Strengthened Assimilate Transport Improves Yield and Quality of Super Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Determination of Grain Yields, Yield Components and Grain Quality
2.3. Plant Height, Stem Tiller Dynamics, and Solar Radiation Interception Rate
2.4. Photosynthesis and Dry Matter Accumulation
2.5. Measurement of Grain Filling Rate
2.6. Soluble Sugar Content Determination
2.7. Measurement of Plant Hormone Content
2.8. Root-Bleeding Sap Determination
2.9. Observation of Vascular Bundles
2.10. Gene Expression Determination
2.11. Data Analysis
3. Results
3.1. Grain Yield and Rice Quality
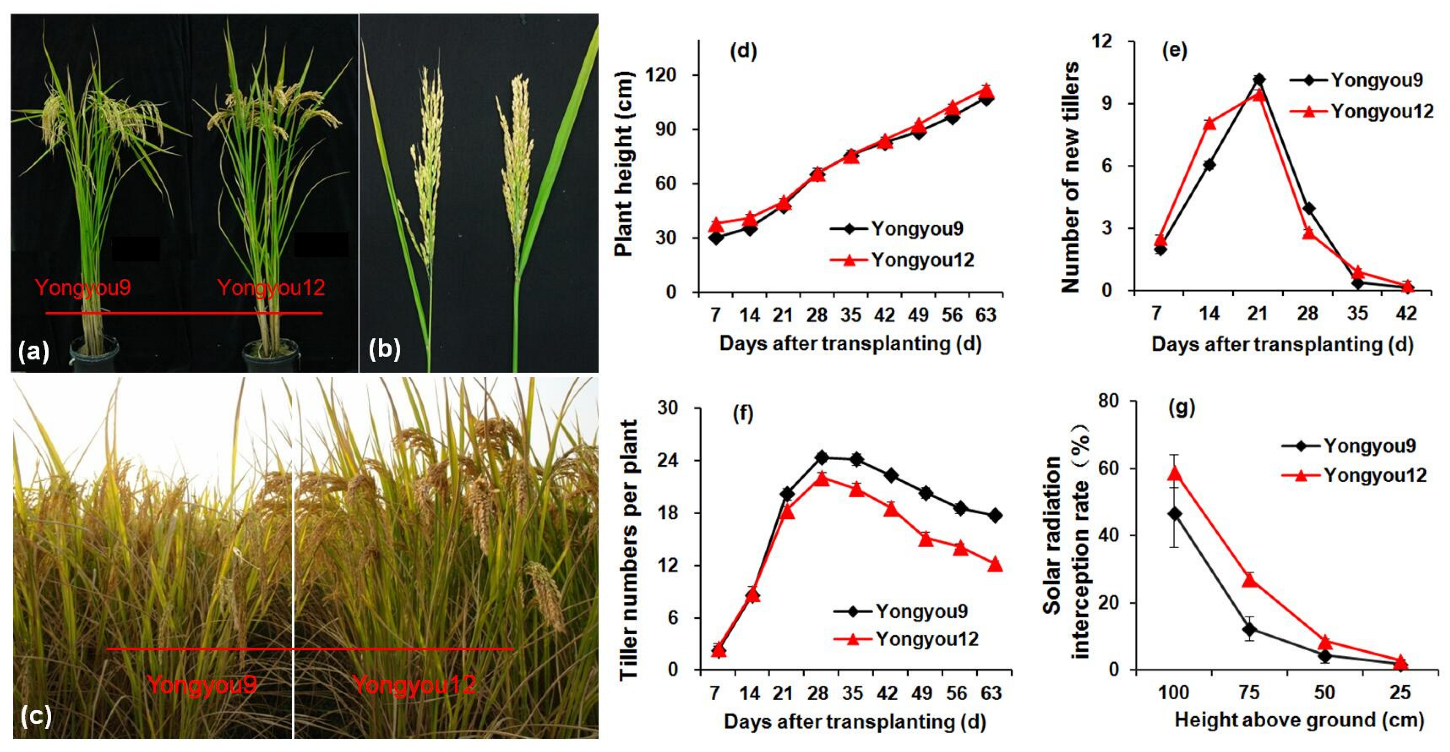
3.2. Plant Morphology and Tillering Dynamics
3.3. Production and Accumulation of Photoassimilates
3.4. Grain-Filling Characteristics
3.5. Plant Hormones
3.6. Soluble Sugar Content
3.7. Gene Expression of SUT and Cell Wall Invertase Genes
3.8. Number and Size of Vascular Bundles
3.9. Root-Bleeding Sap
3.10. Correlation of the Ratio of Panicle Weight to Total Weight/Grain Yield with the Agronomy Traits and Physiological Parameters
3.11. Distribution of Nitrogen, Phosphorus, and Potassium Content in Rice Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, S.; Ali, J.; Zhang, C.; Li, Z.; Zhang, Q. Genomic Breeding of Green Super Rice Varieties and Their Deployment in Asia and Africa. Theor. Appl. Genet. 2020, 133, 1427–1442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FSIN. Globle Report on Food Crises 2020. FSIN 2020. Rome. Available online: https://www.fsinplatfom.org (accessed on 20 January 2022).
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.B.; Mary, A.A.; Michael, J.R.; John, M.M. The science of food security. NPJ Sci. Food 2018, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Ma, G.-H.; Yuan, L.-P. Hybrid rice achievements, development and prospect in China. J. Integr. Agric. 2015, 14, 197–205. [Google Scholar] [CrossRef]
- Du, S.; Wang, S.; Li, C.; Wang, D.; Luo, Y.; Wu, S. Introduction to the progress and problems in ‘‘super’’ rice breeding. Chin. Agric. Sci. Bull. 2006, 8, 195–198. [Google Scholar]
- Liu, K.; Yang, R.; Deng, J.; Huang, L.; Wei, Z.; Ma, G.; Tian, X.; Zhang, Y. High radiation use efficiency improves yield in the recently developed elite hybrid rice Y-liangyou 900. Field Crop. Res. 2020, 253, 107804. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, S.; Man, J. Changes in rice yield and yield stability in China during the past six decades. J. Sci. Food Agric. 2020, 100, 3560–3569. [Google Scholar] [CrossRef]
- Chang, S.; Chang, T.; Song, Q.; Zhu, X.-G.; Deng, Q. Photosynthetic and agronomic traits of an elite hybrid rice Y-Liang-You 900 with a record-high yield. Field Crop. Res. 2016, 187, 49–57. [Google Scholar] [CrossRef]
- Cheung, F. Yield: The search for the rice of the future. Nature 2014, 514, S60–S61. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wu, J.; Shu, Y.; Zhou, P.; Deng, Q. High-yielding cultural techniques of super hybrid rice YLY 900 yielded 15.40 t/hm2 on a 6.84 hm2 scale. Chin. Rice 2014, 20, 1–4. [Google Scholar]
- Chen, Z.; Yang, X.; Song, W.; Khan, A.; Najeeb, U.; Li, P.; Cao, C. Water-saving cultivation plus super rice hybrid genotype improves water productivity and yield. Agron. J. 2020, 112, 1764–1777. [Google Scholar] [CrossRef]
- Liu, K.; Li, T.; Chen, Y.; Huang, J.; Qiu, Y.; Li, S.; Wang, H.; Zhu, A.; Zhuo, X.; Yu, F.; et al. Effects of root morphology and physiology on the formation and regulation of large panicles in rice. Field Crop. Res. 2020, 258, 107946. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. Morphological and physiological traits of roots and their relationships with shoot growth in ‘‘super’’ rice. Field Crop. Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Xu, K.; Zhao, K.; Sun, J.-J.; Wei, H.-H.; Xu, J.-W.; Wei, H.-Y.; Guo, B.-W.; Huo, Z.-Y.; Dai, Q.-G.; et al. Canopy Structure and Photosynthetic Characteristics of Yongyou Series of Indica-Japonica Hybrid Rice under High-yielding Cultivation Condition. Acta Agron. Sin. 2015, 41, 286–296. [Google Scholar] [CrossRef]
- Meng, T.; Wei, H.; Li, X.; Dai, Q.; Huo, Z. A better root morpho-physiology after heading contributing to yield superiority of japonica/indica hybrid rice. Field Crop. Res. 2018, 228, 135–146. [Google Scholar] [CrossRef]
- Meng, T.-Y.; Xu, J.-W.; Shao, Z.-B.; Ge, M.-J.; Zhang, H.-C.; Wei, H.-Y.; Dai, Q.-G.; Huo, Z.-Y.; Xu, K.; Guo, B.-W.; et al. Advantages and Their Formation Characteristics of the Highest Population Productivity of Nitrogen Fertilization in Japonica/Indica Hybrid Rice of Yongyou Series. Acta Agron. Sin. 2015, 41, 1711. [Google Scholar] [CrossRef]
- Wei, H.-H.; Meng, T.-Y.; Li, C.; Zhang, H.-C.; Dai, Q.-G.; Ma, R.-R.; Wang, X.-Y.; Yang, J.-W. Accumulation, Distribution, and Utilization Characteristics of Phosphorus in Yongyou 12 Yielding over 13.5 t ha−1. Acta Agron. Sin. 2016, 42, 886–897. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, T.; Feng, B.; Zhang, C.; Peng, S.; Zhang, X.; Fu, G.; Tao, L. Non-photochemical Quenching Plays a Key Role in Light Acclimation of Rice Plants Differing in Leaf Color. Front. Plant Sci. 2017, 7, 1968. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Xu, G.; Wang, Z.; Zhang, H.; Yang, J.; Zhang, J. Expression of proteins in superior and inferior spikelets of rice during grain filling under different irrigation regimes. Proteomics 2015, 16, 102–121. [Google Scholar] [CrossRef]
- Richards, F.J. A Flexible Growth Function for Empirical Use. J. Exp. Bot. 1959, 10, 290–301. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bollmark, M.; Kubát, B.; Eliasson, L. Variation in Endogenous Cytokinin Content during Adventitious Root Formation in Pea Cuttings. J. Plant Physiol. 1988, 132, 262–265. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, T.; Wang, Z.; Yang, J.; Zhang, J. Involvement of cytokinins in the grain filling of rice under alternate wetting and drying irrigation. J. Exp. Bot. 2010, 61, 3719–3733. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, L.; Wang, Z.; Yang, J.; Zhang, J. Agronomic and physiological performance of high-yielding wheat and rice in the lower reaches of Yangtze River of China. Field Crop. Res. 2012, 133, 119–129. [Google Scholar] [CrossRef]
- Chen, T.; Feng, B.; Fu, W.; Zhang, C.; Tao, L.; Fu, G. Nodes protect against drought stress in rice (Oryza sativa) by mediating hydraulic conductance. Environ. Exp. Bot. 2018, 155, 411–419. [Google Scholar] [CrossRef]
- Eom, J.-S.; Cho, J.-I.; Reinders, A.; Lee, S.-W.; Yoo, Y.; Tuan, P.Q.; Choi, S.-B.; Bang, G.; Park, Y.-I.; Cho, M.-H.; et al. Impaired Function of the Tonoplast-Localized Sucrose Transporter in Rice, OsSUT2, Limits the Transport of Vacuolar Reserve Sucrose and Affects Plant Growth. Plant Physiol. 2011, 157, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Siahpoosh, M.R.; Sanchez, D.H.; Schlereth, A.; Scofield, G.N.; Furbank, R.T.; van Dongen, J.T.; Kopka, J. Modification of OsSUT1 gene expression modulates the salt response of rice Oryza sativa cv. Taipei 309. Plant Sci. 2012, 182, 101–111. [Google Scholar] [CrossRef]
- Yan, S.; Zou, G.; Li, S.; Wang, H.; Liu, H.; Zhai, G.; Guo, P.; Song, H.; Yan, C.; Tao, Y. Seed size is determined by the combinations of the genes controlling different seed characteristics in rice. Theor. Appl. Genet. 2011, 123, 1173–1181. [Google Scholar] [CrossRef]
- Feng, B.; Li, G.; Islam; Fu, W.; Zhou, Y.; Chen, T.; Tao, L.; Fu, G. Strengthened antioxidant capacity improves photosynthesis by regulating stomatal aperture and ribulose-1,5-bisphosphate carboxylase/oxygenase activity. Plant Sci. 2019, 290, 110245. [Google Scholar] [CrossRef]
- Ishimaru, T.; Matsuda, T.; Ohsugi, R.; Yamagishi, T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Funct. Plant Biol. 2003, 30, 1139–1149. [Google Scholar] [CrossRef]
- Jiang, Q.; Du, Y.; Tian, X.; Wang, Q.; Xiong, R.; Xu, G.; Yan, C.; Ding, Y. Effect of panicle nitrogen on grain filling characteristics of high-yielding rice cultivars. Eur. J. Agron. 2016, 74, 185–192. [Google Scholar] [CrossRef]
- Zhu, Q.; Cao, X.; Luo, Y. Growth analysis on the process of grain filling in rice. Acta Agron. Sin. 1988, 14, 182–193. [Google Scholar]
- Mahadi, S.; Nulit, R.; Mohtar, M.; Ibrahim, M.; Ab Ghani, N. Synergistic effect of KCl, thiourea, GA3 and SA on the germination and early seedling growth enhancement of drought-stressed Malaysian indica rice cv. MR220. Biocatal. Agric. Biotechnol. 2020, 29, 101779. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Smith, S.M. Hormone Metabolism and Signaling in Plants; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Gong, Y.; Yang, J.; Yi, Y.; Zhang, J.; Ye, N. Expression profile of the carbon reserve remobilization from the source to sink in rice in response to soil drying during grain filling. Food Energy Secur. 2020, 9, e204. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Wang, J.; Zhang, T.; Huang, J.; Peng, S. High nitrogen input causes poor grain filling of spikelets at the panicle base of super hybrid rice. Field Crop. Res. 2019, 244, 107635. [Google Scholar] [CrossRef]
- Okamura, M.; Arai-Sanoh, Y.; Yoshida, H.; Mukouyama, T.; Adachi, S.; Yabe, S.; Nakagawa, H.; Tsutsumi, K.; Taniguchi, Y.; Kobayashi, N.; et al. Characterization of high-yielding rice cultivars with different grain-filling properties to clarify limiting factors for improving grain yield. Field Crop. Res. 2018, 219, 139–147. [Google Scholar] [CrossRef]
- Huang, M.; Lei, T.; Cao, F.; Chen, J.; Shan, S.; Zou, Y. Grain yield responses to nitrogen rate in two elite double-cropped inbred rice cultivars released 41 years apart. Field Crop. Res. 2020, 259, 107970. [Google Scholar] [CrossRef]
- Sheehy, J.; Dionora, M.; Mitchell, P. Spikelet numbers, sink size and potential yield in rice. Field Crop. Res. 2001, 71, 77–85. [Google Scholar] [CrossRef]
- Huang, M.; Zou, Y.-B.; Jiang, P.; Xia, B.; Ibrahim; Ao, H.-J. Relationship Between Grain Yield and Yield Components in Super Hybrid Rice. Agric. Sci. China 2011, 10, 1537–1544. [Google Scholar] [CrossRef]
- Hua, J.; Zhou, N.; Zhang, W.; Zhang, H.; Huo, Z.; Zhou, P.; Cheng, F.H.; Guo-Ye, L.I.; Huang, D.S.; Chen, Z.P. The structure and formation characteristics of super-high yield population with late Yongyou series of Indica-Japonica hybrid rice in double-cropping rice area. Sci. Agric. Sin. 2015, 48, 1023–1034. [Google Scholar]
- Pan, S.-G.; Huang, S.-Q.; Zhang, F.; Wang, J.-P.; Cai, M.-L.; Cao, C.-G.; Tang, X.-R.; Li, G.-X. Growth and Development Characteristics of Super-High-Yielding Mid-Season Indica Hybrid Rice. Acta Agron. Sin. 2011, 37, 537–544. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wei, H.-H.; Zhang, H.-C.; Sun, J.; Zhang, J.-M.; Li, C.; Lu, H.-B.; Yang, J.-W.; Ma, R.-R.; Xu, J.-F.; et al. Population Characteristics for Super-high Yielding Hybrid Rice Yongyou 12 (>13.5 t ha−1). Acta Agron. Sin. 2014, 40, 2149–2159. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Xiao, F.; Yang, L.; Li, G.; Ding, Y.; Paul, M.J.; Li, W.; Liu, Z. Dynamics of dry matter accumulation in internodes indicates source and sink relations during grain-filling stage of japonica rice. Field Crop. Res. 2020, 263, 108009. [Google Scholar] [CrossRef]
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2013, 65, 1713–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Pan, J.; Cui, K.; Yuan, M.; Hu, Q.; Wang, W.; Mohapatra, P.K.; Nie, L.; Huang, J.; Peng, S. Limitation of Unloading in the Developing Grains Is a Possible Cause Responsible for Low Stem Non-structural Carbohydrate Translocation and Poor Grain Yield Formation in Rice through Verification of Recombinant Inbred Lines. Front. Plant Sci. 2017, 8, 1369. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Li, H.; Wang, K.; Yang, J.; Duan, M.; Zhang, J.; Ye, N. Regulation of gene expression involved in the remobilization of rice straw carbon reserves results from moderate soil drying during grain filling. Plant J. 2019, 101, 604–618. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J. Crop management techniques to enhance harvest index in rice. J. Exp. Bot. 2010, 61, 3177–3189. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Peng, S.; Zhang, Z.; Wang, Z.; Visperas, R.M.; Zhu, Q. Grain and dry matter yields and partitioning of assimilates in japonica/indica hybrids. Crop Sci. 2002, 42, 766–772. [Google Scholar] [CrossRef]
- Xu, D.H.; Wang, X.Y.; Ma, R.R.; Yu, S.M.; Zhu, L.F.; Ouyang, Y.N.; Jin, Q.Y. Analysis on physiological properties of the heavy panicle type of indica-japonica inter-subspecific hybrid rice Yongyou 6. Sci. Agric. Sin. 2010, 43, 4796–4804. [Google Scholar]
- Yuan, L.P. Recent progress in breeding super hybrid rice in China. In Science Progress in China; Lu, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 231–236. [Google Scholar]
- Huang, Z.; Lv, Q.; Xin, Y.; Yuan, L.; Fu, X.; Zhu, L.; Wang, Z. Heterotic performance of the main yield traits in different types of Indica hybrid rice. Food Energy Secur. 2020, 9, e210. [Google Scholar] [CrossRef]











| Variety | Grain Types | A | b | k | m | R2 |
|---|---|---|---|---|---|---|
| Yongyou9 | Superior grains | 27.75 | 2.80 | 0.26 | 1.43 | 0.998 |
| Inferior grains | 23.25 | 4.73 | 0.35 | 1.77 | 0.977 | |
| Yongyou12 | Superior grains | 23.13 | 1.50 | 0.21 | 0.87 | 0.994 |
| Inferior grains | 20.42 | 1.72 | 0.16 | 0.75 | 0.984 |
| Correlated with | Ratio of Panicle Weight to Total Weight | Grain Yield |
|---|---|---|
| Agronomy traits | ||
| Dry weight | ||
| Leaves | 0.862 ** | 0.751 * |
| Sheaths and stems | 0.838 ** | 0.796 * |
| Panicle | 0.984 ** | 0.824 * |
| Total | 0.972 ** | 0.815 * |
| Ratio of panicle to total | / | 0.858 * |
| Plant height | 0.588 | 0.559 |
| Specific leaf weight | 0.406 | 0.530 |
| Physiological parameters | ||
| Net photosynthetic rate | 0.868 ** | 0.942 ** |
| Soluble sugar in panicle | 0.890 ** | 0.905 ** |
| Hormone contents | ||
| GA3 | 0.826 ** | 0.852 * |
| ZR | 0.835 ** | 0.861 * |
| IAA | 0.621 | 0.669 |
| ABA | 0.598 | 0.605 |
| Expression levels of the genes in stem | ||
| SUT1 | 0.918 ** | 0.928 ** |
| SUT2 | 0.925 ** | 0.942 ** |
| CIN2 | 0.921 ** | 0.945 ** |
| Vascular bundle characteristics | ||
| LVB/SVB numbers | 0.904 **/0.915 ** | 0.886 **/0.867 ** |
| LVB/SVB diameter | 0.922 **/0.589 | 0.895 **/0.607 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Yang, X.; Fu, W.; Li, G.; Feng, B.; Fu, G.; Tao, L. Strengthened Assimilate Transport Improves Yield and Quality of Super Rice. Agronomy 2022, 12, 753. https://doi.org/10.3390/agronomy12040753
Chen T, Yang X, Fu W, Li G, Feng B, Fu G, Tao L. Strengthened Assimilate Transport Improves Yield and Quality of Super Rice. Agronomy. 2022; 12(4):753. https://doi.org/10.3390/agronomy12040753
Chicago/Turabian StyleChen, Tingting, Xueqin Yang, Weimeng Fu, Guangyan Li, Baohua Feng, Guanfu Fu, and Longxing Tao. 2022. "Strengthened Assimilate Transport Improves Yield and Quality of Super Rice" Agronomy 12, no. 4: 753. https://doi.org/10.3390/agronomy12040753
APA StyleChen, T., Yang, X., Fu, W., Li, G., Feng, B., Fu, G., & Tao, L. (2022). Strengthened Assimilate Transport Improves Yield and Quality of Super Rice. Agronomy, 12(4), 753. https://doi.org/10.3390/agronomy12040753








