Abstract
As leaf-harvest plants, tea trees show unique nutrient requirements, different from those of corn and other field crops. However, the effects of nitrogen (N), phosphorus (P), and potassium (K) application on the accumulation of quality-related compounds and the mechanisms underlying how nutrients affect tea-leaf metabolism have not been well elucidated. Here, fertilizers with different N, P, K ratios were applied to tea plants in pot experiments, and metabolomics based on gas chromatography-mass spectrometry (GC-MS) combined with multivariate statistical and quantitative detections were conducted to assess the responses of quality-related compounds to NPK in tea leaves. An increased proportion of P and K was beneficial for the accumulation of carbohydrates and catechins in shoots, although the total carbon content did not increase significantly. In contrast, a high proportion of P and K input reduced the relative chlorophyll content in shoots, and the contents of free amino acids such as theanine and glutamic acid negatively correlated with P and K nutrient content. Moreover, the metabolism of malic acid in the tricarboxylic acid cycle was highly promoted by increasing the application of P and K. These results validate our suggestion that the application of high amounts of P and K in tea plantations induces the biased reallocation of photosynthates and carbohydrates to the catechin pathway by promoting malic acid metabolism in young tea shoots, which further affects tea quality. The results of this study provide theoretical ground for tea quality improvement by optimizing fertilization strategies.
1. Introduction
Tea is an important economic crop in Asian and African countries such as China, Japan, India, Sri Lanka, and Kenya. As an important part of tea cultivation and management, fertilization plays a key role in maintaining and promoting tea growth and quality formation. At present, tea farmers are expected to increase the yield of tea through a large amount of fertilization in tea planting, and unreasonable fertilization is widespread. A survey in China found that more than 50% of tea gardens were over-fertilized, and inappropriate compound fertilizers (such as 15-15-15, N:P:K) applied in tea gardens accounted for about 80% of the fertilizers used [1]. This prominent unreasonable fertilizer application causes problems such as tea-quality reduction, soil degradation, and environmental pollution [2,3]. Thus, revealing the relationship between nutrient application in plantations and tea-plant metabolism will provide a theoretical basis for improving tea quality by optimizing fertilization strategies.
Nitrogen (N), phosphorus (P), and potassium (K), as the three most important nutrient elements for plant growth, are also vital for tea quality. Previous studies have suggested that a reasonable application of nitrogen fertilizer can improve the contents of free amino acids [4], caffeine [5], water-solubility extract, chlorophyll [6], and aroma substances [7] in tea plants, while decreasing the content of tea polyphenols [4]. However, the excessive application of nitrogen fertilizer is not conducive to good yield and quality [8,9]. Although the content of P is less than that of N and K, many physiological processes, such as photosynthesis and respiration, are highly dependent on P [10,11]. In addition, P also affects the decomposition and metabolism of minerals and metabolites in tea plants, thereby affecting the yield and quality of tea [12]. Under P deficiency, the concentrations of total polyphenols, flavonoids, total free amino acids, theanine, and aspartate in green tea decrease [12,13,14]. K can be used as an activator of various enzymes in tea plants, participating in the growth and development activities of tea plants, such as promoting photosynthesis, protein synthesis, and the transportation of photosynthate to shoots [15,16]. Under K deficiency, tea biomass, chlorophyll contents, leaf CO2 assimilation rate, and stomatal conductance decreased significantly, while intercellular CO2 concentration increased significantly [15]. The application of K fertilizers can increase the contents of free amino acids, tea polyphenols, isoprene diphosphate, phenylalanine volatile substances, and β-phenylethanol [15,17].
Numerous studies have shown that a specific proportion of N, P, and K are used for organism construction in plants [18]. Therefore, in order to optimize the nutrient ratio, researchers have explored the effects of fertilization formulas on crop yield, quality-component contents, mineral-element absorption, photosynthetic-physiological characteristics, biological characteristics, root activity, antioxidant activity, and other physiological indexes [19,20,21,22,23], as well as glutathione synthetase, nitrate reductase, oxaloacetate transaminase, phenylalanine ammonia-lyase, and other enzyme activities [24,25,26]. For example, in citrus orchards, when the fertilization ratio was N:P:K = 6:2:3, the fruit yield was the highest, and when N:P:K = 3:2:0.15, the fruit quality was the best [27]; for Magnolia wufengensis (Magnoliaceae) seedlings, when N:P:K = 3:2:1, the seedling growth was the most stable [28].
For a long time, fertilization strategies aiming at yield and economic benefits have led to nutrient imbalances. The excessive input of P and K has not only increased the production cost of tea but has also been detrimental to tea quality. To elucidate the effects of N, P, and K application on the accumulation of quality-related compounds and the mechanisms underlying how nutrients affect tea-leaf metabolism, this study aimed to compare the accumulation of tea-quality-related components under different nutrient-input conditions. This study provides a theoretical and reference basis for the directional regulation of tea quality and scientific nutrient management.
2. Materials and Methods
2.1. Plant Materials and Treatments
Three-year-old tea plants [Camellia sinensis ‘Rougui’] were planted in pots at Wuyi University, Fujian. Each treatment included six pots and each pot contained 40 kg soil and one plant. The soil pH applied before the experiment was 4.9, and available NH4+, NO3−, P, and K were 37.7, 28.2, 52, 83 mg/kg, respectively. Plants were irrigated with an automatic micro-sprinkler system every day to ensure an adequate supply of water. Tea plants were supplied with urea, superphosphate calcium, and K sulfate four times, in October, March, May, and July (Table 1). Plants with uniform height and biomass were used for the experiment, and sampling started after 1 year from stably growing plants. Young shoots (a bud with the first leaf) were harvested six times (n = 6; three times for each in two independent experiments). The samples were collected and frozen immediately in liquid nitrogen and stored at −80 °C.

Table 1.
Amount of nitrogen (N), phosphorus (P), and potassium (K) applied in the fertilization treatment.
2.2. Measurement of Shoot Density and Biomass
In spring, the number of shoots per tea plant was investigated to calculate the density of tea shoots. In autumn, the weight of all branches and leaves above ground was calculated as biomass at 30 cm from the root of tea seedlings. To determine the dry weight of plants, fresh samples were dried at 105 °C for 30 min, then dried at 75 °C, and the dry weight of each part was determined.
2.3. Determination of N, P, and K Nutrient Elements in Soil and Leaves
Total P and total K in tea shoots were determined by wet digestion and inductively coupled plasma atomic emission spectroscopy (ICP-AES). The available P in soil was determined by the hydrochloric acid-ammonium fluoride method, and available K was determined by the ammonium acetate extraction method. Organic carbon and total N in tea shoots and soil were measured by the organic elemental analyzer (Vario MACRO cube).
2.4. Quantitative Determination of Catechins, Chlorophylls, and Carotenoids
Catechins, chlorophylls, and carotenoids were analyzed on a reverse-phase high-performance liquid chromatographic system (Waters 2695, Milford, MA, USA) coupled to a DAD detector (Waters 2998, Milford, MA, USA). Separations were performed using a C18 reverse-phase column (250 × 4.6 mm i.d., Luna 5lm, Phenomenex, Torrance, CA, USA), as described by Zhang et al. [29]. The amounts of chlorophyll were determined by SPAD (single-photon avalanche diode, SPAD-520 Plus, Tokyo, Japan). The upper, middle, and lower parts of each leaf were measured, and the average value was obtained.
2.5. Gas-Chromatography-Based Metabolomics
Metabolites were extracted with methanol and 2-chloro-l-phenylalanine as internal standard, following the method described by Zhang et al. [29]. The quality control (QC) sample was prepared by mixing aliquots of all the samples to obtain a pooled sample. Extracts were derivatized online with methoxylamine hydrochloride and BSTFA (with 1% TMCS). The derivatized samples were analyzed on an Agilent 7890B gas-chromatography system coupled to an Agilent 5977AMSD system (Agilent Technologies Inc., Santa Clara, CA, USA). A DB-5MS fused-silica capillary column (30 m × 0.25 mm × 0.25 μm, Agilent J & W Scientific, Folsom, CA, USA) was utilized to separate the derivatives.
2.6. Data Processing
The data converted by AnalysisBaseFileConverter (https://www.reifycs.com/AbfConverter/index.html accessed on 10 December 2019) were imported into the MD-DIAL software for data processing. Metabolites were annotated through LUG database (Untarget database of GC-MS from Lumingbio, Shanghai, China). After alignment with Statistic Compare component, the raw-data array was obtained from raw data with three dimensions data sets, including sample information, peak retention time and peak intensities. Then, all internal standards and any known pseudo-positive peaks were removed and all the internal standards with peak intensity < 0.3 were normalized by subsection (Table S2). Data were transformed by log10, and the resulting data matrix was then imported into R ropls package.
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed to visualize the metabolic difference among experimental groups, after mean centering and unit-variance scaling. Omicshare Tools (https://www.omicshare.com/tools/ accessed on 10 March 2021) were used for the thermal diagram clustering analysis of differential metabolites. Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/kegg/ accessed on 19 March 2021) analysis was used to obtain the results of metabolic pathway enrichment. The hypergeometric test was used to discern the metabolic pathway entries that were significantly enriched in the differential expression of metabolites compared with the whole background. Statistically significant differences among mean values were tested using one-way ANOVA, with a multiple correlation test using false discovery rate (FDR) estimation. Tukey’s post-hoc test was applied for the pairwise comparison of multiple groups’ mean values by SPSS 18.0. Differences were considered significant when p < 0.01.
3. Results
3.1. Effects of Fertilization on Shoot Density, Biomass, and Relative Chlorophyll Content
Compared with the control group leaves without fertilization, the shoot biomass of tea trees in the four groups with different fertilizer ratios was significantly increased (Figure 1), but the shoot biomass of T2, T3, and T4 showed no significant differences from that of T1. The tea-bud density and relative chlorophyll content were significantly affected by four different fertilizer ratios. The tea-bud density of T3 was significantly higher than that of other treatments, while the tea-bud density of T4 was the lowest. Compared with the T0 group, the relative chlorophyll content of tea plants with the four different fertilizer ratios was significantly increased, especially in the T1 group.
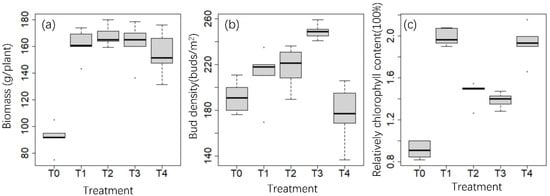
Figure 1.
Changes in biomass (a), bud density (b), and relative chlorophyll content (c) in tea plants under different fertilization treatments.
3.2. The Contents of C, N, P, and K in Soil and Leaves upon Fertilization Treatment
The contents of N, P, and K in the soil increased with the increase in N, P, K nutrient input, respectively (Table 2), indicating that the application of fertilizer with certain nutrient elements effectively increased the content of this nutrient element in the soil. With the increase in the N application rate, the N content in leaves gradually increased (p < 0.05). There were significant differences in N content among different fertilization treatments. With the increase in the P application rate, the P contents of T2–T4 fertilization treatments were significantly higher than that of the control (p < 0.05). There was no significant difference in the K content of T3 and T4 leaves, and the K contents of T1 and T2 leaves were lower than those of the control group leaves without fertilization. The content of C in the leaves of the control group was significantly higher than that of the treatment group, but there were no significant differences between the four treatment groups. The N content in the leaves of each treatment group was significantly higher than that of the control group, and the T1 treatment showed the highest N content.

Table 2.
Concentrations of total C, N, P, and K in soil and plant leaves under different fertilization treatments.
3.3. Catechin Changes under Fertilization Treatments
Compared with the control group without fertilization, the content of most catechins significantly decreased in the T1–T3 group, such as epigallocatechin, epigallocatechin gallate, epicatechin and epicatechin gallate. However, the level of catechins in the T4 group highly increased compared to that in the control group (T0), especially gallocatechin, which was 1.4 times higher. Furthermore, epigallocatechin decreased in T1, T2, and T3 by 46%, 40%, and 55%, respectively, when compared to T0, but significantly increased in T4. When compared to T1, the content of catechins except gallocatechin gallate showed significantly higher levels in T3 and T4. Moreover, the content of most of the catechins showed the lowest level in T1, and the highest level in T4 or T0 (Table 3).

Table 3.
Contents (mg g−1 dry weight) of the main catechins in young tea shoots under different fertilization treatments.
3.4. Overview of Metabolomic Profiling
Differences in the overall profile of total ion chromatography (Figure 2) indicated the effect of fertilization treatments on metabolism in tea plants. In total, 302 metabolites were identified in the five groups of tea-shoot samples by GC-MS (Table S1), including organic acids and derivatives (19%); organic nitrogen compounds (17%); lipids and lipid-like molecules (12%); organoheterocyclic compounds (9%); benzenoids (7%); nucleosides, nucleotides, and analogs (4%); phenylpropanoids and polyketides(4%); and other compounds (28%).
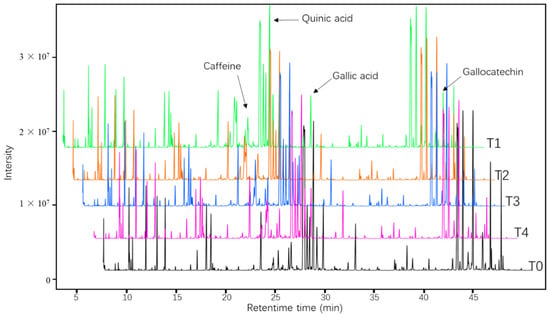
Figure 2.
Total ion chromatograms of metabolites in young tea shoots under recommended amount of nitrogen (N), phosphorus (P), and potassium (K) application (T1), high P application (T2), high K application (T3), high P and K application (T4), and control (T0) treatments analyzed by gas chromatography-mass spectrometry (GC-MS).
To further reveal differences in the metabolite composition of tea plants under different fertilization treatments, multi-dimensional pattern recognition was adopted. In the PCA score plot, samples from the five different groups were stratified (Figure 3A), indicating that metabolites in different groups differed substantially. The explained values of PC1 and PC2 were 41.8% and 18.6%, indicating that about 60.4% of tea metabolites were well-clustered. The T0 group was relatively independent from the other treatment groups and was well-separated from them on the first principal component. T1, T2, T3, and T4 were well-separated on the second principal component. The differential metabolites screened by each comparison group were analyzed by clustering to obtain a clustering heat map (Figure 3B), in which each column represents a set of samples, and each pixel represents a metabolite. Samples from control (T0) and treatment groups (T1/2/3/4) were divided into two categories.
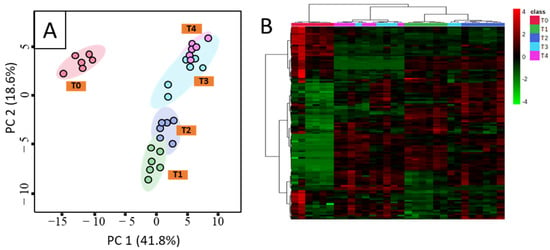
Figure 3.
PCA score plot (A) and heatmap (B) of metabolites in young tea shoots under recommended amount of nitrogen (N), phosphorus (P), and potassium (K) application (T1), high P application (T2), high K application (T3), high P and K application (T4), and control (T0) treatments analyzed by gas chromatography-mass spectrometry (GC-MS). Note: The horizontal scale of heatmap (B) represents the different fertilization treatment groups, and the vertical scale represents the differential metabolites. Color from green to red represents the expression abundance of metabolites from low to high.
3.5. Effect of NPK Application on Tea Shoot Metabolism
To identify the differential metabolite between non-fertilized and fertilized tea plants, as well as different fertilizer treatments, partial least squares discriminant analysis (PLS-DA) was carried out (Figure 4). The prediction parameters of the PLS-DA evaluation model were R2X (the interpretation rate of the established model for X matrix), R2Y (the interpretation rate of the established model for Y matrix), and Q2 (the prediction ability of the model), and their values were 0.784, 0.999, and 0.996, respectively, indicating that the model fitting effect is good. Figure 4 shows that the T1, T2, and T3 (or T4) groups were significantly separated. Results from the permutation test suggested that PLS-DA did not appear to be over-fitted.
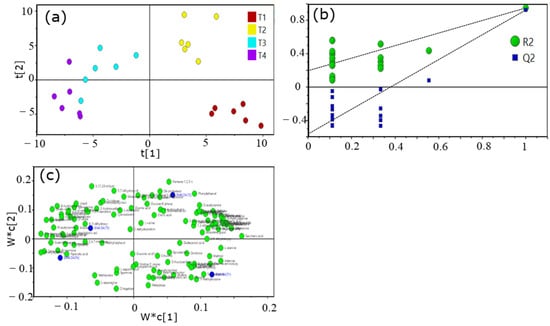
Figure 4.
Score plot (a), permutation test (b), and loading plot (c) from a partial least squares discriminant analysis (PLS-DA) of metabolites in young tea shoots under different fertilization treatments analyzed by gas chromatography−mass spectrometry (GC-MS).
Multi-dimensional analysis and single-dimensional analysis were used to screen the differential metabolites between groups. According to the results of PLS-DA, the double standard of variable importance in projection (VIP) value of VIP > 1 and p < 0.05 were selected to screen the differential metabolites of the samples. A Venn plot visually demonstrated the relationship between the different metabolites in tea plants treated with different fertilizer ratios (Figure 5). Compared with T0, a total of 212 metabolites with significant differences between treatments (VIP > 1, p < 0.05) were identified, and 129 kinds of metabolites were common among them. Compared with the T1 group (the recommended fertilization group), 122 kinds of metabolites were screened out in T2, T3, and T4. Furthermore, only 13 metabolites overlapped between T1 and the other groups. Meanwhile, 19 metabolites were screened from the T2–T1 and T3–T1 groups.
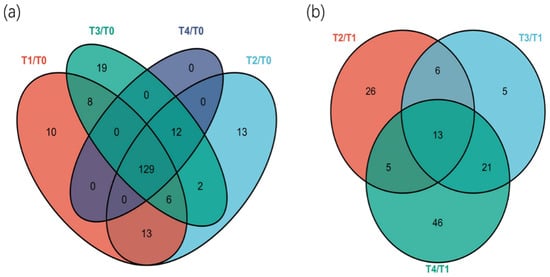
Figure 5.
Venn plot of differentially expressed metabolites in young tea shoots under different fertilization treatments. Each circle represents a comparison group. The number of overlapping parts of each circle represents the number of common differential metabolites between the comparison groups, and the number of non-overlapping parts represents the number of specific differential metabolites between the comparison groups.
3.6. Changes in Quality-Related Compound Accumulation under Fertilization Treatments
The metabolites significantly changed by fertilization were mapped to the biological pathways included in the KEGG database. There were 13 pathways significantly enriched (Table 4), including 7 metabolic pathways, 4 biosynthetic pathways, 1 conversion pathway, and 1 degradation pathway, respectively. Among the significant metabolic pathways selected, the synthesis and metabolism of amino acids (amino-acid biosynthesis; arginine biosynthesis; glycine, serine, and threonine metabolism; lysine biosynthesis; lysine degradation; and phenylalanine metabolism) and carbohydrate synthesis and metabolism (pentose, glucuronic acid conversion, starch and sucrose metabolism, carbon metabolism) fluctuated significantly with fertilization treatment. Compared with T0, the phenylalanine metabolism pathway and flavonoid biosynthesis pathway were significantly up-regulated in T3 and T4, while there was no significant change in T1 and T2.

Table 4.
Significantly different metabolic pathways under different fertilization conditions.
The main differentially expressed metabolites involved in the synthesis and metabolism of amino acids were glycine, serine, phenylpyruvate acid and oxoglutaric acid. Compared with T0, the contents of glycine, serine, phenylpyruvate in the fertilization group increased, while the content of oxoglutaric acid in the fertilization group decreased. The main differentially expressed metabolites involved in the synthesis and metabolism of carbohydrates were glucose, trehalose, glycine, serine, inositol galactoside, glutaric acid and xylose lactone. Compared with T0, the contents of glucose, trehalose, glycine, serine in the fertilization group increased, while the content of oxoglutaric acid and xyloselactone in the fertilization group decreased (Table 5).

Table 5.
Major differentially expressed metabolites in young tea shoots under different fertilization conditions.
Compared with T0, the total amount of amino acids, carbohydrates in all four groups was increased. Compared with the content of amino acids in T0, the contents of Saccharopine and L-glutamine acid increased the most, being 17080–26603 times and 53–76 times higher, respectively, followed by Citrulline and L-alanine. This reflected that, compared with no fertilization, each fertilization treatment had a significant effect on promoting the accumulation of amino-acid contents in tea plants. However, compared with the normal fertilization treatment T1, excessive P and K inputs decreased the accumulation of amino acids in tea plants.
Among carbohydrates and their derivatives, the contents of trehalose and trehalose-6-phosphate in the treatment group were significantly increased, compared with the content in T0, being 3.73–5.13 times and 3.25–3.71 times, respectively. This reflected that, compared with no fertilization, each fertilization treatment had a significant effect on promoting the accumulation of carbohydrates and their derivatives in tea plants. Moreover, the promotion effect of excessive P and K on the accumulation of carbohydrates and their derivatives was the most obvious.
3.7. Correlations between Leaf and Soil N, P, and K Contents and Main Quality-Component Contents
Under different fertilization treatments, the correlation analysis between the contents of C, N, P, and K in plant shoots and soil and the main quality components of shoots showed that the total N content in plants was significantly positively correlated with N-containing substances such as amino acids and caffeine in shoots, but negatively correlated with flavonoid contents (Figure 6). Similar to plant N, total soil N content also showed a similar pattern. The correlation analysis of PK content in plants and soil with the main quality components of shoots showed that the P/K ratio in plants was significantly negatively correlated with amino-acid contents in shoots, and negatively correlated with flavonoid contents. The P/K ratio in plants is significantly negatively correlated with amino-acid contents in tea shoots, but negatively correlated with flavonoid contents. It is noteworthy that the P/K ratio in soil and catechin components showed a significant negative correlation. Differing from the P/K ratio in plants, there was no correlation between P/K in soil and flavonoids in shoots. The P/K ratio in the soil is negatively correlated with amino-acid contents in shoots, but this correlation was not obvious.
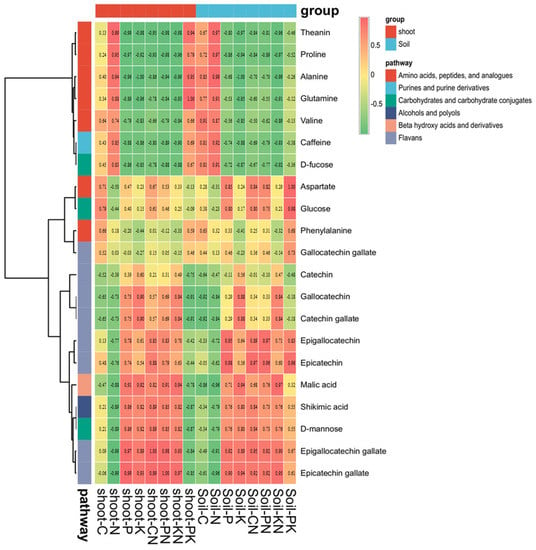
Figure 6.
Correlation between leaf (soil) mineral nutrients and quality-related compounds in young tea shoots under different fertilization treatments.
4. Discussion
4.1. A Reasonable Input of P and K Is Beneficial to the Accumulation of Tea Quality Components
The goals of nutrient management in plant cultivation are higher quality, yield, and efficiency. Reasonable fertilization has been proven to have a great effect on improving tea quality and yield [30]. In this study, fertilization also had the most-obvious effect on the improvement in tea-bud density, thereby increasing tea yield. Additionally, compared with the non-fertilized group, the relative content of chlorophyll in tea leaves upon each fertilization combination was also increased, to varying degrees. The increase in chlorophyll will promote the photosynthesis and nutrient accumulation of tea plants, thereby improving the yield and quality of tea [31]. The fluctuation in tea-bud density confirmed that fertilization promoted the yield of tea, while the subsequent determination of metabonomics confirmed that the reasonable input of P and K promoted the accumulation of tea quality components. In this study, a total of 13 metabolic pathways were enriched, as detected by the differently accumulated metabolites in the fertilization treatment group. Notably, amino-acid and carbohydrate metabolism significantly fluctuated with fertilization treatment. For example, the fertilization groups showed a significantly increased accumulations of amino acids, including glutamic acid, alanine, and glycine, in tea shoots when compared to the non-fertilized group. The increased accumulation of amino acids improves the freshness of tea and makes tea quality better [32].
4.2. The Accumulation of N-Containing Metabolites in Shoots Was Inhibited by High-Proportion P and K Application
Previous studies showed that excessive application of nitrogen fertilizers was not conducive to good yield and quality [8,9]. In this study, when fertilization combinations were compared, the recommended-fertilization-proportion group (T1) had the highest relative chlorophyll content. In this group, the applied amount of K was 90 kg/hm2. The excessive application of P and K fertilizer reduced the relative chlorophyll content in tea shoots. Chlorophyll is necessary for photosynthesis, and it is also highly connected to tea quality. Therefore, excessive input of P and K fertilizers will have a negative impact on the photosynthesis of tea plants, and also affect tea color [33]. Compared with non-fertilized tea plants, the accumulation of caffeine, an N-containing metabolite, significantly increased in young tea shoots treated with high levels of P and K. Considering its contribution to the bitter taste in tea infusion [34,35], unreasonable fertilization decreases tea quality. Compared with the recommended fertilization ratio (T1), the increase in P and K fertilizer had a negative impact on the further transformation of pyruvate into amino acids, thereby reducing the accumulation of amino acids in tea shoots. The accumulation of glucose, pyruvic acid, and phenylalanine significantly increased with the increase in P and K fertilizers, while the accumulation of alanine, methylalanine, glutamine decreased with the increase in P and K fertilizers (Figure 7). These results suggest that the influence of increasing P and K fertilizer on the accumulation of amino acids and carbohydrates is mainly executed by affecting the metabolization of pyruvic acid.
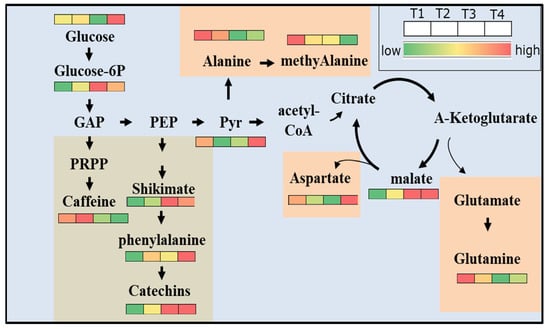
Figure 7.
Schematic presentation of the analysis of the phenylalanine metabolism/flavonoid biosynthesis pathway in young tea shoots under different fertilization treatments.
4.3. High Proportion of P and K Promote Carbohydrate and Flavonoid Accumulation
Catechins are the most abundant polyphenols in tea and affect the bitterness and convergence of tea infusion. Compared with non-fertilized tea plants, the accumulation of catechins in tea shoots under the T1–T3 treatments was significantly decreased. However, the accumulation of catechins in tea shoots under the T4 treatment was significantly increased, suggesting that flavonoid metabolism could be highly promoted by P and K nutrition deficiency and redundancy (Figure 7). The increase in the content of catechins can improve the antioxidant level of tea, but may not be very friendly to some people who pursue a light tea flavor for its bitterness and convergence of tea infusion [36,37]. Notably, the accumulation of malic acid shows a similar pattern as most flavonoids. The correlation analysis also validates that the changes in malic acid are highly correlated with catechins. Particularly, differing from all other amino acids, the changes in aspartic acid (which is biosynthesized from malic acid in the tricarboxylic acid cycle) under fertilizer treatment are also highly associated with catechin contents. Taken together, these results suggest that the effect of excessive P and K fertilizer on catechin accumulation may be related to malic-acid metabolism in the tricarboxylic acid cycle.
5. Conclusions
We have revealed the effects of N, P, and K nutrient application on the metabolism of quality-related components using metabolomics. Our results showed that high P and K inputs reduced the contents of chlorophyll and free amino acids while increasing the accumulation of sugars and catechins in shoots, although the total carbon content did not change significantly. This suggested that high P and K in tea promoted the metabolism and distribution of new shoots photosynthates to carbohydrates and catechins. The results of this study provide a theoretical basis for optimizing tea-fertilization strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12051086/s1, Table S1: Differentially accumulated metabolites in young tea shoots under different fertilization conditions; Table S2: Metabolites analyzed by gas chromatography-mass spectrometry (GC-MS).
Author Contributions
Q.Z. and M.L. gathered samples, and Q.Z. participated in the study design; K.W., Q.Z., H.Z., Y.S. and M.L. performed data analysis; K.W., M.C. and Q.Z. interpreted the results and drafted the manuscript. M.L. and J.R. gave guidance on experimental design. J.R. conceived of the study, and provided funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Zhejiang Provincial Natural Science Foundation of China (LY21C150005), the National Natural Science Foundation of China (32072627), the Chinese Academy of Agricultural Sciences through Agricultural Sciences Innovation Project (CAAS-ASTIP-2017-TRICAAS), and the China Agriculture Research System of MOF and MARA (CARS-19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was financially supported by the Chinese Academy of Agricultural Sciences through Agricultural Sciences Innovation Project (CAAS-ASTIP-2017-TRICAAS) and China Agriculture Research System of MOF and MARA (CARS-19).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruan, J.Y.; Ma, L.F.; Yi, X.Y.; Shi, Y.Z.; Ni, K.; Liu, M.Y.; Zhang, Q.F. Integrated nutrient management in tea plantation to reduce chemical fertilizer and increase nutrient use efficiency. J. Tea Sci. 2020, 40, 85–95. [Google Scholar]
- Liu, M.Y.; Burgos, A.; Ma, L.F.; Zhang, Q.F.; Tang, D.D.; Ruan, J.Y. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wu, L.Q.; Wang, D.H.; Fu, J.Y.; Shen, C.; Li, X.; Liu, M.Y.; Zhang, L.P.; Zhang, L.; Fan, L.C.; et al. Soil acidification in Chinese tea plantations. Sci. Total Environ. 2020, 715, 136963. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.Y.; Haerdter, R.; Gerendas, J. Impact of nitrogen supply on carbon/nitrogen allocation: A case study on amino acids and catechins in green tea Camellia sinensis (L.) O. Kuntze plants. Plant Biol. 2010, 12, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Kwach, B.O.; Owuor, P.O.; Kamau, D.M.; Msomba, S.W.; Uwimana, M.A. Variations in the precursor of plain black tea quality parameters due to location of production and nitrogen fertilizer rates in eastern Africa clonal tea leaves. Exp. Agric. 2016, 52, 266–278. [Google Scholar] [CrossRef][Green Version]
- Lin, Z.H.; Zhong, Q.S.; Chen, C.S.; Ruan, Q.C.; Chen, Z.H.; You, X.M. Carbon dioxide assimilation and photosynthetic electron transport of tea leaves under nitrogen deficiency. Bot. Stud. 2016, 57, 37. [Google Scholar] [CrossRef]
- Wang, H.B.; Chen, X.T.; Wang, Y.H.; Ye, J.H.; Jia, X.L.; Zhang, Q.; He, H.B. Effects of tea garden soil on aroma components and related gene expression in tea leaves. J. Appl. Bot. Food Qual. 2020, 93, 105–111. [Google Scholar]
- Ma, L.F.; Yang, X.D.; Shi, Y.Z.; Yi, X.Y.; Ji, L.F.; Cheng, Y.; Ni, K.; Ruan, J.Y. Response of tea yield, quality and soil bacterial characteristics to long-term nitrogen fertilization in an eleven-year field experiment. Appl. Soil Ecol. 2021, 166, 103976. [Google Scholar] [CrossRef]
- Sun, L.L.; Liu, Y.; Wu, L.Q.; Liao, H. Comprehensive Analysis Revealed the Close Relationship between N/P/K Status and Secondary Metabolites in Tea Leaves. ACS Omega 2019, 4, 176–184. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chen, L.S.; Chen, R.B.; Zhang, F.Z.; Jiang, H.X.; Tang, N. CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport probed by the JIP-test, of tea leaves in response to phosphorus supply. BMC Plant Biol. 2009, 9, 43. [Google Scholar] [CrossRef]
- Salehi, S.Y.; Hajiboland, R. A high internal phosphorus use efficiency in tea (Camellia sinensis L.) plants. Asian J. Plant Sci. 2008, 7, 30–36. [Google Scholar] [CrossRef]
- Ding, Z.T.; Jia, S.S.; Wang, Y.; Xiao, J.; Zhang, Y.F. Phosphate stresses affect ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kc, S.; Liu, M.Y.; Zhang, Q.F.; Fan, K.; Shi, Y.Z.; Ruan, J.Y. Metabolic Changes of Amino Acids and Flavonoids in Tea Plants in Response to Inorganic Phosphate Limitation. Int. J. Mol. Sci. 2018, 19, 3683. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.M.; Yuan, X.Y.; He, H. The Distribution of Phosphorus Forms in Wuyi Rock Region and Its Effect on Tea Quality-Related Constituents in Tea Garden Soil. Pol. J. Environ. Stud. 2021, 30, 4331–4341. [Google Scholar] [CrossRef]
- Ruan, J.Y.; Ma, L.F.; Shi, Y.Z. Potassium management in tea plantations: Its uptake by field plants, status in soils, and efficacy on yields and quality of teas in China. J. Plant Nutr. Soil Sci. 2013, 176, 450–459. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhong, Q.S.; Chen, C.S.; Chen, Z.H.; You, X.M. Effects of different potassium level on leaf photosynthesis of tea seedling. J. Tea Sci. 2013, 33, 261–267. [Google Scholar]
- Yuan, L.; Wang, S.S.; Wang, Z.H.; Huang, J.Q. Tea-grown soils and tea quality in Sichuan and Chongqing, China. Pedosphere 2000, 10, 45–52. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Rhodes, R.; Miles, N.; Hughes, J.C. Interactions between potassium, calcium and magnesium in sugarcane grown on two contrasting soils in South Africa. Field Crops Res. 2018, 223, 1–11. [Google Scholar] [CrossRef]
- Krouk, G.; Kiba, T. Nitrogen and Phosphorus interactions in plants: From agronomic to physiological and molecular insights. Curr. Opin. Plant Biol. 2020, 57, 104–109. [Google Scholar] [CrossRef]
- Jiang, D.; Dai, T.; Jing, Q.; Cao, W.; Zhou, Q.; Zhao, H.; Fan, X. Effects of long-term fertilization on leaf photosynthetic characteristics and grain yield in winter wheat. Photosynthetica 2004, 42, 439–446. [Google Scholar] [CrossRef]
- Sani, M.N.H.; Hasan, M.; Uddain, J.; Subramaniam, S. Impact of application of Trichoderma and biochar on growth, productivity and nutritional quality of tomato under reduced N-P-K fertilization. Ann. Agric. Sci. 2020, 65, 107–115. [Google Scholar] [CrossRef]
- Barzegar, T.; Mohammadi, S.; Ghahremani, Z. Effect of nitrogen and potassium fertilizer on growth, yield and chemical composition of sweet fennel. J. Plant Nutr. 2020, 43, 1189–1204. [Google Scholar] [CrossRef]
- Bukhori, M.F.M.; Izzat, M.K.; Saiman, M.Z.; Majid, N.A.; Jaafar, H.Z.E.; Ghasemzadeh, A.; Sinniah, U.R. Preliminary Study on the Effect of Nitrogen and Potassium Fertilization, and Evapotranspiration Replacement Interaction on Primary and Secondary Metabolites of Gynura procumbens Leaves. Pertanika J. Trop. Agric. Sci. 2020, 43, 391–413. [Google Scholar]
- Farahmandi, S.R.; Samavat, S.; Mostafavi, M.; Mohammadi Torkashvand, A.; Kalate Jari, S. Combined foliar-applied L-glutamic acid, nitrogen, and potassium improve plant growth, physio-chemical attributes, minerals, and longevity of gerbera (Gerbera jamesonii). J. Plant Nutr. 2022, 45, 951–962. [Google Scholar] [CrossRef]
- Razmavar, Z.; Naderi, R.; Abdossi, V.; Ladanmoghadam, A.; Nematollahi, F. The Response of Plant Growth and Physio-biochemical Properties Inedible Flowers of Pelargonium peltatum L. to Soil Applied Potassium and Selenium. J. Med. Plants By-Prod. 2021, 10, 217–225. [Google Scholar]
- Li, Z.G.; Zhang, R.H.; Xia, S.J.; Wang, L.; Liu, C.; Zhang, R.Q.; Fan, Z.H.; Chen, F.; Liu, Y. Interactions between N, P and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Deng, S.X.; Shi, K.K.; Ma, J.; Zhang, L.L.; Ma, L.Y.; Jia, Z.K. Effects of Fertilization Ratios and Frequencies on the Growth and Nutrient Uptake of Magnolia wufengensis (Magnoliaceae). Forests 2019, 10, 65. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Liu, M.Y.; Mumm, R.; Vos, R.C.H.; Ruan, J.Y. Metabolomics reveals the within-plant spatial effects of shading on tea plants. Tree Physiol. 2021, 41, 317–330. [Google Scholar] [CrossRef]
- Gebrewold, A.Z. Review on integrated nutrient management of tea (Camellia sinensis L.). Cogent Food Agric. 2018, 4, 1543536. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Xu, X.F.; Zhang, Y.X.; Ni, W.Z. Improving albino tea quality by foliar application of glycinebetaine as a green egulator under lower temperature conditions. J. Agric. Food Chem. 2021, 69, 1242–1250. [Google Scholar] [CrossRef]
- Wei, K.; Ruan, L.; Li, H.; Wu, L.; Wang, L.; Cheng, H. Estimation of the effects of major chemical components on the taste quality of green tea. Int. Food Res. J. 2019, 26, 869–876. [Google Scholar]
- Yu, X.L.; Hu, S.; He, C.; Zhou, J.T.; Qu, F.F.; Ai, Z.Y.; Chen, Y.Q.; Ni, D.J. Chlorophyll Metabolism in Postharvest Tea (Camellia sinensis L.) Leaves: Variations in Color Values, Chlorophyll Derivatives, and Gene Expression Levels under Different Withering Treatments. J. Agric. Food Chem. 2019, 67, 10624–10636. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.G.; Yeo, A.S.L.; Low, M.Y.; Zhou, W.B. Identifying key non-volatile compounds in ready-to-drink green tea and their impact on taste profile. Food Chem. 2014, 155, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cheng, Y.F.; Deng, C.; Ma, Y.; Wang, Z.W.; Chen, X.H.; Xue, L.B. Comparative transcriptome analysis of eggplant (Solanum melongena L.) and turkey berry (Solanum torvum Sw.): Phylogenomics and disease resistance analysis. BMC Genom. 2014, 15, 412. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedula, V.S.P.; Prakash, I. The aroma, taste, color and bioactive constituents of tea. J. Med. Plants Res. 2011, 5, 2110–2124. [Google Scholar]
- Narukawa, M.; Kimata, H.; Noga, C.; Watanabe, T. Taste characterisation of green tea catechins. Int. J. Food Sci. Technol. 2010, 45, 1579–1585. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).