Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seeds
2.2. Germination Assay
2.3. Measurements of Physiological and Biochemical Indictors
2.4. Statistical Analysis
3. Results
3.1. Evaluation of Seed Vigor
3.2. Evaluation of Phytohormones Changes under Aging Treatment
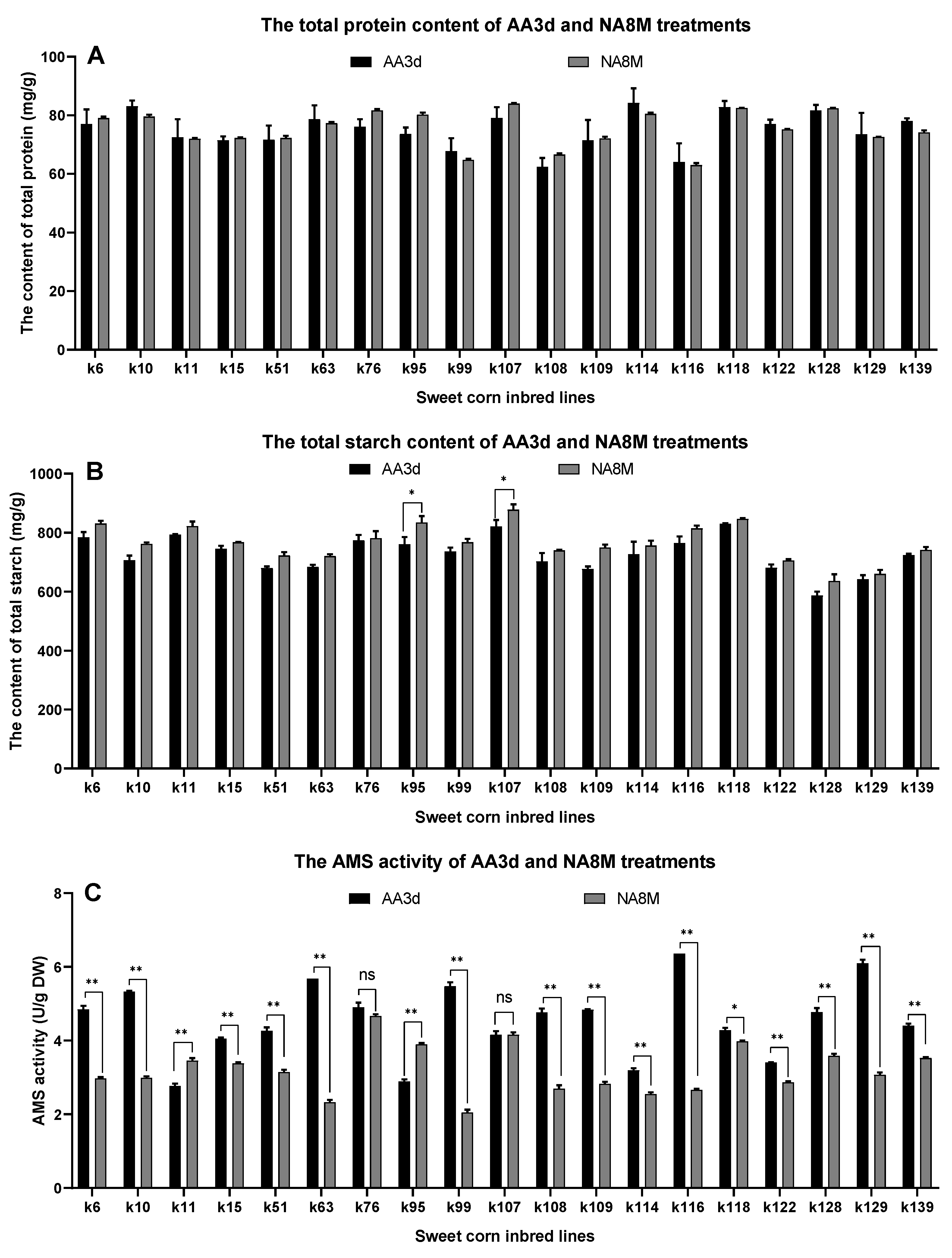
3.3. Evaluation of Total Protein and Total Starch Content under Aging
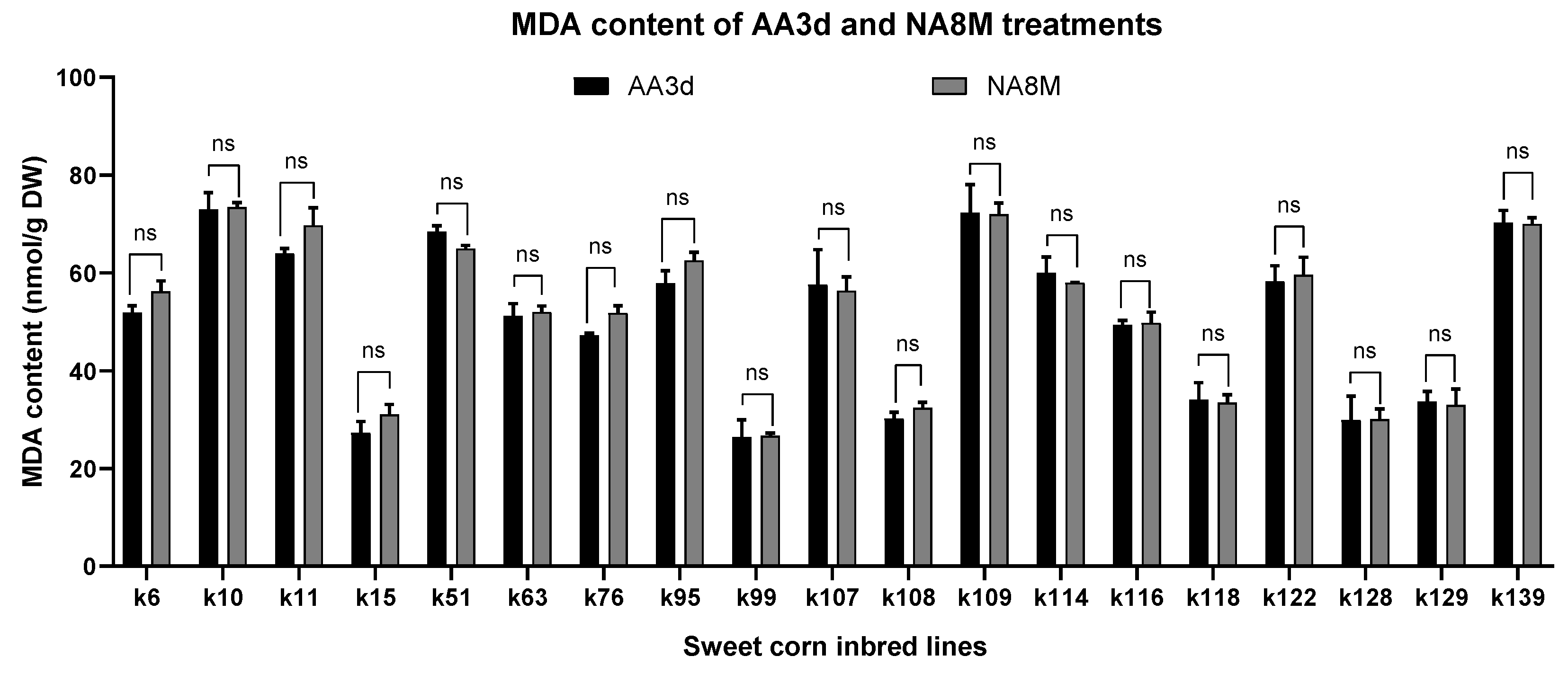
3.4. Evaluation of Lipid Peroxidation
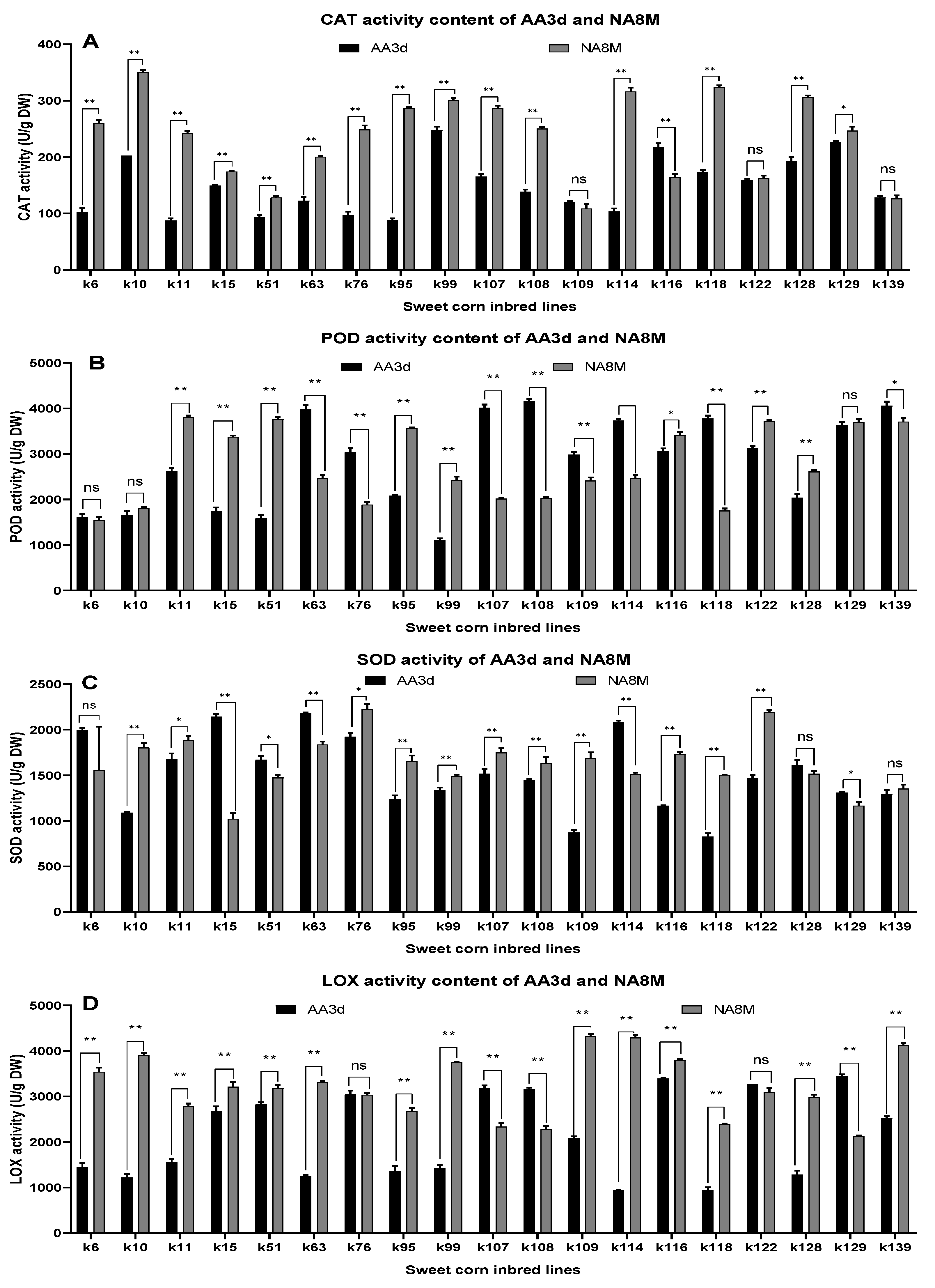
3.5. Evaluation of the Activity of Antioxidant Enzymes
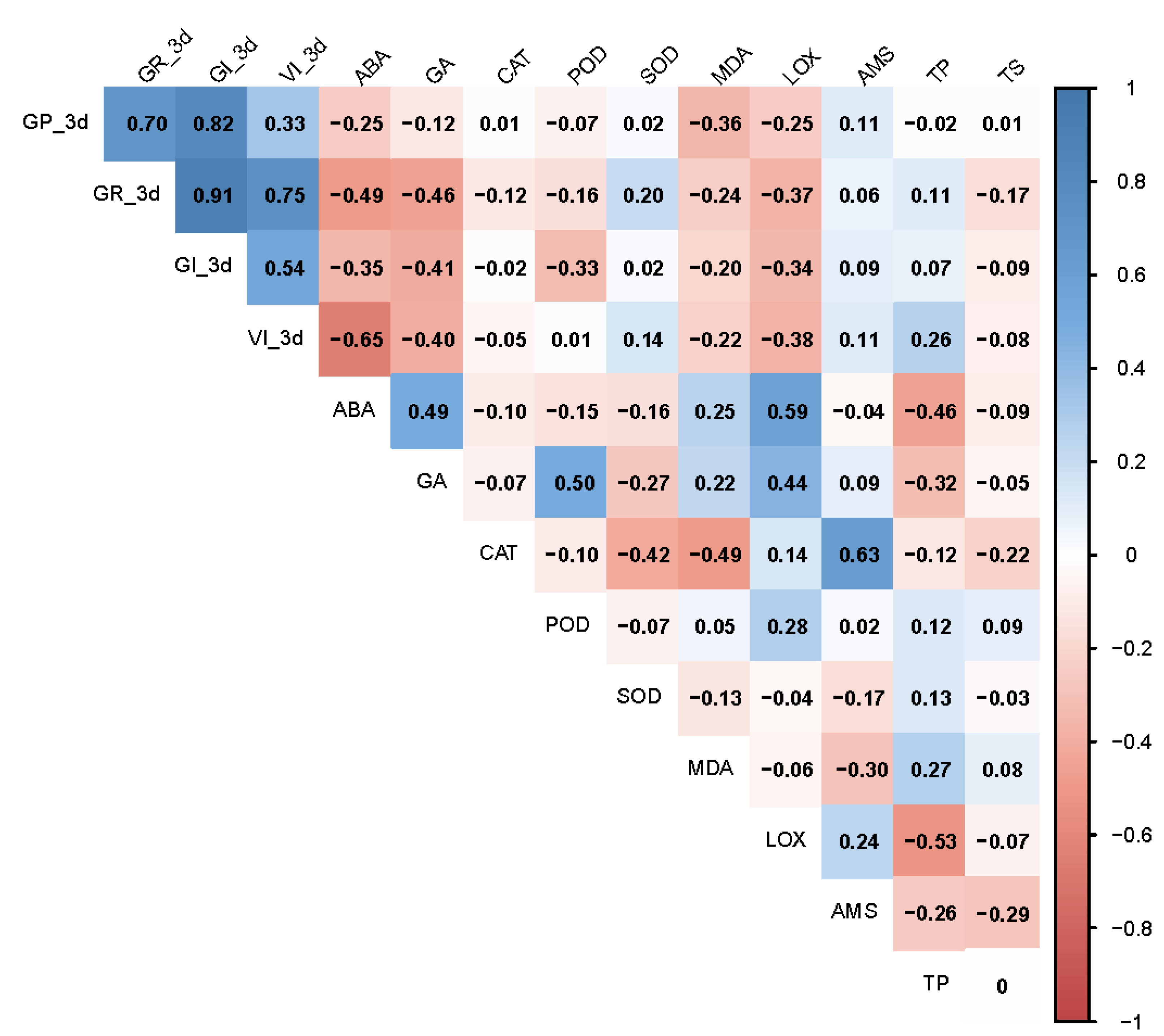
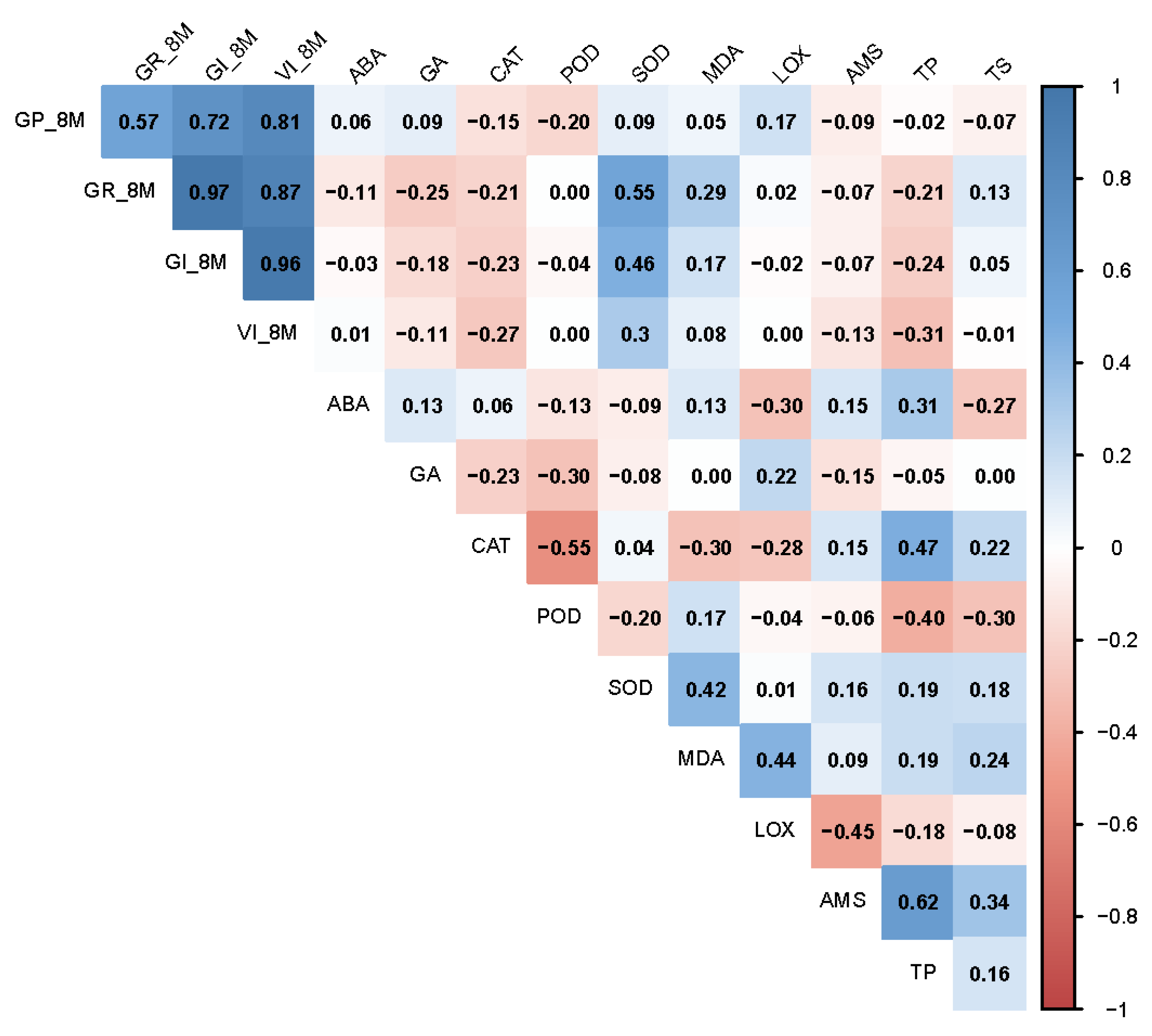
3.6. Correlation of Seed Vigor Indexes and Physiological Indexes in Artificially Aged Seeds
3.7. Correlation of Seed Vigor Indexes and Physiological Indexes in Naturally Aged Seeds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hallauer, A.R. Specialty Corns, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Fan, L.; Yan, Q. Research progress on the causes of low seed vigor and seed treatment technology of sweet corn. Chin. Agric. Sci. Bull. 1996, 12, 24–26. [Google Scholar]
- Mcdonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar] [CrossRef]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.; Belghazi, M.; Job, C.; Job, D. Proteome-wide characterization of seed aging in Arabidopsis: A comparison between artificial and natural aging protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [Green Version]
- Baskin, C.C.; Baskin, J.M. Breaking Seed Dormancy during Dry Storage: A Useful Tool or Major Problem for Successful Restoration via Direct Seeding? Plants 2020, 9, 636. [Google Scholar] [CrossRef]
- Kameswara Rao, N.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar] [CrossRef]
- Xia, F.; Wang, X.; Li, M.; Mao, P. Mitochondrial structural and antioxidant system responses to aging in oat (Avena sativa L.) seeds with different moisture contents. Plant Physiol. Biochem. 2015, 94, 122–129. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D. Temperature sensitivity of the low-moisture-content limit to negative seed longevity--moisture content relationships in hermetic storage. Ann. Bot. 2006, 97, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Simpson, W.R.; Song, M.L.; Bao, G.S.; Niu, X.L.; Zhang, Z.H.; Xu, H.F.; Liu, X.; Li, Y.L.; Li, C.J. Effects of seed moisture content and Epichloe endophyte on germination and physiology of Achnatherum inebrians. S. Afr. J. Bot. 2020, 134, 407–414. [Google Scholar] [CrossRef]
- Filho, C.H.; Goneli, A.; Masetto, T.E.; Martins, E.; Oba, G.C. The effect of drying temperatures and storage of seeds on the growth of soybean seedlings. J. Seed 2016, 38, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Harrington, J.F. Seed Storage and Longevity; Kozlowski, T.T., Ed.; Seed Biology; Academic Press: New York, NY, USA, 1972; Volume III, pp. 145–245. [Google Scholar]
- Jiang, F.L.; Bo, L.P.; Xu, J.J.; Wu, Z. Changes in respiration and structure of non-heading Chinese cabbage seeds during gradual artificial aging. Sci. Hortic.-Amst. 2018, 238, 14–22. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Salehi, B.; Kumar, M.; Sahrifi-Rad, M.; Coviello, E.; Iriti, M.; Sharifi-Rad, J. Accelerated ageing induces physiological and biochemical changes in tomato seeds involving MAPK pathways. Sci. Hortic.-Amst. 2019, 248, 20–28. [Google Scholar] [CrossRef]
- Xia, F.; Chen, L.; Sun, Y.; Mao, P. Relationships between ultrastructure of embryo cells and biochemical variations during ageing of oat (Avena sativa L.) seeds with different moisture content. Acta Physiol. Plant 2015, 37, 1825. [Google Scholar] [CrossRef]
- Radha, B.N.; Channakeshava, B.C.; Hullur, N.; Pandurange, G.; Bhanuprakash, K.; Ramachandrappa, B.K.; Munirajappa, R. Effect of seed ageing on protein quality and quantity in maize. Int. J. Bioassays 2014, 3, 1708–1713. [Google Scholar]
- Pairochteerakul, P.; Jothityangkoon, D.; Ketthaisong, D.; Simla, S.; Lertrat, K.; Suriharn, B. Seed Germination in Relation to Total Sugar and Starch in Endosperm Mutant of Sweet Corn Genotypes. Agronomy 2018, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Morscher, F.; Kranner, I.; Arc, E.; Bailly, C.; Roach, T. Glutathione redox state, tocochromanols, fatty acids, antioxidant enzymes and protein carbonylation in sunflower seed embryos associated with after-ripening and ageing. Ann. Bot. 2015, 116, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M.; Polle, A. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive Oxygen Species and Antioxidants in Postharvest Vegetables and Fruits. Int. J. Food Sci. 2020, 2020, 8817778. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, X.; Jia, S.; Li, M.; Mao, P. Transcriptomic analysis reveals the changes of energy production and AsA-GSH cycle in oat embryos during seed ageing. Plant Physiol. Biochem. 2020, 153, 40–52. [Google Scholar] [CrossRef]
- Aalen, R.B. Peroxiredoxin antioxidants in seed physiology. Seed Sci. Res. 1999, 9, 285–295. [Google Scholar] [CrossRef]
- Machado Neto, N.; Custodio, C.; Takaki, M. Evaluation of naturally and artificially aged seeds of Phaseolus vulgaris L. Seed Sci. Technol. 2001, 29, 137–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, L.; Zhu, S.; Liu, J.; Yang, R.; Wang, Q.; Li, X.; Feng, F. Changes of vigor, physiological characteristics and genetic diversities of artificially aged sweet corn seeds. J. South China Agric. Univ. 2018, 39, 25–30. [Google Scholar]
- Guan, Y.J.; Hu, J.; Wang, Z.F.; Zhu, S.J.; Wang, J.C.; Knapp, A. Time series regression analysis between changes in kernel size and seed vigor during developmental stage of sh2 sweet corn (Zea mays L.) seeds. Sci. Hortic.-Amst. 2013, 154, 25–30. [Google Scholar] [CrossRef]
- Friendly, M. Corrgrams: Exploratory displays for correlation matrices. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, B.; Wu, J.; Wu, Y.; Xuan, H.; Zhu, X. Study on Accelerated Aging Test of Rice Seeds with High Temperature and Humidity. J. Chin. Cereals Oils Assoc. 2010, 25, 8–12. [Google Scholar]
- Kapoor, N.; Arya, A.; Siddqui, A.; Kumar, H.; Amir, A. Physiological and Biochemical Changes During Seed Deterioration in Aged Seeds of Rice (Oryza sativa L.). Am. J. Plant Physiol. 2011, 6, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Basra, S.M.A.; Ahmad, N.; Khan, M.M.; Iqbal, N.; Cheema, M.A. Assessment of cottonseed deterioration during accelerated ageing. Seed Sci. Technol. 2003, 31, 531–540. [Google Scholar] [CrossRef]
- Suresh, A.; Shah, N.; Kotecha, M.; Robin, P. Evaluation of biochemical and physiological changes in seeds of Jatropha curcas L. Under natural aging, accelerated aging and saturated salt accelerated aging. Sci. Hortic.-Amst. 2019, 255, 21–29. [Google Scholar] [CrossRef]
- Giurizatto, M.; Ferrarese-Filho, O.; Ferrarese, M.; Dias, A.; Gonçalves, M.; Cardoso, C. α-Tocopherol levels in natural and artificial aging of soybean seeds. Acta Scientiarum. Agron. 2012, 34, 339–343. [Google Scholar] [CrossRef]
- Priestley, D.A.; Leopold, A.C. Lipid changes during natural aging of soybean seeds. Physiol. Plant. 1983, 59, 467–470. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, W.; Ren, Y.; Jiang, Y.; Sun, A.; Qian, Y.; Zhang, Y.; He, N.; Hang, N.T.; Liu, Z.; et al. Genetic dissection of seed storability using two different populations with a same parent rice cultivar N22. Breed Sci. 2015, 65, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Man, Q.; Pan, J.; Lei, Y.; Yu, H.; Wu, M.; Zhang, W.; Liu, L. Differences in Properties of Seed Vigor Between Artificially and Naturally Aged Soybean Seeds. Seed 2015, 34, 14–18. [Google Scholar]
- Lee, Y.P.; Baek, K.; Lee, H.; Kwak, S.; Bang, J.; Kwon, S. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, M.; Wu, H.; Zhao, T.; Wu, P.; Cao, D. High Drying Temperature Accelerates Sunflower Seed Deterioration by Regulating the Fatty Acid Metabolism, Glycometabolism, and Abscisic Acid/Gibberellin Balance. Front. Plant Sci. 2021, 12, 628251. [Google Scholar] [CrossRef]
- Xue, X.; Du, S.; Jiao, F.; Xi, M.; Wang, A.; Xu, H.; Jiao, Q.; Zhang, X.; Jiang, H.; Chen, J.; et al. The regulatory network behind maize seed germination: Effects of temperature, water, phytohormones, and nutrients. Crop J. 2021, 9, 718–724. [Google Scholar] [CrossRef]
- Bueso, E.; Muñoz-Bertomeu, J.; Campos, F.; Brunaud, V.; Martínez, L.; Sayas, E.; Ballester, P.; Yenush, L.; Serrano, R. ARABIDOPSIS THALIANA HOMEOBOX25 uncovers a role for Gibberellins in seed longevity. Plant Physiol. 2014, 164, 999–1010. [Google Scholar] [CrossRef] [Green Version]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a better monitoring of seed ageing under ex situ seed conservation. Conserv. Physiol. 2015, 3, v26. [Google Scholar] [CrossRef] [Green Version]


| Germination Potential (%) | Germination Rate (%) | Germination Index | Vigor Index | ||
|---|---|---|---|---|---|
| mean | Control | 61.84 ± 3.61 a | 90.84 ± 1.75 a | 5.10 ± 0.21 a | 36.69 ± 2.49 b |
| AA3d | 31.16 ± 3.94 c | 60.53 ± 4.80 c | 2.15 ± 0.19 d | 11.81 ± 1.25 d | |
| AA6d | 13.89 ± 3.09 e | 30.63 ± 5.54 d | 1.18 ± 0.23 e | 3.42 ± 0.88 e | |
| NA4M | 67.61 ± 4.14 a | 88.11 ± 2.61 a | 5.25 ± 0.18 a | 39.64 ± 2.68 a | |
| NA6M | 52.42 ± 6.75 b | 76.00 ± 4.62 b | 4.51 ± 0.34 b | 28.18 ± 3.53 c | |
| NA8M | 22.79 ± 4.77 d | 55.58 ± 5.64 c | 3.17 ± 0.39 c | 14.45 ± 3.21 d | |
| Variance component | inbred lines | 2210.00 ** | 2045.00 ** | 8.36 ** | 714.00 ** |
| treatments | 18,495.00 ** | 19,683.00 ** | 106.10 ** | 8134.00 ** | |
| Interaction | 497.50 ** | 477.20 ** | 1.66 ** | 150.10 ** |
| Treatments | Max | Min | Mean | Coefficient of Variation | Correlation Coefficient |
|---|---|---|---|---|---|
| AA3d | 72.91 | 26.40 | 50.66 | 0.32 | 0.9878 ** |
| NA8M | 73.44 | 26.69 | 51.74 | 0.31 |
| Traits | Mean | Variance Component | |||
|---|---|---|---|---|---|
| AA3d | NA8M | Inbred Lines | Treatments | Interaction | |
| CAT activity | 148.35 ± 49.91 | 236.26 ± 72.98 (ns) | 9945.00 ** | 146,819.00 ** | 6037.00 ** |
| POD activity | 2844.66 ± 998.95 | 2764.92 ± 804.67 (ns) | 1,598,798.00 ** | 120,800.00 ** | 1,767,739.00 ** |
| SOD activity | 1518.63 ± 404.84 | 1632.42 ± 315.21 (ns) | 254,752.00 ** | 245,995.00 ** | 255,639.00 ** |
| LOX activity | 2158.24 ± 920.67 | 3219.39 ± 681.86 (ns) | 966,162.00 ** | 21,395,065.00 ** | 1,715,268.00 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yang, R.; Ji, Z.; Zhang, H.; Zheng, W.; Zhang, H.; Feng, F. Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy 2022, 12, 1028. https://doi.org/10.3390/agronomy12051028
Wang B, Yang R, Ji Z, Zhang H, Zheng W, Zhang H, Feng F. Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy. 2022; 12(5):1028. https://doi.org/10.3390/agronomy12051028
Chicago/Turabian StyleWang, Bo, Ruichun Yang, Zhaoqian Ji, Huaxing Zhang, Wenbo Zheng, Huihui Zhang, and Faqiang Feng. 2022. "Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging" Agronomy 12, no. 5: 1028. https://doi.org/10.3390/agronomy12051028
APA StyleWang, B., Yang, R., Ji, Z., Zhang, H., Zheng, W., Zhang, H., & Feng, F. (2022). Evaluation of Biochemical and Physiological Changes in Sweet Corn Seeds under Natural Aging and Artificial Accelerated Aging. Agronomy, 12(5), 1028. https://doi.org/10.3390/agronomy12051028





