Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematodes
2.2. Insects
2.3. Laboratory, Greenhouse, and Field Trials
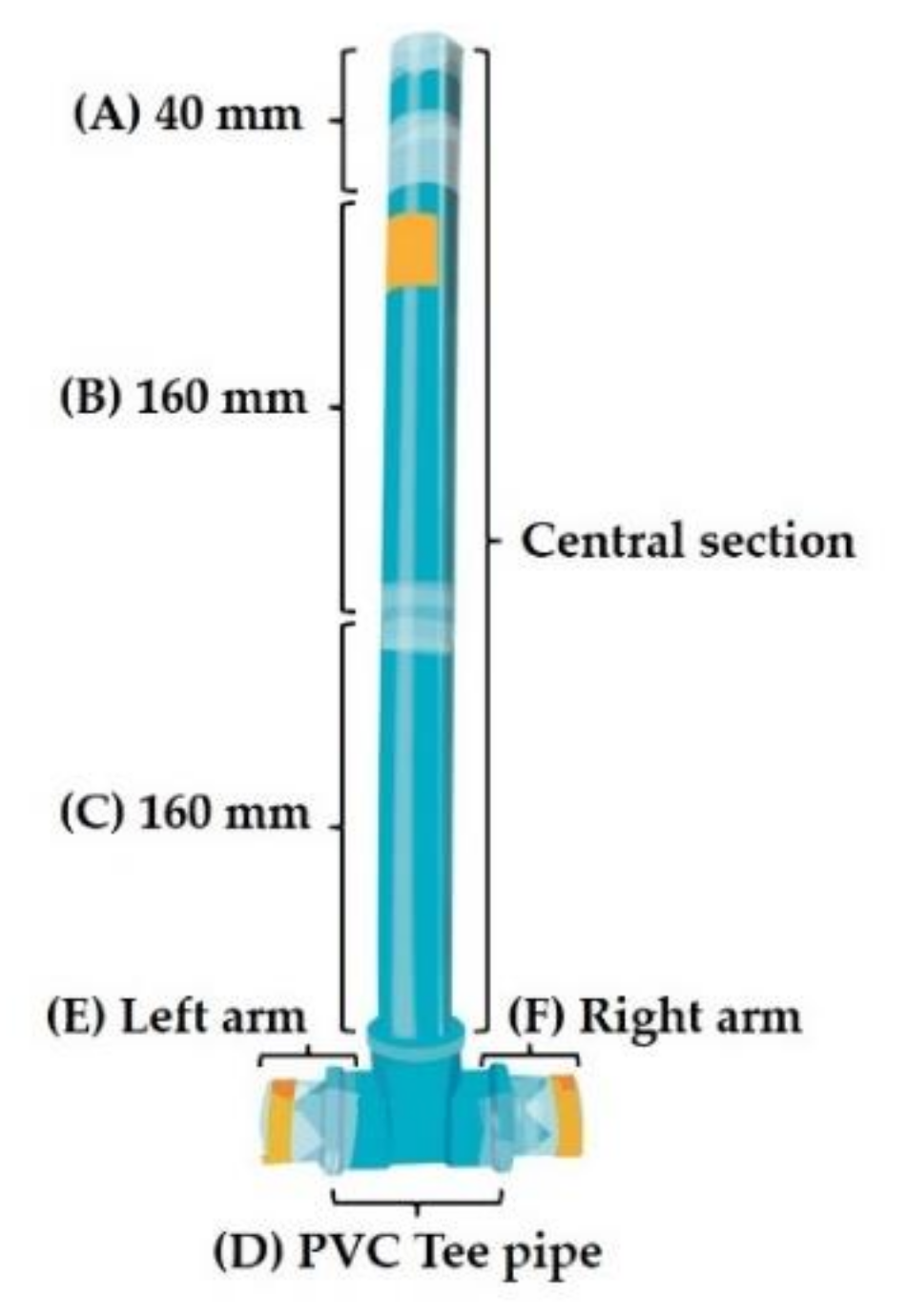
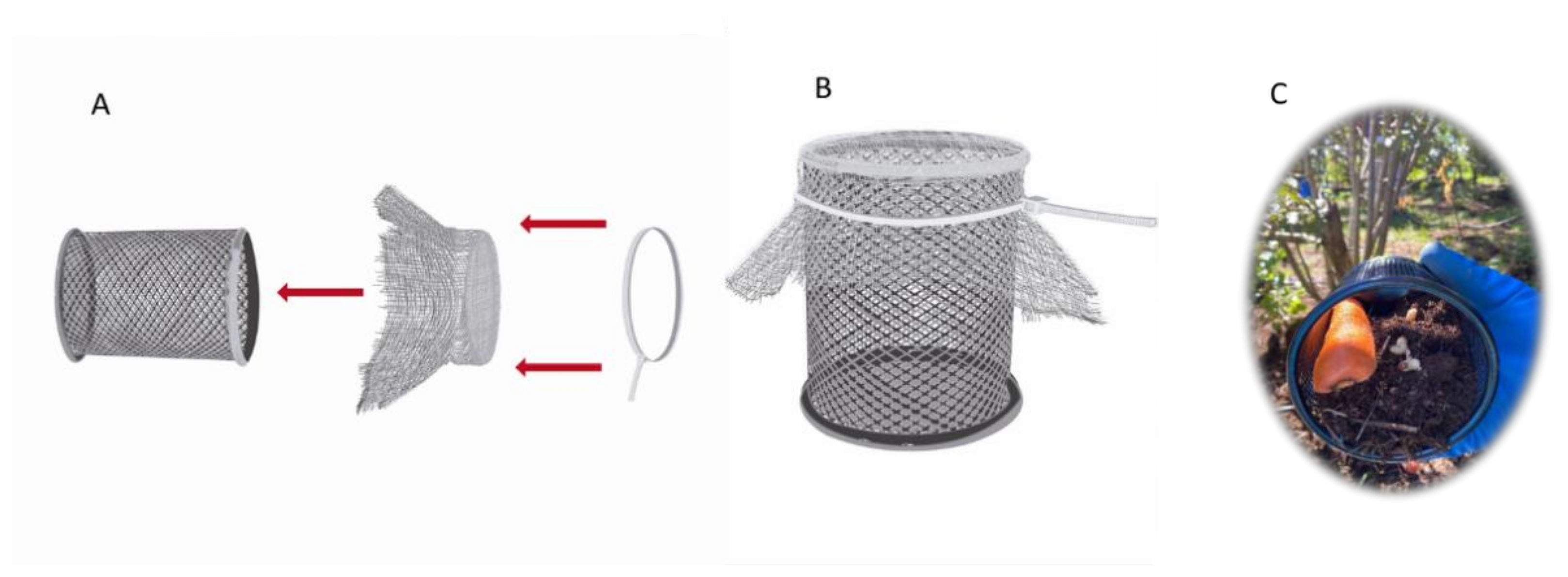
2.4. Artificial Selection of IJs Using an Odor Stimulus
2.5. Comparison of Selected IJs vs. the Original Stock
2.6. Efficacy of the Selected IJs in the Greenhouse
2.7. Efficacy of the Selected IJs in the Field
2.8. Data Analysis
3. Results
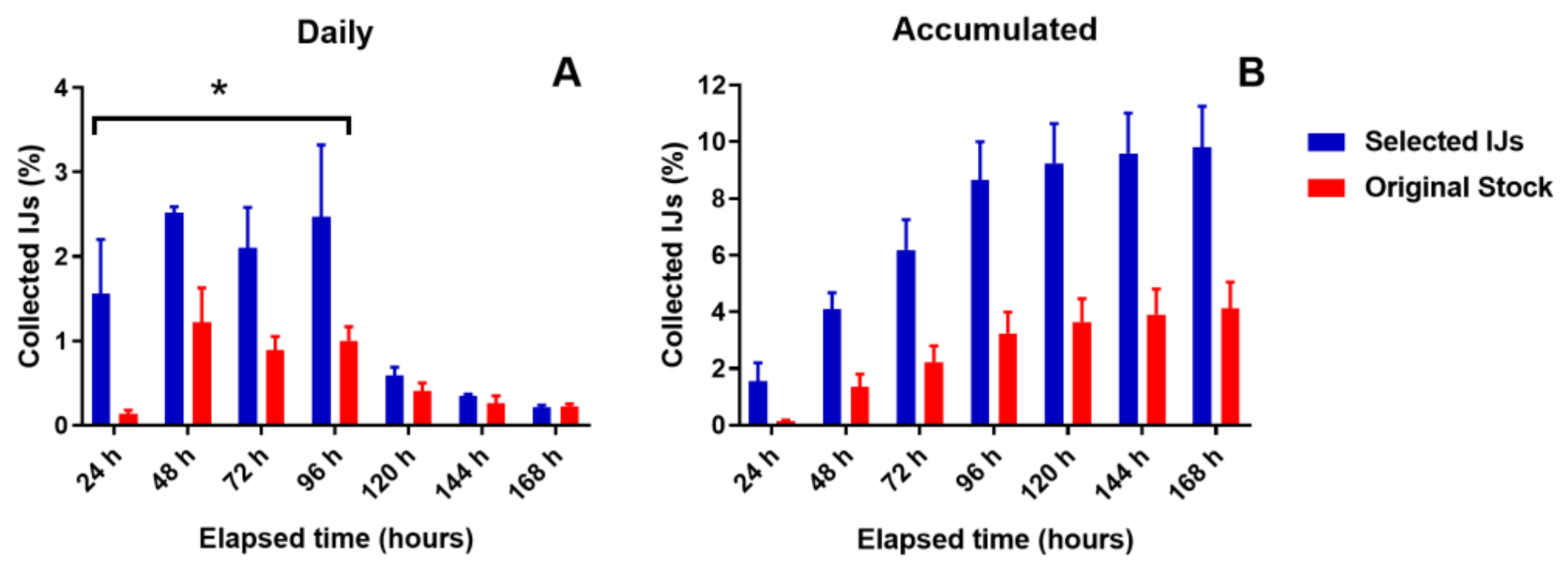
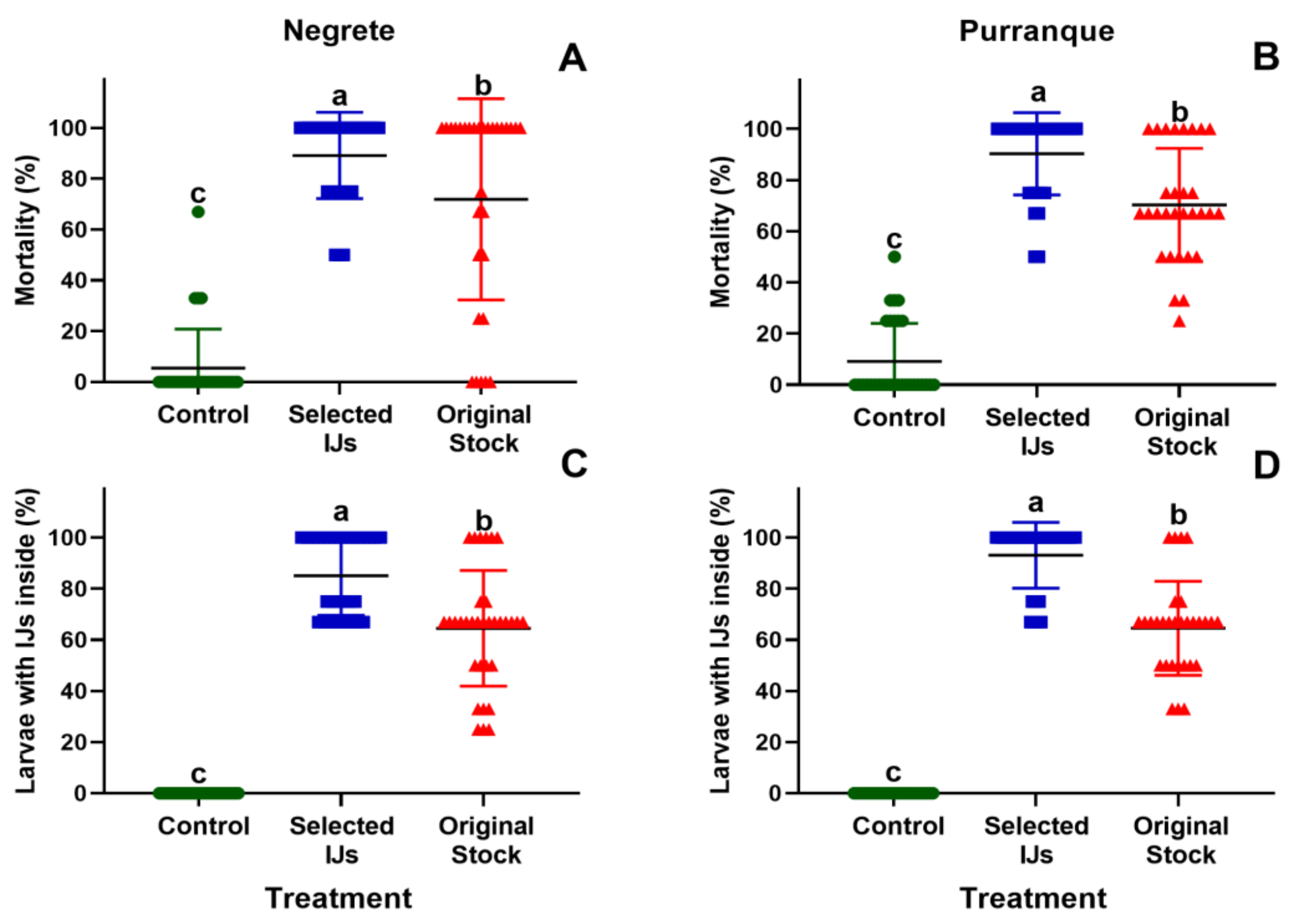
3.1. Comparison of Selected IJs vs. the Original Stock in the Laboratory
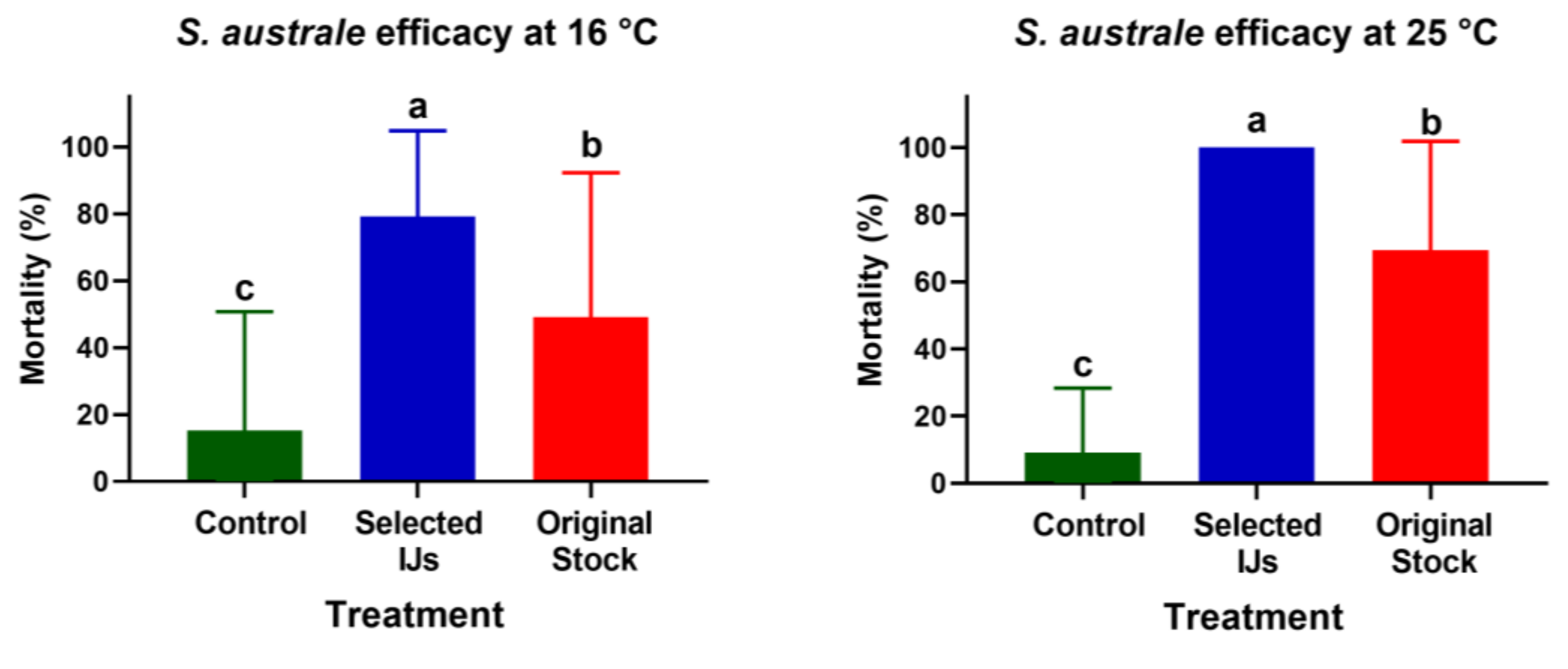
3.2. Efficacy of the Selected IJs in the Greenhouse
3.3. Efficacy of the Selected IJs in the Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kuschel, G.S. La subfamilia Aterpinae en América. Rev. Chil. De Entomol. 1951, 1, 205–244. [Google Scholar]
- Del Río, M.G.; Klasmer, P.; Lanteri, A.A. Gorgojos (Coleoptera: Curculionidae) perjudiciales para frutos rojos en la Argentina. Rev. Soc. Entomol. Argent. 2010, 69, 101–110. [Google Scholar]
- Lencinas, M.V.; Zamora, F.J.; Martínez, G.J. Ataque de Aegorhinus nodipennis (Curculionidae) en forestaciones y huertas de estancias de Tierra del Fuego: ¿una invasión incipiente? Anales del Instituto de la Patagonia 2021, 49, 1–8. [Google Scholar] [CrossRef]
- Artigas, J.N. Entomología Económica: Insectos de Interés Agrícola, Forestal, Médico y Veterinario (Nativos, Introducidos y Susceptibles de ser Introducidos); Universidad de Concepción: Concepción, Chile, 1994; Volume 2, p. 1126. [Google Scholar]
- Klein, C.; Waterhouse, D.F. The distribution and importance of arthropods associated with agriculture and forestry in Chile. Monograph 2000, 68, 1–234. [Google Scholar]
- Aguilera, A. Plagas del frambueso en la IX Región de Chile. IPA Carillanca 1994, 4, 23–32. [Google Scholar]
- Zavala, A.; Elgueta, M.; Abarzúa, J.; Aguilera, A.; Quiroz, A.; Rebolledo, R. Diversity and distribution of the Aegorhinus genus in the La Araucanía Region of Chile, with special reference to A. superciliosus and A. nodipennis. Ciencia e investigación agraria 2011, 38, 367–377. [Google Scholar] [CrossRef]
- Cisternas, E. Curculionidos. Insectos plagas de berries. Ficha Técnica INIA 2002, 47, 14–15. [Google Scholar]
- Cisternas, E. Cabritos asociados al arándano. INIA La Cruz Ficha Técnica 2015, 62, 1–2. [Google Scholar]
- Aguilera, A.; Rebolledo, R. Estadios larvarios de Aegorhinus superciliosus (Guerin, 1830) (Coleoptera:Curculionidae). Rev.Chilena Ent. 2001, 28, 5–8. [Google Scholar]
- Aguilera, A. Descripción de las principales plagas subterráneas. In Manejo Integrado de Plagas Subterráneas en Avellano Europeo; Ellena, M., Abel González, G., Eds.; Boletín INIA—Instituto de Investigaciones Agropecuarias: Vilcún, Chile, 2012; Volume 237, p. 110. [Google Scholar]
- Aguilera, A. Control selectivo de plagas en frutales de la zona sur. Seminario ed Protección Vegetal INIA Carillanca 1995, 45, 141–188. [Google Scholar]
- Fundación para la Innovación Agraria. Resultados y lecciones en biocontrol del cabrito de los frutales con nemátodos entomopatógenos: Proyecto de innovación en Regiones del Biobío, de La Araucanía, de Los Ríos y de Los Lagos. Rep. FIA 2011, 1, 28. [Google Scholar]
- Aguilera, A. Plagas del arándano en Chile. In Seminario: El cultivo del arándano; Lobos, W., Ed.; INIA Carillanca: Temuco, Chile, 1988; pp. 109–131. [Google Scholar]
- Aguilera, A. Insectos fitófagos cuarentenarios asociados a frutales menores en la Novena Región. IPA Carillanca 1990, 9, 7–11. [Google Scholar]
- Ellena, M.; Gonzalez, A.; Aguilera, A. Manejo integrado de plagas subterráneas en Avellano europeo (con especial referencia a Aegohinus superciliosus y A. nodipennis). Boletín INIA 2012, 1, 237. [Google Scholar]
- Kaya, H.K.; Gaugler, R. Entomopathogenic Nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Lacey, L.A.; Unruh, T.R. Entomopathogenic Nematodes for Control of Codling Moth,Cydia pomonella (Lepidoptera: Tortricidae): Effect of Nematode Species, Concentration, Temperature, and Humidity. Biol. Control. 1998, 13, 190–197. [Google Scholar] [CrossRef]
- Shapiro, D.I.; McCoy, C.W. Effects of culture method and formulation on the virulence of Steinernema riobrave (Rhabditida: Steinernematidae) to Diaprepes abbreviatus (Coleoptera: Curculionidae). J. Nematol. 2000, 32, 281–288. [Google Scholar]
- Yan, X.; Han, R.; Moens, M.; Chen, S.; De Clercq, P. Field evaluation of entomopathogenic nematodes for biological control of striped flea beetle, Phyllotreta striolata (Coleoptera: Chrysomelidae). BioControl 2013, 58, 247–256. [Google Scholar] [CrossRef]
- Bathon, H. Impact of entomopathogenic nematodes on non-target hosts. In Biocontrol Science and Technology; Taylor and Francis Ltd.: Oxford, UK, 1996; pp. 421–434. [Google Scholar]
- Rovesti, L.; Deseo, K.V. Compatibility of chemical pesticides with the entomopathogenic nematodes: Steinernema carpocapsae Weiser and Steinernema feltiae Filipjev (Nematoda: Steinernematidae). Nematologica 1990, 36, 237–245. [Google Scholar] [CrossRef]
- Campbell, J.F.; Lewis, E.E. Entomopathogenic nematode host-search strategies. In The Behavioural Ecology of Parasites; CABI: Wallingford, UK, 2002; pp. 13–38. [Google Scholar]
- Forst, S.; Dowds, B.; Boemare, N.; Stackebrandt, E. Xenorhabdus and Photorhabdus Spp.: Bugs ThatKill Bugs. Annu. Rev. Microbiol. 1997, 51, 47–72. [Google Scholar] [CrossRef]
- France, A. Uso de nematodos entomapatogenos para el control de insectos. In Manejo de Burrito de la vid, Naupactus Xanthographus (Germar) y otros Curculiónidos Asociados a Vides; Paola Luppichini, B., Natalia Olivares, P., Ernesto Cisternas, A., Eds.; Dirección: La Cruz, Región de Valparaíso, Chile, 2013; Volume 260, p. 79. [Google Scholar]
- Edgington, S.; Buddie, A.G.; Tymo, L.; Hunt, D.J.; Nguyen, K.B.; France, A.I.; Merino, L.M.; Moore, D. Steinernema australe n. sp. (Panagrolaimomorpha: Steinernematidae), a new entomopathogenic nematode from Isla Magdalena, Chile. Nematology 2009, 11, 699–717. [Google Scholar] [CrossRef]
- Lephoto, T.E.; Gray, V.M. First report of the isolation of entomopathogenic nematode Steinernema australe (Rhabditida: Steinernematidae) from South Africa. S. Afr. J. Sci. 2019, 115, 115. [Google Scholar] [CrossRef]
- Lephoto, T.E.; Gray, V.M. Desiccation stress tolerance of Steinernema australe and Heterorhabditis bacteriophora. Arch. Phytopathol. Plant Prot. 2021, 54, 611–624. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Kaya, H.K.; Taormino, S.P. Infectivity of Entomopathogenic Nematodes (Rhabditida: Steinernematidae) at Different Soil Depths and Moistures. J. Invertebr. Pathol. 1995, 65, 193–199. [Google Scholar] [CrossRef]
- Edgington, S.; Gowen, S.R. Ecological characterisation of Steinernema australe (Panagrolaimomorpha: Steinernematidae) an entomopathogenic nematode from Chile. Russ. J. Nematol. 2010, 18, 9–18. [Google Scholar]
- Gaugler, R.; Campbell, J.F.; McGuire, T.R. Selection for host-finding in Steinernema feltiae. J. Invertebr. Pathol. 1989, 54, 363–372. [Google Scholar] [CrossRef]
- Gaugler, R.; McGuire, T.; Campbell, J. Genetic Variability among Strains of the Entomopathogenic Nematode Steinernema feltiae. J. Nematol. 1989, 21, 247–253. [Google Scholar]
- Boff, M.I.C.; Zoon, F.C.; Smits, P.H. Orientation of Heterorhabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer. Entomologia Experimentalis et Applicata 2001, 98, 329–337. [Google Scholar] [CrossRef]
- Santhi, V.S.; Ment, D.; Salame, L.; Soroker, V.; Glazer, I. Genetic improvement of host-seeking ability in the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora toward the Red Palm Weevil Rhynchophorus ferrugineus. Biol. Control. 2016, 100, 29–36. [Google Scholar] [CrossRef]
- Grewal, P.S.; Gaugler, R.; Wang, Y. Enhanced cold tolerance of the entomopathogenic nematode Steinernema feltiae through genetic selection. Ann. Appl. Biol. 1996, 129, 335–341. [Google Scholar] [CrossRef]
- Nimkingrat, P.; Khanam, S.; Strauch, O.; Ehlers, R.-U. Hybridisation and selective breeding for improvement of low temperature activity of the entomopathogenic nematode Steinernema Feltiae. BioControl 2013, 58, 417–426. [Google Scholar] [CrossRef]
- Nimkingrat, P.; Strauch, O.; Ehlers, R.U. Hybridisation and genetic selection for improving desiccation tolerance of the entomopathogenic nematode Steinernema feltiae. Biocontrol Sci. Technol. 2013, 23, 348–361. [Google Scholar] [CrossRef]
- Salame, L.; Glazer, I.; Chubinishvilli, M.T.; Chkhubianishvili, T. Genetic improvement of the desiccation tolerance and host-seeking ability of the entomopathogenic nematode Steinernema feltiae. Phytoparasitica 2010, 38, 359–368. [Google Scholar] [CrossRef]
- Mukuka, J.; Strauch, O.; Hoppe, C.; Ehlers, R.U. Improvement of heat and desiccation tolerance in Heterorhabditis bacteriophora through cross-breeding of tolerant strains and successive genetic selection. BioControl 2010, 55, 511–521. [Google Scholar] [CrossRef]
- Bal, H.K.; Taylor, R.A.J.; Grewal, P.S. Ambush foraging entomopathogenic nematodes employ ‘sprinters’ for long-distance dispersal in the absence of hosts. J. Parasitol. 2014, 100, 422–432. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Campos-Herrera, R.; Kaplan, F.; Duncan, L.W.; Rodriguez-Saona, C.; Koppenhöfer, A.M.; Stelinski, L.L. Subterranean, herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE 2012, 7, e38146. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Willett, D.; Rivera, M.J.; Ali, J.G. ‘Tuning’ communication among four trophic levels of the root biome to facilitate biological control. Biol. Control. 2019, 131, 49–53. [Google Scholar] [CrossRef]
- Ali, J.G.; Alborn, H.T.; Stelinski, L.L. Constitutive and induced subterranean plant volatiles attract both entomopathogenic and plant parasitic nematodes. J. Ecol. 2011, 99, 26–35. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The Use of Push-Pull Strategies in Integrated Pest Management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga M, X.; Campos-Herrera, R.; Jaffuel, G.; Röder, G.; Turlings, T.C.J. Diffusion of the maize root signal (E)-β-caryophyllene in soils of different textures and the effects on the migration of the entomopathogenic nematode Heterorhabditis megidis. Rhizosphere 2017, 3, 53–59. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Stock, P.; Goodrich-Blair, H. Nematode parasites, pathogens and associates of insects and invertebrates of economic importance. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Elsevier Ltd.: New York, NY, USA, 2012; pp. 373–426. [Google Scholar]
- Singh, P.; Moore, R. Handbook of Insect Rearing; Elsevier: New York, NY, USA, 1985; Volume I. [Google Scholar]
- Hiltpold, I.; Baroni, M.; Toepfer, S.; Kuhlmann, U.; Turlings, T.C.J. Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J. Exp. Biol. 2010, 213, 2417–2423. [Google Scholar] [CrossRef]
- Mauleon, H.; Briand, S.; Laumond, C.; Bonifassi, É. Utilisation d’enzymes digestives pour l’étude du parasitisme des Steinernema et des Heterorhabditis envers les larves d’insectes. Fundam. Appl. Nematol. 1993, 16, 185–191. [Google Scholar]
- Navarro, P.D.; McMullen, J.G.; Stock, S.P. Effect of dinotefuran, indoxacarb, and imidacloprid on survival and fitness of two arizona-native entomopathogenic nematodes against Helicoverpa zea (Lepidoptera: Noctuidae). Nematropica 2014, 44, 64–73. [Google Scholar]
- Baiocchi, T.; Lee, G.; Choe, D.H.; Dillman, A.R. Host seeking parasitic nematodes use specific odors to assess host resources. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Hiltpold, I.; Baroni, M.; Toepfer, S.; Kuhlmann, U.; Turlings, T.C.J. Selective breeding of entomopathogenic nematodes for enhanced attraction to a root signal did not reduce their establishment or persistence after field release. Plant Signal. Behav. 2010, 5, 1450–1452. [Google Scholar] [CrossRef][Green Version]
- Bal, H.K.; Michel, A.P.; Grewal, P.S. Genetic selection of the ambush foraging entomopathogenic nematode, Steinernema carpocapsae for enhanced dispersal and its associated trade-offs. Evol. Ecol. 2014, 28, 923–939. [Google Scholar] [CrossRef]
- Grewal, P.S.; Lewis, E.E.; Gaugler, R.; Campbell, J.F. Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 1994, 108, 207–215. [Google Scholar] [CrossRef]
- Griffin, C.T. Perspectives on the behavior of entomopathogenic nematodes from dispersal to reproduction: Traits contributing to nematode fitness and biocontrol Efficacy. J. Nematol. 2012, 44, 177–184. [Google Scholar] [PubMed]
- Glaser, R.W.; Fox, H. A Nematode Parasite of the Japanese Beetle (Popillia japonica Newm.). Science 1930, 71, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, R.; Campbell, J.F.; McGuire, T.R. Fitness of a genetically improved entomopathogenic nematode. J. Invertebr. Pathol. 1990, 56, 106–116. [Google Scholar] [CrossRef]
- Stuart, R.J.; Lewis, E.E.; Gaugler, R. Selection alters the pattern of emergence from the host cadaver in the entomopathogenic nematode, Steinernema glaseri. Parasitology 1996, 113, 183–189. [Google Scholar] [CrossRef]
- Elawad, S.A.; Gowen, S.R.; Hague, N.G.M. Progeny production of Steinernema abbasi in lepidopterous larvae. Int. J. Pest Manag. 2001, 47, 17–21. [Google Scholar] [CrossRef]
- Navarro, P.D.; McMullen, J.G.; Stock, S.P. Interactions between the entomopathogenic nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae) and the saprobic fungus Fusarium oxysporum (Ascomycota: Hypocreales). J. Invertebr. Pathol. 2014, 115, 41–47. [Google Scholar] [CrossRef]
- Elgueta, M. Las Especies de Curculionoidea (Insecta: Coleoptera) de Interés Agrícola en Chile. Museo Nacional de Historia Natural 1993, 1–80. [Google Scholar]
- Luppichini, P.; France, A.; Urtubia, I.; Olivares, N.; Cisternas, A. Manejo de Burrito de la vid, Naupactus xanthographus (Germar) y otros curculiónidos asociados a vides; Boletín INIA—Instituto de Investigaciones Agropecuarias: Temuco, Chile, 2013; Volume 260, p. 77. [Google Scholar]
- Aguilera, A.; Galdames, R.; Ellena, M.; González, A.; Sandoval, P. Protección del cultivo plagas, enfermedades y desordenes fisiológicos del Avellano Europeo. In El Avellano Europeo en Chile. Una Década de Recopilación e Investigación; Ellena, M., Ed.; Boletín INIA—Instituto de Investigaciones Agropecuarias: Temuco, Chile, 2018; Volume 36, p. 425. [Google Scholar]
- Johnigk, S.-A. Endotokia matricida in ermaphrodites of spp. and the effect of the food supply. Nematology 1999, 1, 717–726. [Google Scholar]
- Ryder, J.J.; Griffin, C.T. Density-dependent fecundity and infective juvenile production in the entomopathogenic nematode, Heterorhabditis megidis. Parasitology 2002, 125, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G. Taxonomy and Biology of Steinernematidae and Heterorhabditidae. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 23–61. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, P.D.; Palma-Millanao, R.; Ceballos, R.; Monje, A.J. Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process. Agronomy 2022, 12, 1128. https://doi.org/10.3390/agronomy12051128
Navarro PD, Palma-Millanao R, Ceballos R, Monje AJ. Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process. Agronomy. 2022; 12(5):1128. https://doi.org/10.3390/agronomy12051128
Chicago/Turabian StyleNavarro, Patricia D., Rubén Palma-Millanao, Ricardo Ceballos, and Almendra J. Monje. 2022. "Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process" Agronomy 12, no. 5: 1128. https://doi.org/10.3390/agronomy12051128
APA StyleNavarro, P. D., Palma-Millanao, R., Ceballos, R., & Monje, A. J. (2022). Steinernema australe Enhanced Its Efficacy against Aegorhinus nodipennis (Coleoptera: Curculionidae) Larvae in Berry Orchards after an Artificial Selection Process. Agronomy, 12(5), 1128. https://doi.org/10.3390/agronomy12051128





