Characterization of Source–Sink Traits and Carbon Translocation in Maize Hybrids under High Plant Density
Abstract
:1. Introduction
2. Materials and Methods
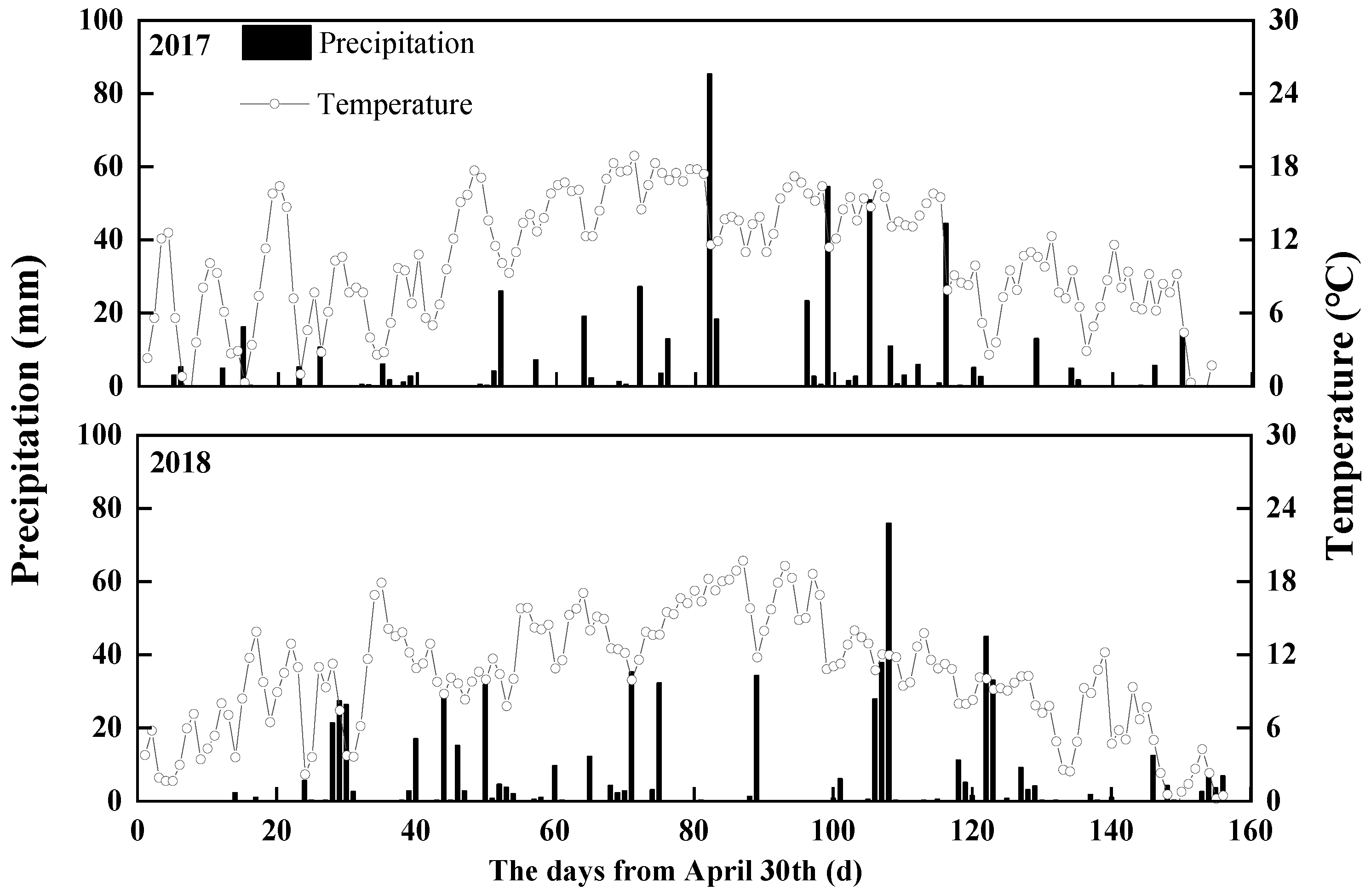
2.1. Site Description
2.2. Experiment Design and Field Management
2.3. Data Collection
2.3.1. Dry Matter (DM) and Carbon (C) Content
2.3.2. Leaf Area Index (LAI) and Net Photosynthetic Rate (Pn) of Ear Leaf
2.3.3. Enzyme Activity and Hormone Content in Grain
2.3.4. Kernel Dry Weight Accumulation and Kernel Volume
2.3.5. Area and Number of Vascular Bundle
2.3.6. Floret Number, Grain Yield and Sink Capacity
2.4. Statistical Analysis
3. Results
3.1. Number of Florets, Grain Yield, and Sink Capacity
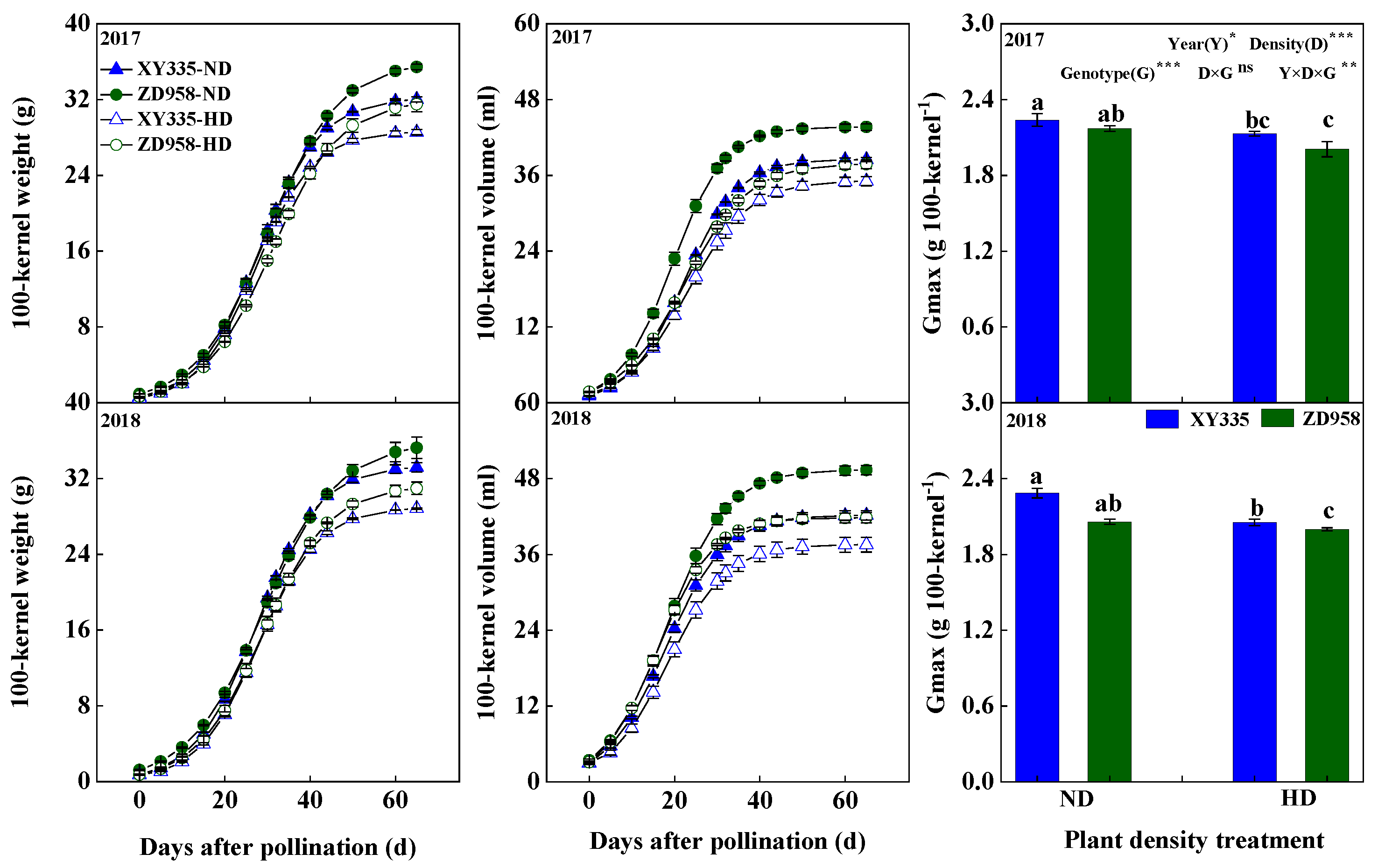
3.2. Kernel Weight, Kernel Volume, and Maximum Grain-Filling Rate (Gmax)
3.3. Leaf Area Index (LAI) and Net Photosynthetic Rate (Pn) of Ear Leaf
3.4. Dry Matter Accumulation and Distribution in Different Organs of Maize
3.5. Leaf and Grain Carbon © Contents, and Matter Transport Efficiency
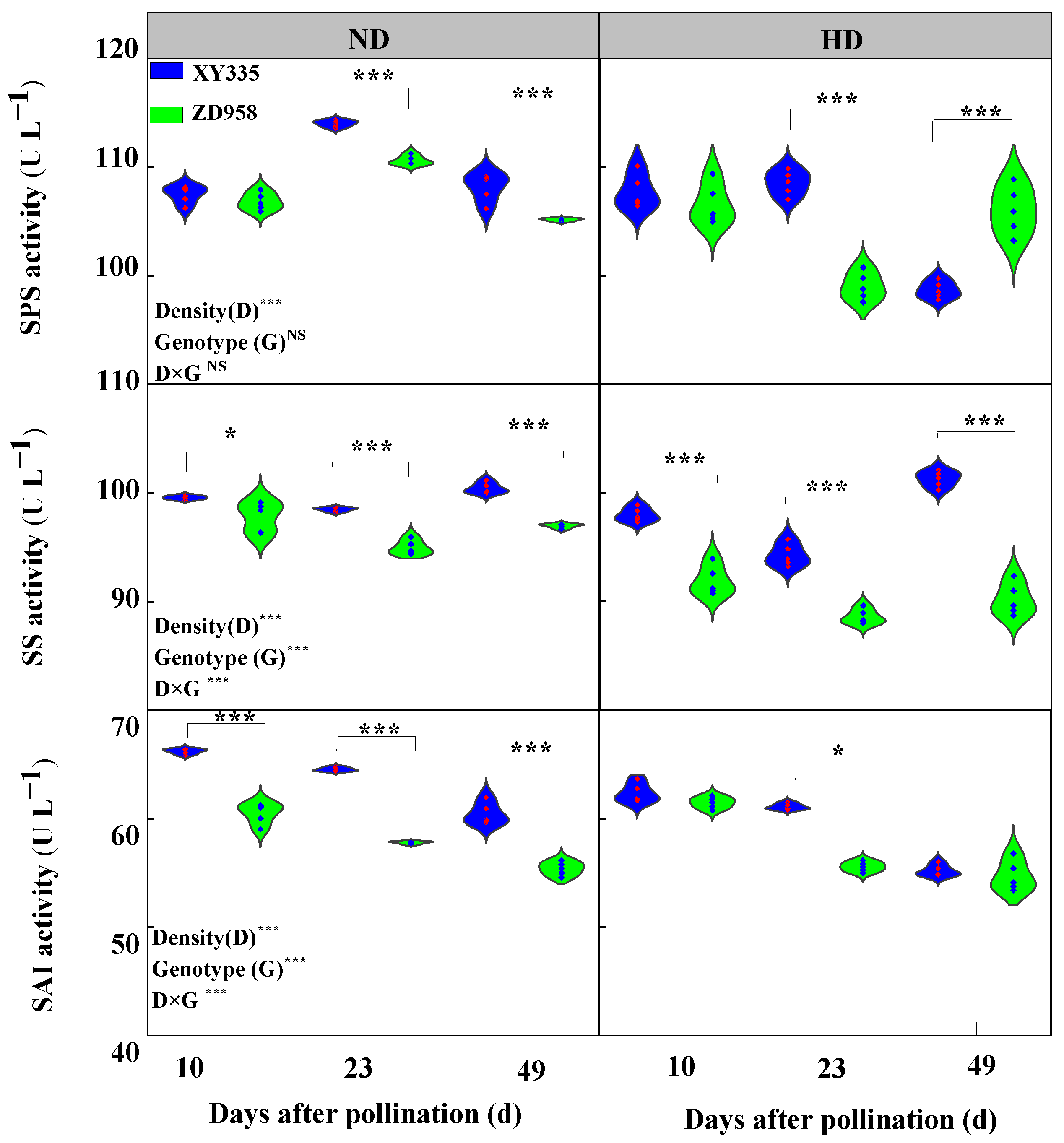
3.6. Sucrose Synthase (SS), Sucrose Phosphate Synthase (SPS), and Soluble Acid Invertase (SAI) Enzyme Activity in Grain
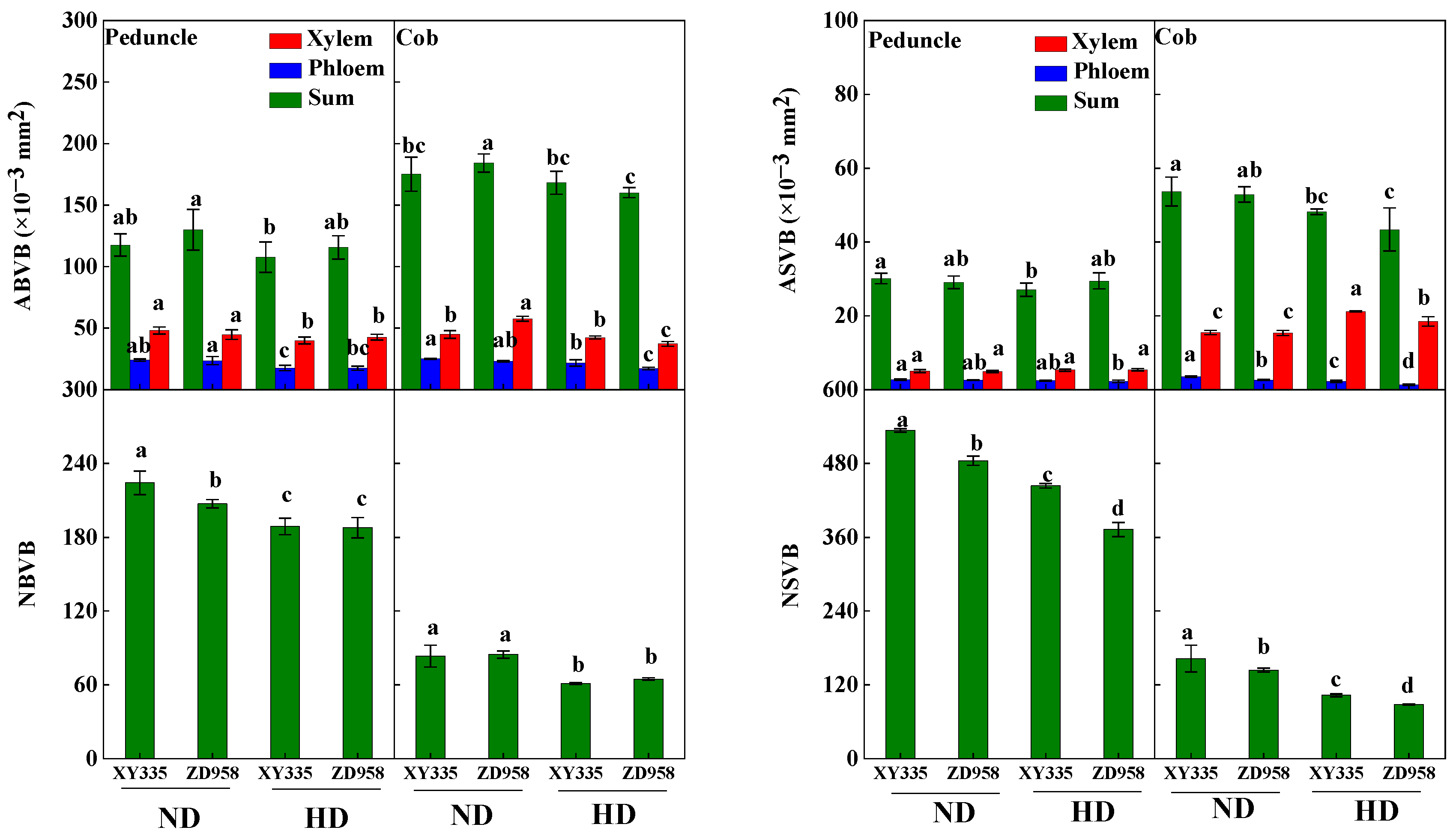
3.7. Vascular Bundle Structure in Internodes
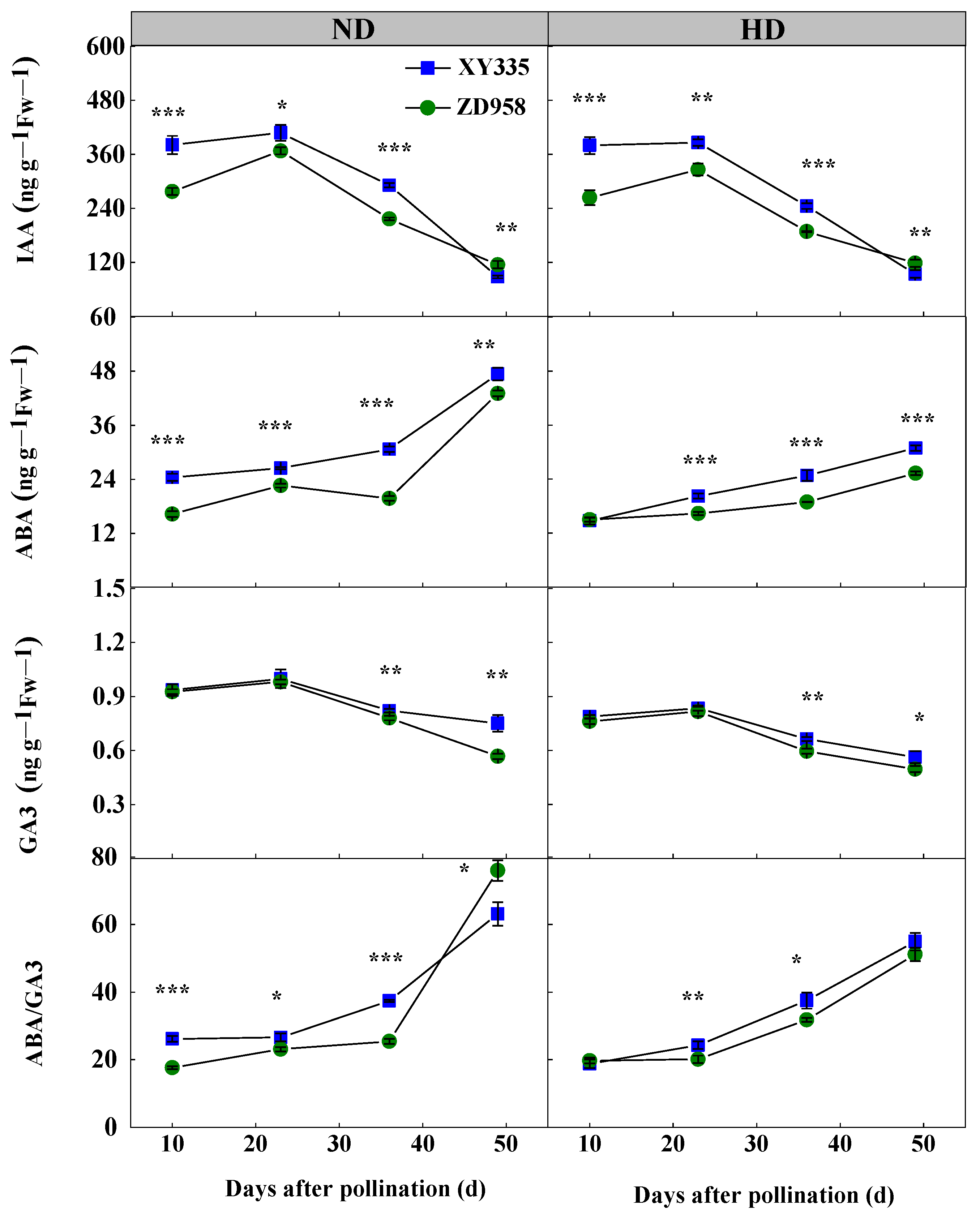
3.8. Hormone Content in Grain
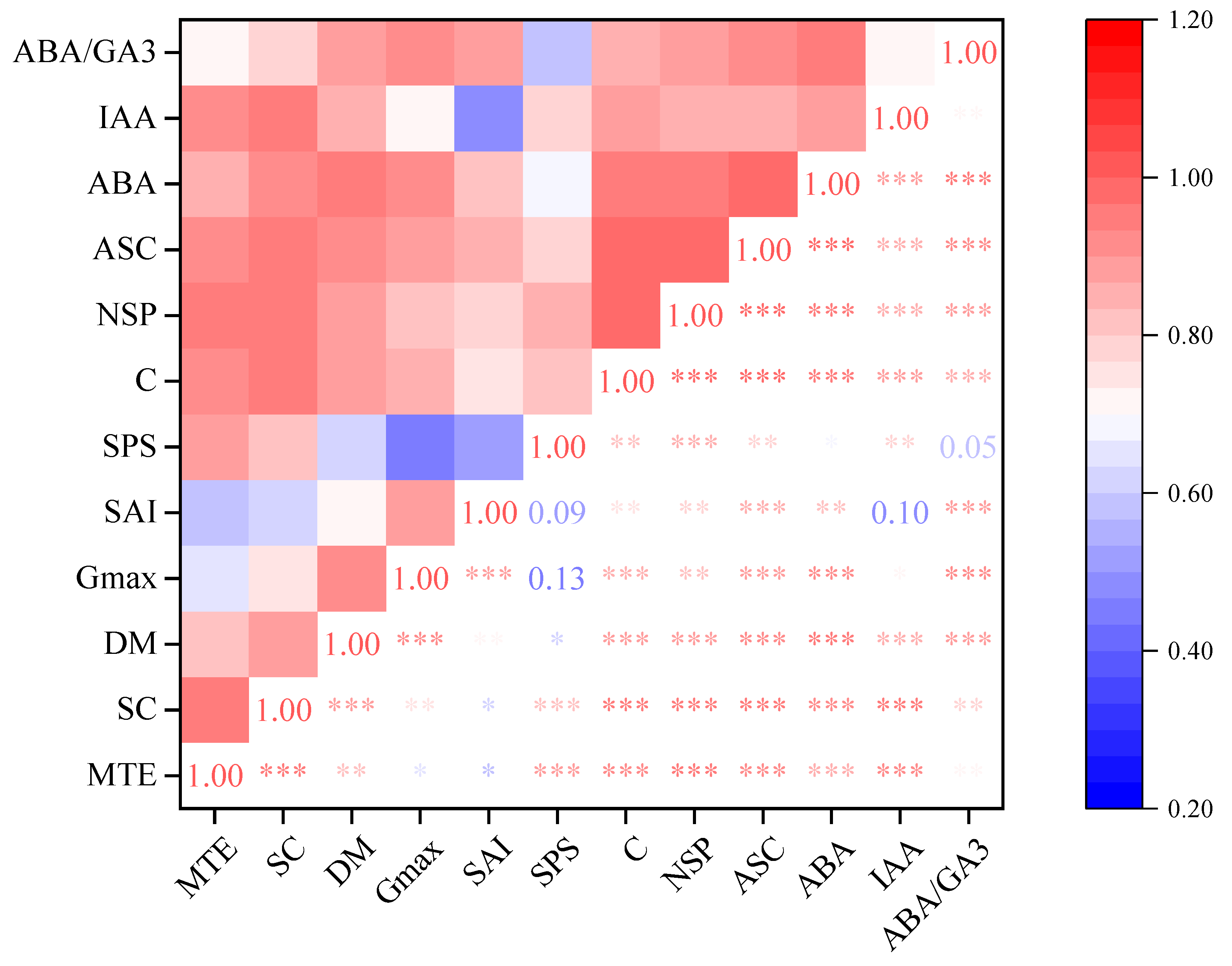
3.9. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sangoi, L. Understanding plant density effects on maize growth and development: An important issue to maximize grain yield. Cienc. Rural. 2001, 31, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.N.; Gu, L.M.; Dong, S.T.; Zhang, J.W.; Liu, P.; Zhao, B. Optimum leaf removal increases canopy apparent photosynthesis, 13C-photosynthate distribution and grain yield of maize crops grown at high density. Field Crops Res. 2015, 170, 32–39. [Google Scholar] [CrossRef]
- Borras, L.; Westgate, M.E.; Astini, J.P.; Echarte, L. Coupling time to silking with plant growth rate in maize. Field Crops Res. 2007, 102, 73–85. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Koutroubas, S.D. review of maize hybrids’ dependence on high plant populations and its implications for crop yield stability. Field Crops Res. 2004, 88, 103–114. [Google Scholar] [CrossRef]
- Borras, L.; Slafer, G.; Otegui, M. Seed dry weight response to source–sink manipulations in wheat, maize, and soybean: A quantitative reappraisal. Field Crops Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Egli, D.B. Is there a role for sink size in understanding maize population yield relationships? Crop Sci. 2015, 55, 2453. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E.A. Dissection of physiological processes underlying grain yield in maize by examining genetic improvement and heterosis. Maydica 2006, 51, 399–408. [Google Scholar] [CrossRef]
- Ho, L.C. Metabolism and compartmentation of imported sugars in sink organs in relation to sink strength. Annu. Rev. Plant Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Faust, T.F.; Kumar, A.; Schubert, S. Reduced sink activity in growing shoot tissues of maize under salt stress of the first phase may be compensated by increased PEP-carboxylase activity. J. Agron. Crop Sci. 2016, 202, 384–393. [Google Scholar] [CrossRef]
- Ercoli, L.; Lulli, L.; Mariotti, M.; Masoni, A.; Arduini, I. Post-anthesis dry matter and nitrogen dynamics in durum wheat as affected by nitrogen supply and soil water availability. Eur. J. Agronmy. 2008, 28, 138–147. [Google Scholar] [CrossRef]
- Otegui, M.E.; Andrade, F.H.; Suero, E.E. Growth, water use, and kernel abortion of maize subjected to drought at silking. Field Crops Res. 1995, 40, 87–94. [Google Scholar] [CrossRef]
- Chen, K.; Camberato, J.J.; Tuinstra, M.R.; Kumudini, S.V.; Tollenaar, T.J.M. Vyn Genetic improvement in density and nitrogen stress tolerance traits over 38 years of commercial maize hybrid release. Field Crops Res. 2016, 196, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Kamara, A.Y.; Menkir, A.; Abubakar, A.W.; Tofa, A.I.; Ademulegun, T.D.; Omoigui, L.O.; Kamai, N. Maize hybrids response to high plant density in the Guinea savannah of Nigeria. J. Crop Improv. 2021, 35, 1–20. [Google Scholar] [CrossRef]
- Lalonde, S.; Tegede, M.; Throne-Holst, M.; Frommer, W.B.; Patrick, J.W. Phloem loading and unloading of sugars and amino acids. Plant Cell Environ. 2003, 26, 37–56. [Google Scholar] [CrossRef] [Green Version]
- Hirel, B.; Berti, P.; Quilleré, I.; Bourdoncle, W.; Attagnant, C.; Dellay, C.; Gouy, A.; Cadiou, S.; Retailliau, C.; Falque, M.; et al. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001, 125, 1258–1270. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Lee, J.; Kichey, T. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of kernel production. Plant Cell. 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [Green Version]
- Causse, M.; Rocher, J.P.; Pelleschi, S.; Barriére, Y.; de Vienne, D.; Priou-l, J.L. Sucrose Phosphate Synthase: An enzyme with heterotic activity correlated with Maize Growth. Crop Sci. 1995, 35, 995–1001. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Lou, H.L.; Li, Y.R. Soluble acid invertase and sucrose phosphate synthase: Key enzymes in regulating sucrose accumulation in sugarcane stalk. J. Sugar Tech. 2009, 13, 28–33. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Z.H.; Wang, Z.Q.; Yang, J.C.; Zhang, J.H. Pre-anthesis nonstructural carbohydrate reserve in the stem enhances the sink strength of inferior spikelets during grain filling of rice. Field Crop Res. 2011, 123, 170–182. [Google Scholar] [CrossRef]
- He, P.; Zhou, W.; Jin, J.Y. Carbon and nitrogen metabolism related to grain formation in two different senescent types of maize. J. Plant Nutr. 2004, 27, 295–311. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Hill, J. Effect on yield and dry-matter distribution of the stay-green characteristic in cultivars of forage maize grown in England. Grass Forage Sci. 2003, 58, 258–264. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, Y.; Zhao, M.; Qi, H.; Li, C.F. Nitrogen supply regulates vascular bundle structure and matter transport characteristics of spring maize under high plant density. Front. Plant Sci. 2021, 11, 602739. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, J.; Zhou, Y. Structural responses of vascular bundles in different maize stems to water deficit during seedling stage: A compensating development. Res. Crop. 2014, 15, 532–538. [Google Scholar] [CrossRef]
- Shane, M.W.; McCully, M.E.; Canny, M.J. The vascular system of maize stems revisited: Implications for water transport and xylem safety. Ann. Bot. 2000, 86, 245–258. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Chen, Q.Y.; Xu, G.H.; Xu, D.Y.; Tian, J.G.; Tian, F. Identification and fine mapping of quantitative trait loci for the number of vascular bundle in maize stem. J. Integr. Plant Biol. 2016, 58, 81–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.G.; Liang, H.W.; Gao, J.L.; Yu, X.F.; Sun, J.Y.; Su, Z.J.; Hu, S.P.; Yu, S.B.; Li, Y.J.; Wei, S.L.; et al. Relationship of sink activity with endogenous hormones and polyamine contents in inferior kernels of maize. Acta Agron. Sin. 2017, 43, 1196–1204. [Google Scholar] [CrossRef]
- Xu, Y.J.; Gu, D.J.; Zhang, B.B.; Zhang, H.; Wang, Z.Q.; Yang, J.C. Hormone contents in kernels at different positions on an ear and their relationship with endosperm development and kernel filling in maize. Acta Agron. Sin. 2013, 39, 1452–1461. [Google Scholar] [CrossRef]
- Liu, Y.; Sui, Y.W.; Gu, D.D.; Wen, X.X.; Chen, Y.; Li, C.J.; Liao, Y.C. Effects of conservation tillage on grain filling and hormonal changes in wheat under simulated rain fall conditions. Field Crops Res. 2013, 144, 43–51. [Google Scholar] [CrossRef]
- Ober, E.S.; Setter, T.L. Water deficit induces abscisic-acid accumulation in endosperm of maize viviparous mutants. Plant Physiol. 1992, 98, 353–356. [Google Scholar] [CrossRef] [Green Version]
- Mahouachi, J.; Gómez, G.A.; Primo-Millo, E.; Talon, M. Antagonistic changes between abscisic acid and gibberellins in citrus fruits subjected to a series of different water conditions. J. Plant Growth Regul. 2005, 24, 179–187. [Google Scholar] [CrossRef]
- Pirasteh, A.H.; Emam, Y.; Pessarakli, M. Changes in endogenous hormonal status in corn (Zea mays) hybrid under drought stress. J. Plant Nutr. 2013, 36, 1695–1707. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Chen, J.; Lin, S.S.; Li, Z.; Cheng, R.H.; Fang, C.X.; Chen, H.F.; Lin, W.X. Proteomic and phosphorproteomic determination of ABA’s effects on grain filling of Oryza sativa L. inferior spikelets. Plant Sci. 2012, 185, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.S.; Wang, X.Y.; Li, G.H.; Qin, Y.Y.; Jiang, D.; Dong, S.T. Plant density and nitrogen supply affect the grain-filling parameters of maize kernels located in different ear positions. Front. Plant Sci. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Cheng, F.; Zhang, G. The relationship between grain filling and hormone content as affected by genotype and source-sink relation. Plant Growth Regul. 2006, 49, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xiao, C.X.; Wu, D.L.; Xia, T.T.; Chen, Q.W.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Effects of nitrogen application rate on grain yield and grain nitrogen concentration in two maize hybrids with contrasting nitrogen remobilization efficiency. Eur. J. Agron. 2015, 62, 79–89. [Google Scholar] [CrossRef]
- Edreira, J.I.; Mayer, R.; Luis, I.; Otegui, M.E. Heat stress in temperate and tropical maize hybrids: Kernel growth, water relations and assimilate availability for grain filling. Field Crops Res. 2014, 166, 162–172. [Google Scholar] [CrossRef]
- Liu, X.W.; Wang, X.L.; Wang, X.Y.; Gao, J.; Luo, N.; Meng, Q.F.; Wang, P. Dissecting the critical stage in the response of maize kernel set to individual and combined drought and heat stress around flowering. Environ. Exp. Bot. 2020, 179, 104213. [Google Scholar] [CrossRef]
- Yagioka, A.; Hayashi, S.; Kimiwada, K.; Kondo, M. Sink production and grain-filling ability of a new high-yielding rice variety, Kitagenki. Field Crops Res. 2021, 260, 107991. [Google Scholar] [CrossRef]
- Campos, H. Estatística Experimental Não-Paramétrica, 4th ed.; Departamento de Matemáticae Estatística-ESALQ: Piracicaba, Brazil, 1983. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book: New York, NY, USA, 1997. [Google Scholar]
- Yang, H.S.; Dobermann, A.; Lindquist, J.L.; Walters, D.T.; Arkebauer, T.J. Hybrid maize a maize simulation model that combines two crop modeling approaches. Field Crops Res. 2004, 87, 131–154. [Google Scholar] [CrossRef] [Green Version]
- Gambín, B.L.; Borrás, L.; Otegui, M.E. Kernel weight dependence upon plant growth at different grain-filling stages in maize and sorghum. Aust. J. Agric. Res. 2008, 59, 280–290. [Google Scholar] [CrossRef]
- Chen, Y.L.; Xiao, C.X.; Chen, X.C.; Li, Q.; Zhang, J.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Characterization of the plant traits contributed to high grain yield and high grain nitrogen concentration in maize. Field Crops Res. 2014, 159, 1–9. [Google Scholar] [CrossRef]
- Seebauer, J.R.; Singletary, G.W.; Krumpelman, P.M.; Ruffo, M.L.; Below, F.E. Relationship of source and sink in determining kernel composition of maize. J. Exp. Bot. 2010, 61, 511–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.J.; Schreiber, B.M.N.; Roessler, J.A. Kernel Sink Capacity in Maize: Genotypic and Maternal Regulation. Crop Sci. 1996, 36, 301–306. [Google Scholar] [CrossRef]
- Gambín, B.L.; Borras, L.; Otegui, M.E. Source–sink relations and kernel weight differences in maize temperate hybrids. Field Crops Res. 2006, 95, 316–326. [Google Scholar] [CrossRef]
- Shen, L.X.; Huang, Y.K.; Li, T. Top-grain filling characteristics at an early stage of maize (Zea mays L.) with different nitrogen use efficiencies. J. Integr. Agric. 2017, 16, 626–639. [Google Scholar] [CrossRef] [Green Version]
- Gambín, L.B.; Borrás, L. Plasticity of sorghum kernel weight to increased assimilate availability. Field Crops Res. 2007, 100, 272–284. [Google Scholar] [CrossRef]
- Sala, R.G.; Westgate, M.E.; Andrade, F.H. Source/sink ratio and the relationship between maximum water content, maximum volume, and final dry weight of maize kernels. Field Crops Res. 2007, 101, 19–25. [Google Scholar] [CrossRef]
- Lizaso, J.I.; Westgate, M.E.; Batchelor, W.D.; Fonseca, A. Predicting potential kernel set in maize from simple flowering characteristics. Crop Sci. 2003, 43, 892–903. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Sucrose-cleaving enzymes and carbohydrate pools in Lilium longiflorum floral organs. Physiol. Plant. 1998, 103, 541–550. [Google Scholar] [CrossRef]
- Zi, Y.; Ding, J.F.; Song, J.M.; Humphreys, G.; Peng, Y.X.; Li, C.Y.; Zhu, X.K.; Guo, W.S. Grain yield, starch content and activities of key enzymes of waxy and nonwaxy wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4548. [Google Scholar] [CrossRef]
- Fahy, B.; Siddiqui, H.; David, L.C.; Powers, S.J.; Borrill, P.; Uauy, C.; Smith, A.M. Final grain weight is not limited by the activity of key starch-synthesising enzymes during grain filling in wheat. J. Exp. Bot. 2018, 69, 5461–5475. [Google Scholar] [CrossRef] [PubMed]
- Hans, W.; Ljudmilla, B.; Ulrich, W. Sugar import and metabolism during seed development. Trends Plant Sci. 1997, 2, 169–174. [Google Scholar] [CrossRef]
- Bihmidine, S.; Hunter, C.T.; Johns, C.E.; Koch, K.E.; Braun, D.M. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front. Plant Sci. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Li, Z.; Mo, F.; Liao, Y.; Liu, Y. Removal of superior wheat kernels promotes filling of inferior kernels by changing carbohydrate metabolism and sink strength. Crop J. 2021, 9, 1375–1385. [Google Scholar] [CrossRef]
- D’Andrea, K.E.; Piedra, C.V.; Mandolino, C.I.; Cirilo, A.G.; Otegui, M.E. Contribution of reserves to kernel weight and grain yield determination in maize: Phenotypic and genotypic variation. Crop Sci. 2016, 56, 697–706. [Google Scholar] [CrossRef]
- Antonietta, M.; Fanello, D.; Acciaresi, D.; Guiamet, H.A. Senescence and yield responses to plant density in stay green and earlier senescing maize hybrids from Argentina. Field Crops Res. 2014, 155, 111–119. [Google Scholar] [CrossRef]
- Teresa, F.M.; Igor, S.; José, M.E.; Begoña, G.M. Late nitrogen fertilization affects carbohydrate mobilization in wheat. J. Plant Nutr. Soil Sci. 2010, 173, 907–919. [Google Scholar] [CrossRef]
- Ning, P.; Yang, L.; Li, C.G.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.J.; Wang, L.C.; Gu, W.R.; Wang, Y.J.; Zhang, J.H. Increasing photosynthetic performance and post-silking N uptake by moderate decreasing leaf source of maize under high planting density. J. Integr. Agr. 2021, 20, 494–510. [Google Scholar] [CrossRef]
- Khan, N.A.; Murayama, S.; Ishimine, Y.; Tsuzuki, E.; Nakamura, I. Physio morphological studies of F1 hybrids in rice (Oryza sativa L.). Photosynthetic ability and yield. Plant Prod. Sci. 1998, 1, 233–239. [Google Scholar] [CrossRef]
- Piao, L.; Qi, H.; Li, C.F.; Zhao, M. Optimized tillage practices and row spacing to improve grain yield and matter transport efficiency in intensive spring maize. Field Crops Res. 2016, 198, 258–268. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Abscisic acid and ethylene interact in wheat grains in response to soil drying during grain filling. New Phytol. 2006, 171, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in theresponse of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lur, H.S.; Setter, T.L. Endosperm development of maize defective kernel (dek) mutants. Auxin and cytokinin levels. Ann. Bot. 1993, 72, 1–6. [Google Scholar] [CrossRef]
- Boyer, J.S. Drought decision-making. J. Exp. Bot. 2010, 61, 3493–3497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Mambelli, S.; Setter, T.L. Abscisic acid catabolism in maize kernels in response to water deficit at early endosperm development. Ann. Bot. 2002, 90, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mambelli, S.; Setter, T.L. Inhibition of maize endosperm cell division and endoreduplication by exogenously applied abscisic acid. Physiol. Plant. 1998, 104, 266–272. [Google Scholar] [CrossRef]
- Travaglia, C.; Cohen, A.C.; Reinoso, H.; Castillo, C.; Bottini, R. Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J. Plant Growth Regul. 2007, 26, 285–289. [Google Scholar] [CrossRef]
- Kende, H.; Zeevaart, J.A.D. The five ‘classical’ plant hormones. Plant Cell. 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [Green Version]

| Year | Genotype | Plant Density (Plant ha−1) | 100-KW (g) | Number of Florets (No. Plant−1) | KNP (No. Plant−1) | Sink Capacity (g Plant−1) | Grain Yield (t ha−1) |
| 2017 | XY335 | ND | 32.8b | 723.3a | 651.5a | 213.5a | 10.9c |
| HD | 28.5c | 626.0b | 594.2b | 169.4c | 13.9a | ||
| ZD958 | ND | 33.8a | 636.7b | 546.4c | 184.8b | 9.3d | |
| HD | 32.5b | 559.7c | 484.0d | 157.1d | 12.5b | ||
| 2018 | XY335 | ND | 29.3c | 784.7a | 696.3a | 204.1a | 10.4c |
| HD | 27.7d | 705.3b | 645.7b | 178.6c | 13.8a | ||
| ZD958 | ND | 32.4a | 722.0b | 612.0c | 198.1b | 9.3d | |
| HD | 31.0b | 615.3c | 541.3d | 168.0d | 12.4b | ||
| ANOVA | Year (Y) | *** | *** | *** | ** | ** | |
| Density (D) | *** | *** | *** | *** | ** | ||
| Genotype (G) | *** | *** | *** | *** | *** | ||
| D × G | NS | NS | NS | NS | NS | ||
| Y × D × G | NS | * | *** | ** | * | ||
| Year | Genotype | Plant Density (Plant ha−1) | Total DM (g Plant−1) | Pre-Silking DM (g Plant−1) | Post-Silking DM (g Plant−1) | DM Distribution in Different Organs at Maturity (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Stem | Leaf | Grain | Cob | ||||||
| 2017 | XY335 | ND | 364.0a | 192.7a | 171.3a | 28.0b | 7.1c | 58.7a | 6.2ab |
| HD | 318.0c | 158.4c | 159.5b | 26.4b | 9.1b | 57.5a | 7.0a | ||
| ZD958 | ND | 348.8b | 171.9b | 176.9ab | 31.0a | 9.1b | 54.8b | 5.1c | |
| HD | 274.5d | 130.9d | 143.7c | 26.1b | 13.5a | 54.8b | 5.7bc | ||
| 2018 | XY335 | ND | 387.0a | 157.2a | 229.8a | 26.0c | 11.4b | 57.3a | 5.4a |
| HD | 290.8c | 120.0c | 170.8c | 27.9b | 12.0b | 55.7b | 4.5b | ||
| ZD958 | ND | 333.8b | 134.4b | 199.4b | 29.2a | 11.3b | 53.7c | 5.8a | |
| HD | 281.5d | 118.4c | 163.1c | 29.4a | 13.0a | 53.5c | 4.0b | ||
| ANOVA | Year (Y) | NS | *** | *** | NS | *** | *** | *** | |
| Density (D) | *** | *** | *** | * | *** | NS | NS | ||
| Genotype (G) | *** | *** | NS | *** | *** | *** | ** | ||
| D × G | NS | NS | NS | ** | *** | NS | NS | ||
| Y × D× G | *** | ** | *** | NS | NS | NS | NS | ||
| Genotype | Plant Density (Plant ha−1) | SLC (g) | MLC (g) | LCTE (%) | G-C (g) | Peduncle Bleeding Sap (mg h −1) | MTE (mg mm −2 h −1) |
|---|---|---|---|---|---|---|---|
| XY335 | ND | 24.7a | 10.4c | 57.9a | 89.5a | 1005.0a | 23.7a |
| HD | 16.5b | 13.7ab | 17.0c | 71.3c | 588.0c | 18.3b | |
| ZD958 | ND | 23.1a | 12.5b | 45.9b | 81.3b | 936.7b | 23.1a |
| HD | 16.3b | 15.0a | 8.0d | 62.6d | 468.3d | 14.4c | |
| ANOVA | Density (D) | *** | *** | *** | *** | *** | *** |
| Genotype (G) | NS | ** | *** | *** | *** | ** | |
| D × G | NS | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Qi, H.; Zhao, M.; Zhou, W.; Wang, X.; Gong, X.; Jiang, Y.; Li, C. Characterization of Source–Sink Traits and Carbon Translocation in Maize Hybrids under High Plant Density. Agronomy 2022, 12, 961. https://doi.org/10.3390/agronomy12040961
Ren H, Qi H, Zhao M, Zhou W, Wang X, Gong X, Jiang Y, Li C. Characterization of Source–Sink Traits and Carbon Translocation in Maize Hybrids under High Plant Density. Agronomy. 2022; 12(4):961. https://doi.org/10.3390/agronomy12040961
Chicago/Turabian StyleRen, Hong, Hua Qi, Ming Zhao, Wenbin Zhou, Xinbing Wang, Xiangwei Gong, Ying Jiang, and Congfeng Li. 2022. "Characterization of Source–Sink Traits and Carbon Translocation in Maize Hybrids under High Plant Density" Agronomy 12, no. 4: 961. https://doi.org/10.3390/agronomy12040961
APA StyleRen, H., Qi, H., Zhao, M., Zhou, W., Wang, X., Gong, X., Jiang, Y., & Li, C. (2022). Characterization of Source–Sink Traits and Carbon Translocation in Maize Hybrids under High Plant Density. Agronomy, 12(4), 961. https://doi.org/10.3390/agronomy12040961







