Scholar] [CrossRef]
- Solecka, J.; Ziemska, J.; Rajnisz, A.; Laskowska, A.; Guśpiel, A. Actinomycetes—Occurrence and production of biologically active compounds. Postępy Mikrobiol. Adv. Microbiol. 2013, 52, 83–91. [Google Scholar]
- Józefowska, A.; Zaleski, T.; Zarzycki, J.; Frączek, K. Do Mowing Regimes Affect Plant and Soil Biological Activity in the Mountain Meadows of Southern Poland? J. Mt. Sci. 2018, 15, 2409–2421. [Google Scholar] [CrossRef]
- Kizilova, A.K.; Stepanov, A.L.; Makarov, M.I. Biological Activity of Alpine Mountain-Meadow Soils in the Teberda Reserve. Eurasian Soil Sci. 2006, 39, 67–70. [Google Scholar] [CrossRef]
- Chmolowska, D.; Elhottová, D.; Krištůfek, V.; Kozak, M.; Kapustka, F.; Zubek, S. Functioning Grouped Soil Microbial Communities According to Ecosystem Type, Based on Comparison of Fallows and Meadows in the Same Region. Sci. Total Environ. 2017, 599–600, 981–991. [Google Scholar] [CrossRef]
- Ilmarinen, K.; Mikola, J.; Nissinen, K.; Vestberg, M. Role of Soil Organisms in the Maintenance of Species-Rich Seminatural Grasslands through Mowing. Restor. Ecol. 2009, 17, 78–88. [Google Scholar] [CrossRef]
- Creamer, R.E.; Schulte, R.P.O.; Stone, D.; Gal, A.; Krogh, P.H.; Lo Papa, G.; Murray, P.J.; Pérès, G.; Foerster, B.; Rutgers, M.; et al. Measuring Basal Soil Respiration across Europe: Do Incubation Temperature and Incubation Period Matter? Ecol. Indic. 2014, 36, 409–418. [Google Scholar] [CrossRef]
- Bei, Q.; Moser, G.; Müller, C.; Liesack, W. Seasonality Affects Function and Complexity but Not Diversity of the Rhizosphere Microbiome in European Temperate Grassland. Sci. Total Environ. 2021, 784, 9. [Google Scholar] [CrossRef]
- Hammerl, V.; Kastl, E.-M.; Schloter, M.; Kublik, S.; Schmidt, H.; Welzl, G.; Jentsch, A.; Beierkuhnlein, C.; Gschwendtner, S. Influence of Rewetting on Microbial Communities Involved in Nitrification and Denitrification in a Grassland Soil after a Prolonged Drought Period. Sci. Rep. 2019, 9, 2280. [Google Scholar] [CrossRef]
- Jurburg, S.D.; Natal-da-Luz, T.; Raimundo, J.; Morais, P.V.; Sousa, J.P.; van Elsas, J.D.; Salles, J.F. Bacterial Communities in Soil Become Sensitive to Drought under Intensive Grazing. Sci. Total Environ. 2018, 618, 1638–1646. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Berlin, Germany, 2008; ISBN 978-3-540-74922-6. [Google Scholar]
- Binet, M.N.; Sage, L.; Malan, C.; Clément, J.C.; Redecker, D.; Wipf, D.; Geremia, R.A.; Lavorel, S.; Mouhamadou, B. Effects of Mowing on Fungal Endophytes and Arbuscular Mycorrhizal Fungi in Subalpine Grasslands. Fungal Ecol. 2013, 6, 248–255. [Google Scholar] [CrossRef]
- Bielinska, E.J.; Gruszecki, T. The Impact of Extensive Grazing of Sheep on the Enzymatic Activity of Soils Selected Habitats Natura 2000 (in Polish). Zesz. Probl. Postępów Nauk Rol. 2011, 567, 11–19. [Google Scholar]
- Bini, D.; dos Santos, C.A.; do Carmo, K.B.; Kishino, N.; Andrade, G.; Zangaro, W.; Nogueira, M.A. Effects of Land Use on Soil Organic Carbon and Microbial Processes Associated with Soil Health in Southern Brazil. Eur. J. Soil Biol. 2013, 55, 117–123. [Google Scholar] [CrossRef]
- Bielinska, E.J.; Mocek-Płóciniak, A. Phosphatases in Soil Environmental (in Polish). Monography; Wyd. Uniwersytetu Przyrodniczego w Pozaniu: Poznań, Poland, 2009. [Google Scholar]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.A.; Mahmood, R.; Ali, S. Soil Urease Inhibition by Various Plant Extracts. PLoS ONE 2021, 16, e0258568. [Google Scholar] [CrossRef]
- Jesmin, T.; Margenot, A.J.; Mulvaney, R.L. A Comprehensive Method for Casein-Based Assay of Soil Protease Activity. Commun. Soil Sci. Plant Anal. 2022, 53, 507–520. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; DeLeon, E.R.; Arif, M.; Arif, F.; Arora, N.; Straub, K.D. Catalase as a Sulfide-Sulfur Oxido-Reductase: An Ancient (and Modern?) Regulator of Reactive Sulfur Species (RSS). Redox Biol. 2017, 12, 325–339. [Google Scholar] [CrossRef]
- Hadwan, M.H. Simple Spectrophotometric Assay for Measuring Catalase Activity in Biological Tissues. BMC Biochem. 2018, 19, 7. [Google Scholar] [CrossRef]
- Bielińska, E.J.; Futa, B.; Chmielewski, S.; Patkowski, K.; Gruszecki, T.M. Quantification of Biodiversity Related to the Active Protection of Grassland Habitats in the Eastern Lublin Region of Poland Based on the Activity of Soil Enzymes. Pol. J. Soil Sci. 2017, 50, 55. [Google Scholar] [CrossRef]
- Domżał, H.; Bielinska, E.J. Physicochemical and Chemical Properties of Soils. Acta Agrophysica 2007, 145, 65–77. [Google Scholar]
- Glina, B.; Piernik, A.; Mocek-Płóciniak, A.; Maier, A.; Glatzel, S. Drivers Controlling Spatial and Temporal Variation of Microbial Properties and Dissolved Organic Forms (DOC and DON) in Fen Soils with Persistently Low Water Tables. Glob. Ecol. Conserv. 2021, 27, e01605. [Google Scholar] [CrossRef]
- Futa, B.; Patkowski, K.; Bielińska, E.J.; Gruszecki, T.M.; Pluta, M.; Kulik, M.; Chmielewski, S. Sheep and Horse Grazing in a Large-Scale Protection Area and Its Positive Impact on Chemical and Biological Soil Properties. Pol. J. Soil Sci. 2017, 49, 111–122. [Google Scholar] [CrossRef][Green Version]
- Futa, B.; Tajchman, K.; Steiner-Bogdaszewska, Ż.; Drozd, L.; Gruszecki, T.M. Preliminary Results of Effect of Rotational Grazing of Farmed Red Deer (Cervus Elaphus) on the Biochemical Status of Soil. Agronomy 2021, 11, 558. [Google Scholar] [CrossRef]
- Wolińska, A.; Stepniewska, Z.; Szymańska, E. Dehydrogenase Activity of Soil Microorganisms and the Total DNA Level in Soil of Different Use. J. Agric. Sci. Technol. 2013, 3, 613–622. [Google Scholar]
- Qin, Y.; Niu, D.; Kang, J.; Zhou, Y.; Li, X. Effects of Livestock Exclusion on Soil Physical and Biochemical Properties of a Desert Rangeland. Pol. J. Environ. Stud. 2015, 24, 2587–2595. [Google Scholar] [CrossRef]
- Herold, N.; Schöning, I.; Gutknecht, J.; Alt, F.; Boch, S.; Müller, J.; Oelmann, Y.; Socher, A.S.; Wilcke, W.; Wubet, T.; et al. Soil Property and Management Effects on Grassland Microbial Communities across a Latitudinal Gradient in Germany. Appl. Soil Ecol. 2014, 73, 41–50. [Google Scholar] [CrossRef]
- Garcia, M.R.L.; Sampaio, A.A.M.; Nahas, E. Impact of Different Grazing Systems for Bovine Cattle on the Soil Microbiological and Chemical Characteristics. Rev. Bras. Zootec. 2011, 40, 1568–1575. [Google Scholar] [CrossRef]
- Cui, J.; Holden, N.M. The Relationship between Soil Microbial Activity and Microbial Biomass, Soil Structure and Grassland Management. Soil Tillage Res. 2015, 146, 32–38. [Google Scholar] [CrossRef]
- Olivera, N.L.; Prieto, L.; Carrera, A.L.; Cisneros, H.S.; Bertiller, M.B. Do Soil Enzymes Respond to Long-Term Grazing in an Arid Ecosystem? Plant Soil 2014, 378, 35–48. [Google Scholar] [CrossRef]
- Maryskevych, O.; Shpakivska, I. Impact of the Pastoral Land Use on Soil Properties in Skolivski Beskydy Mts. (Ukrainian Part of the Eastern Carpathians). Rocz. Bieszcz. 2011, 19, 349–357. [Google Scholar]
- Józefowska, A.; Zaleski, T.; Zarzycki, J. Does the Different Mowing Regime Affect Soil Biological Activity and FLoristic Composition of Thermophilous Pieniny Meadow? In Proceedings of the Geophysical Research Abstracts, Vienna, Austria, 17–22 April 2016; Volume 18. [Google Scholar]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical Properties in Managed Grassland Soils in a Temperate Humid Zone: Modifications of Soil Quality as a Consequence of Intensive Grassland Use. Biol. Fertil. Soils 2009, 45, 711–722. [Google Scholar] [CrossRef]
- Gilmullina, A.; Rumpel, C.; Blagodatskaya, E.; Chabbi, A. Management of Grasslands by Mowing versus Grazing—Impacts on Soil Organic Matter Quality and Microbial Functioning. Appl. Soil Ecol. 2020, 156, 103701. [Google Scholar] [CrossRef]
- Cui, H.; Sun, W.; Delgado-Baquerizo, M.; Song, W.; Ma, J.-Y.; Wang, K.; Ling, X. Contrasting Effects of N Fertilization and Mowing on Ecosystem Multifunctionality in a Meadow Steppe. Soil Ecol. Lett. 2020, 2, 268–280. [Google Scholar] [CrossRef]
- Yu, P.; Liu, S.; Han, K.; Guan, S.; Zhou, D. Conversion of Cropland to Forage Land and Grassland Increases Soil Labile Carbon and Enzyme Activities in Northeastern China. Agric. Ecosyst. Environ. 2017, 245, 83–91. [Google Scholar] [CrossRef]
- Vranová, V.; Formánek, P.; Rejšek, K.; Pavelka, M. Impact of Land-Use Change on Proteolytic Activity of Mountain Meadows. Soil Water Res. 2009, 4, 122–125. [Google Scholar] [CrossRef]
- Molik, E.; Ślezińska-Iwanicz, R.; Nahajło, K. Large-scale sheep grazing as an example of centuries-old management by methods of sustainable development in the Silesian and Zywiec Beskids. Wiadomości. Zootech. 2018, 56, 132–137. [Google Scholar]
| Use of Grassland | Enzyme | Enzyme Response | Reference |
|---|---|---|---|
| Extensive grazing | Dehydrogenases | increase | [72,81,84,85,86] |
| Alkaline phosphatase | increase | [64,84,87,88] | |
| Acid phosphatase | increase | [64,72,84,88,89] | |
| Urease | increase | [64,72,81,84,85,89,90] | |
| Proteases | no response | [90,91] | |
| Catalase | decrease | [87,92] | |
| Mowing | Dehydrogenases | increase | [62,86,93,94] |
| Alkaline phosphatase | increase | [64,95,96,97] | |
| Acid phosphatase | increase | [64,95] | |
| Urease | decrease | [97] | |
| Proteases | decrease | [94,98] | |
| Catalase | increase | [92,94,97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencel, J.; Mocek-Płóciniak, A.; Kryszak, A. Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review. Agronomy 2022, 12, 1136. https://doi.org/10.3390/agronomy12051136
Mencel J, Mocek-Płóciniak A, Kryszak A. Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review. Agronomy. 2022; 12(5):1136. https://doi.org/10.3390/agronomy12051136
Chicago/Turabian StyleMencel, Justyna, Agnieszka Mocek-Płóciniak, and Anna Kryszak. 2022. "Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review" Agronomy 12, no. 5: 1136. https://doi.org/10.3390/agronomy12051136
APA StyleMencel, J., Mocek-Płóciniak, A., & Kryszak, A. (2022). Soil Microbial Community and Enzymatic Activity of Grasslands under Different Use Practices: A Review. Agronomy, 12(5), 1136. https://doi.org/10.3390/agronomy12051136






