Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity
Abstract
1. Introduction
2. Materials and Methods
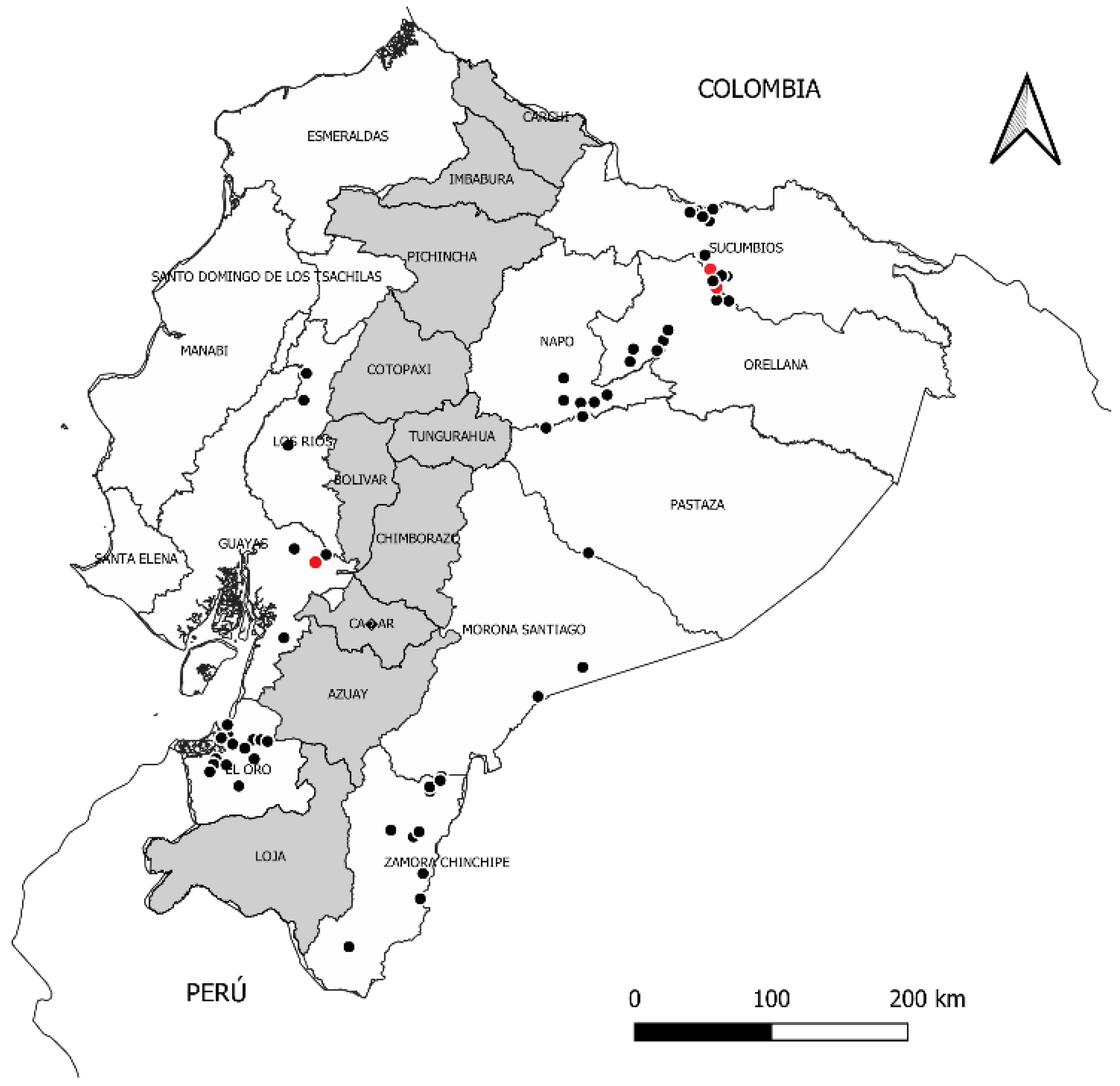
2.1. Isolation of M. roreri Strains and Monosporic Cultures
2.2. M. roreri’s Sensitivity to Azoxystrobin
2.3. DNA Extraction and Amplification of Simple Sequence Repeats (Microsatellite Markers SSRs)
2.4. Sensitivity and Genetic Analyses
3. Results
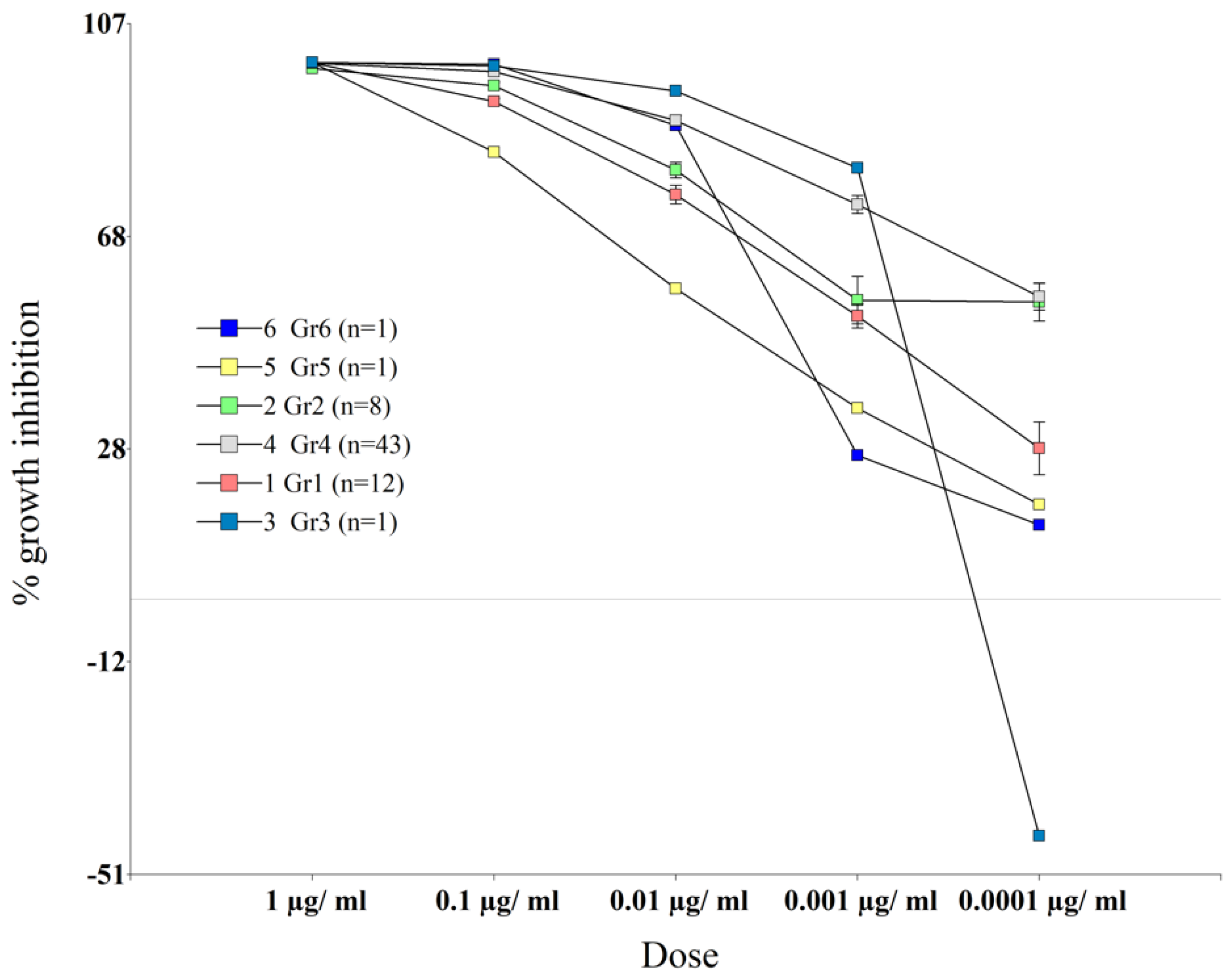
3.1. M. roreri Sensitivity to Fungicides
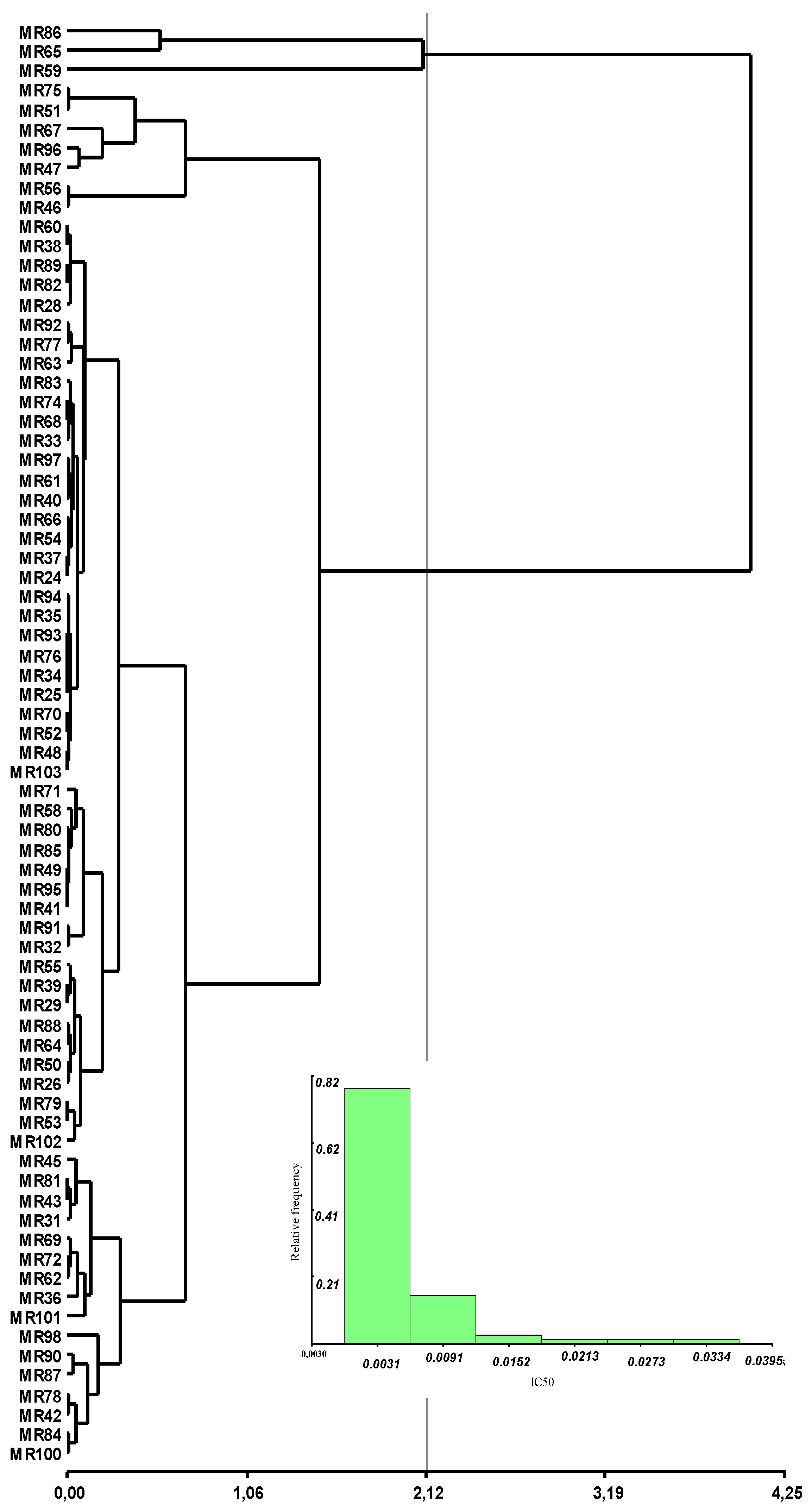
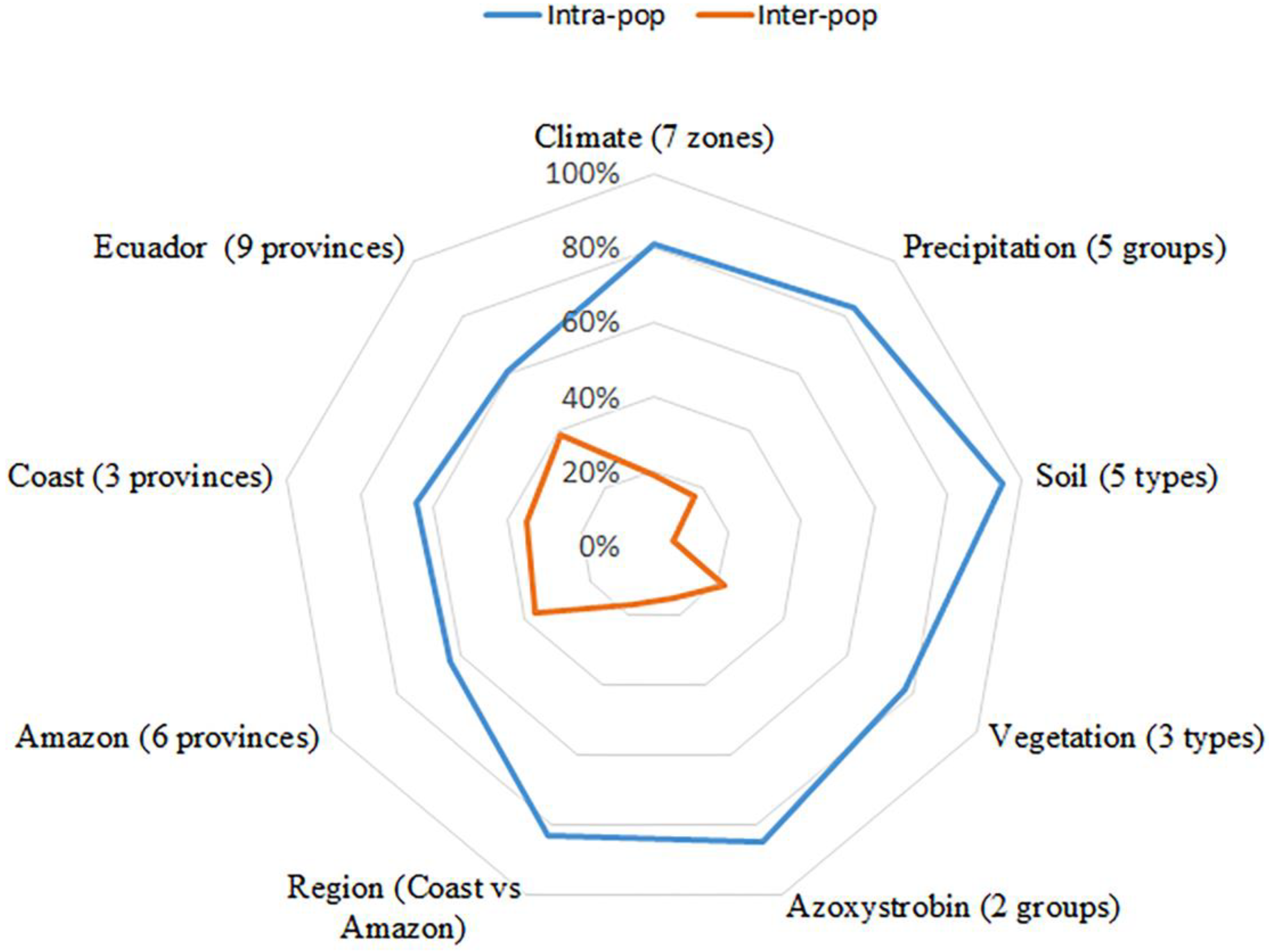
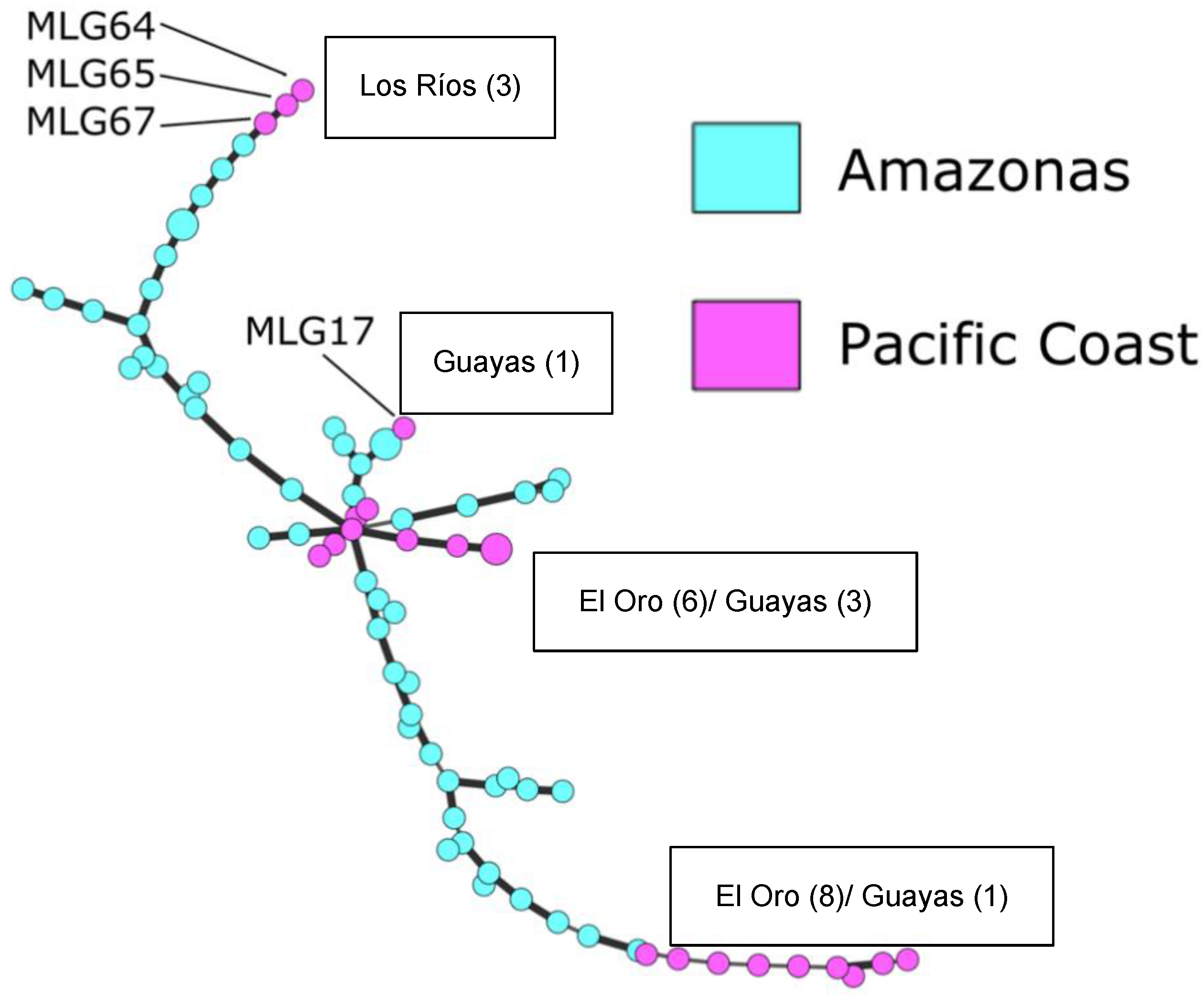
3.2. Genetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, H.C.; Holmes, K.A.; Phillips, W.; Wilkinson, M.J. What’s in a Name: Crinipellis, the Final Resting Place for the Frosty Pod Rot Pathogen of Cocoa? Mycologist 2002, 16, 148–152. [Google Scholar] [CrossRef]
- Fowler, R.; Lopez, G. The Cacao Industry of Ecuador; Foreign Agriculture Report; US Government Printing Office: Washington, DC, USA, 1949.
- Enríquez, G. Cacao Orgánico: Guía Para Productores Ecuatorianos, 2nd ed.; INIAP: Quito, Ecuador, 2010; Volume 54, ISBN 9978-43-493-3.
- Evans, H.C. Frosty Pod Rot (Moniliophthora roreri). In Cacao Diseases: A History of Old Enemies and New Encounters; Springer: Cham, Switzerland, 2016; pp. 63–96. ISBN 978-3-319-24789-2. [Google Scholar]
- Johnson, E.S.; Rutherford, M.A.; Edgington, S.; Flood, J.; Crozier, J.; Cafá, G.; Buddie, A.G.; Offord, L.; Elliott, S.M.; Christie, K.V. First Report of Moniliophthora roreri Causing Frosty Pod Rot on Theobroma cacao in Jamaica. New Dis. Rep. 2017, 36, 2. [Google Scholar] [CrossRef]
- Parra, D.; Martínez, S.P.; Sosa, D.; Rumbos, R.; Gutiérrez, B.; Moya, A. Avances en las investigaciones venezolanas sobre enfermedades del cacao. Rev. Estud. Transdiscipl. 2009, 1, 56–75. [Google Scholar]
- Pegg, K.G.; Coates, L.M.; O’Neill, W.T.; Turner, D.W. The Epidemiology of Fusarium Wilt of Banana. Front. Plant Sci. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Rorer, J.B. Asociacion de Agricultores del Ecuador Enfermedades y Plagas del Cacao en el Ecuador y Metodos Modernos Apropiados al Cultivo del Cacao, Informe Presentado al Presidente y Miembros de la Asociación de Agricultores del Ecuador; El Diario Ilustrado: Guayaquil, Ecuador, 1918. [Google Scholar]
- Sánchez, V.; Zambrano, J.; Iglesias, C. La Cadena de Valor del Cacao en América Latina y el Caribe; INIAP, Estación Experimental Santa Catalina: Quito, Ecuador, 2019; ISBN 978-9942-36-465-4.
- Pico, J.T.; Paredes, N.J.; Subía, C.R.; Suárez, C.; Caicedo, V.C.; Fernández, F.M. Efecto de Prácticas de Manejo Sobre la Incidencia de Moniliophthora roreri (Cif & Par) y Rendimiento en el Cultivo de Cacao (Theobroma cacao L.), 1st ed.; AGN LATAM /INIAP, Estación Experimental Central de la Amazonía: Sacha, Ecuador, 2019; ISBN 978-9942-38-269-6. [Google Scholar]
- Phillips-Mora, W.; Amores, F. Moniliasis del Cacao. CropLife Latin America. Available online: https://www.croplifela.org/es/plagas/listado-de-plagas/moniliasis-del-cacao (accessed on 5 November 2021).
- Krauss, U.; Hidalgo, E.; Bateman, R.; Adonijah, V.; Arroyo, C.; García, J.; Crozier, J.; Brown, N.A.; Martijn, G.; Holmes, K.A. Improving the Formulation and Timing of Application of Endophytic Biocontrol and Chemical Agents against Frosty Pod Rot (Moniliophthora roreri) in Cocoa (Theobroma cacao). Biol. Control 2010, 54, 230–240. [Google Scholar] [CrossRef]
- Tirado-Gallego, P.A.; Lopera-Alvarez, A.; Ríos-Osorio, L.A. Estrategias de Control de Moniliophthora roreri y Moniliophthora Perniciosa En Theobroma cacao L.: Revisión Sistemática. Rev. Corpoica Cienc. Tecnol. Agropecu. 2016, 17, 417–430. [Google Scholar] [CrossRef]
- Torres De La Cruz, M.; Fredy, C.; García, O.; Ortiz, D.T.; Aguilera, A.M.; Nava Díaz, C.; De La Cruz, T.; Cf, G.; Ortíz, T.; Aguilera, M.; et al. Efecto Del Azoxystrobin Sobre Moniliophthora roreri, Agente Causal de La Moniliasis Del Cacao (Theobroma cacao). Rev. Mex. Fitopatol. 2013, 31, 65–69. [Google Scholar]
- Bateman, R.P.; Hidalgo, E.; Garcia, J.; Arroyo, C.; ten Hoopen, G.M.; Adonijah, V.; Krauss, U. Application of Chemical and Biological Agents for the Management of Frosty Pod Rot (Moniliophthora roreri) in Costa Rican Cocoa (Theobroma cacao). Ann. Appl. Biol. 2005, 147, 129–138. [Google Scholar] [CrossRef]
- Krauss, U.; Ten Hoopen, G.; Hidalgo, E.; Martinez, A.; Arroyo, C.; García, J.; Portuguez, A.; Sánchez, V. Manejo Integrado de La Moniliasis (Moniliophthora roreri) Del Cacao (Theobroma cacao) En Talamanca, Costa Rica. Agrofor. En Las Américas 2003, 10, 52–58. [Google Scholar]
- Brent, K.; Hollomon, D. Fungicide Resistance: The Assessment of Risk, 2nd ed.; Global Crop Protection Federation: Brussels, Belgium, 2007. [Google Scholar]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Thomas, E.; van Zonneveld, M.; Loo, J.; Hodgkin, T.; Galluzzi, G.; van Etten, J. Present Spatial Diversity Patterns of Theobroma cacao L. in the Neotropics Reflect Genetic Differentiation in Pleistocene Refugia Followed by Human-Influenced Dispersal. PLoS ONE 2012, 7, e47676. [Google Scholar] [CrossRef]
- Zarrillo, S.; Gaikwad, N.; Lanaud, C.; Powis, T.; Viot, C.; Lesur, I.; Fouet, O.; Argout, X.; Guichoux, E.; Salin, F.; et al. The Use and Domestication of Theobroma cacao during the Mid-Holocene in the Upper Amazon. Nat. Ecol. Evol. 2018, 2, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.S.; Joyce, R.A.; Hall, G.R.; Hurst, W.J.; McGovern, P.E. Chemical and Archaeological Evidence for the Earliest Cacao Beverages. Proc. Natl. Acad. Sci. USA 2007, 104, 18937–18940. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Shao, J.; Strem, M.D.; Phillips-Mora, W.; Zhang, D.; Meinhardt, L.W.; Bailey, B.A. Combination of RNAseq and SNP Nanofluidic Array Reveals the Center of Genetic Diversity of Cacao Pathogen Moniliophthora roreri in the Upper Magdalena Valley of Colombia and Its Clonality. Front. Microbiol. 2015, 6, 850. [Google Scholar] [CrossRef] [PubMed]
- Phillips-Mora, W.; Aime, M.C.; Wilkinson, M.J. Biodiversity and Biogeography of the Cacao (Theobroma cacao) Pathogen Moniliophthora roreri in Tropical America. Plant Pathol. 2007, 56, 911–922. [Google Scholar] [CrossRef]
- Bailey, B.; Evans, H.; Phillips-Mora, W.; Ali, S.; Meinhardt, L. Moniliophthora roreri, Causal Agent of Cacao Frosty Pod Rot. Mol. Plant Pathol. 2018, 19, 1580–1594. [Google Scholar] [CrossRef]
- Maridueña-Zavala, M.G.; Villavicencio-Vásquez, M.E.; Cevallos-Cevallos, J.M.; Peralta, E.L. Molecular and Morphological Characterization of Moniliophthora roreri Isolates from Cacao in Ecuador. Can. J. Plant Pathol. 2016, 38, 460–469. [Google Scholar] [CrossRef]
- Ram, A. Biology, epidemiology and control of moniliasis (Moniliophthora roreri) of cacao. Ph.D. Thesis, University of London, London, UK, 1989. [Google Scholar]
- Maridueña-Zavala, M.G.; Quevedo, A.; Aguaguiña, K.; Serrano, L.; Sosa del Castillo, D. Colección de cultivos microbianos CIBE (CCM-CIBE): Una colección para la investigación. Bionatura 2021, 6, 1664–1668. [Google Scholar] [CrossRef]
- Cenis, J.L. Rapid Extraction of Fungal DNA for PCR Amplification. Nucleic Acids Res. 1992, 20, 2380. [Google Scholar] [CrossRef]
- Melo, B.L.B.; de Souza, J.T.; Santos, R.M.F.; Rehner, S.A.; Solis, K.H.; Suarez, C.; Hebbar, P.K.; Lemos, L.S.L.; Gramacho, K.P. Development of Microsatellites for the Cacao Frosty Pod Rot Pathogen, Moniliophthora roreri. For. Pathol. 2014, 44, 320–324. [Google Scholar] [CrossRef]
- Dia, M.; Wehner, T.C.; Arellano, C. RGxE: An R Program for Genotype x Environment Interaction Analysis. Am. J. Plant Sci. 2017, 8, 1672–1698. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant Analysis of Principal Components: A New Method for the Analysis of Genetically Structured Populations. BMC Genet. 2010, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Portilla Farfán, F. Agroclimatología del Ecuador, 1st ed.; Editorial Abya-Yala: Quito, Ecuador, 2018; ISBN 978-9978-10-310-4. [Google Scholar]
- Qin, C.-F.; He, M.-H.; Chen, F.-P.; Zhu, W.; Yang, L.-N.; Wu, E.-J.; Guo, Z.-L.; Shang, L.-P.; Zhan, J. Comparative Analyses of Fungicide Sensitivity and SSR Marker Variations Indicate a Low Risk of Developing Azoxystrobin Resistance in Phytophthora infestans. Sci. Rep. 2016, 6, 20483. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.M.; Stevenson, W.R. Aggressiveness and Fungicide Sensitivity of Alternaria dauci from Cultivated Carrot. Plant Dis. 2010, 94, 405–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amaya-Márquez, D.; Espinoza-Lozano, F.; Villavicencio-Vasquez, M.E.; Sosa del Castillo, D.; Perez-Martinez, S. Inhibition and Stimulation of Mycelial Growth of Moniliophthora roreri by Flutolanil in Populations of Ecuador. Acta Agron. 2021, 70, 3. [Google Scholar] [CrossRef]
- Gaceta Oficial Colombia. El Cacao. Instrucción Popular 1850. Año XIX (N° 1.148): 429–430. Available online: https://books.google.com.hk/books?id=7rszAQAAMAAJ&pg=PA425&lpg=PA425&dq=Gaceta+Oficial+Colombia+.+El+Cacao.+Instrucci%C3%B3n+popular+1850:+429%E2%80%93430.&source=bl&ots=pg68wXUSUz&sig=ACfU3U0oWCW6zfdQd8x1jIDpLgQccil_lQ&hl=en&sa=X&redir_esc=y#v=onepage&q=Gaceta%20Oficial%20Colombia%20.%20El%20Cacao.%20Instrucci%C3%B3n%20popular%201850%3A%20429%E2%80%93430.&f=false (accessed on 7 April 2022).
- Phillips-Mora, W. Origin, Biogeography, Genetic Diversity and Taxonomic Affinities of the Cacao (Theobroma cacao L.) Fungus Moniliophthora roreri (Cif.) Evans et al. as Determined Using Molecular, Phytopathological and Morpho-Physiological Evidence. PhD Thesis, The University of Reading, Reading, UK, 2003. [Google Scholar]
- Allen, J.A. Recolecciones de Cacao Silvestre de la Region Amazónlca Ecuatoriana. Proyecto Amazonas. Convenio INIAP-CCCA Reporte final-Primera Fase; INIAP-Estación Experimental Tropical Pichilingue: Quito, Ecuador, 1985; Volume 15. [Google Scholar]
- Evans, H.C. A Reassessment of Moniliophthora (Monilia) Pod Rot of Cocoa. Cocoa Grow. Bull. 1986, 37, 34–43. [Google Scholar]
- Ciferri, R.; Parodi, E. Descrizione del fungo che causa la “Moniliasi” del cacao. Phytopathol. Z. 1933, 6, 539–542. [Google Scholar]
- Hiraoka, M.; Yamamoto, S. Agricultural Development in the Upper Amazon of Ecuador. Geogr. Rev. 1980, 70, 423–445. [Google Scholar] [CrossRef]

| Population | N | H | G | Gnorm | Lambda | Hexp |
|---|---|---|---|---|---|---|
| Amazon | 54 | 3.96 | 51.27 | 0.93 | 0.98 | 0.68 |
| Coast | 22 | 3.03 | 20.17 | 0.92 | 0.95 | 0.57 |
| Total | 76 | 4.29 | 71.43 | 0.93 | 0.99 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Lozano, F.; Amaya-Márquez, D.; Pinto, C.M.; Villavicencio-Vásquez, M.; Sosa del Castillo, D.; Pérez-Martínez, S. Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy 2022, 12, 1119. https://doi.org/10.3390/agronomy12051119
Espinoza-Lozano F, Amaya-Márquez D, Pinto CM, Villavicencio-Vásquez M, Sosa del Castillo D, Pérez-Martínez S. Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy. 2022; 12(5):1119. https://doi.org/10.3390/agronomy12051119
Chicago/Turabian StyleEspinoza-Lozano, Fernando, Darlyn Amaya-Márquez, C. Miguel Pinto, Mirian Villavicencio-Vásquez, Daynet Sosa del Castillo, and Simón Pérez-Martínez. 2022. "Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity" Agronomy 12, no. 5: 1119. https://doi.org/10.3390/agronomy12051119
APA StyleEspinoza-Lozano, F., Amaya-Márquez, D., Pinto, C. M., Villavicencio-Vásquez, M., Sosa del Castillo, D., & Pérez-Martínez, S. (2022). Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy, 12(5), 1119. https://doi.org/10.3390/agronomy12051119






