Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
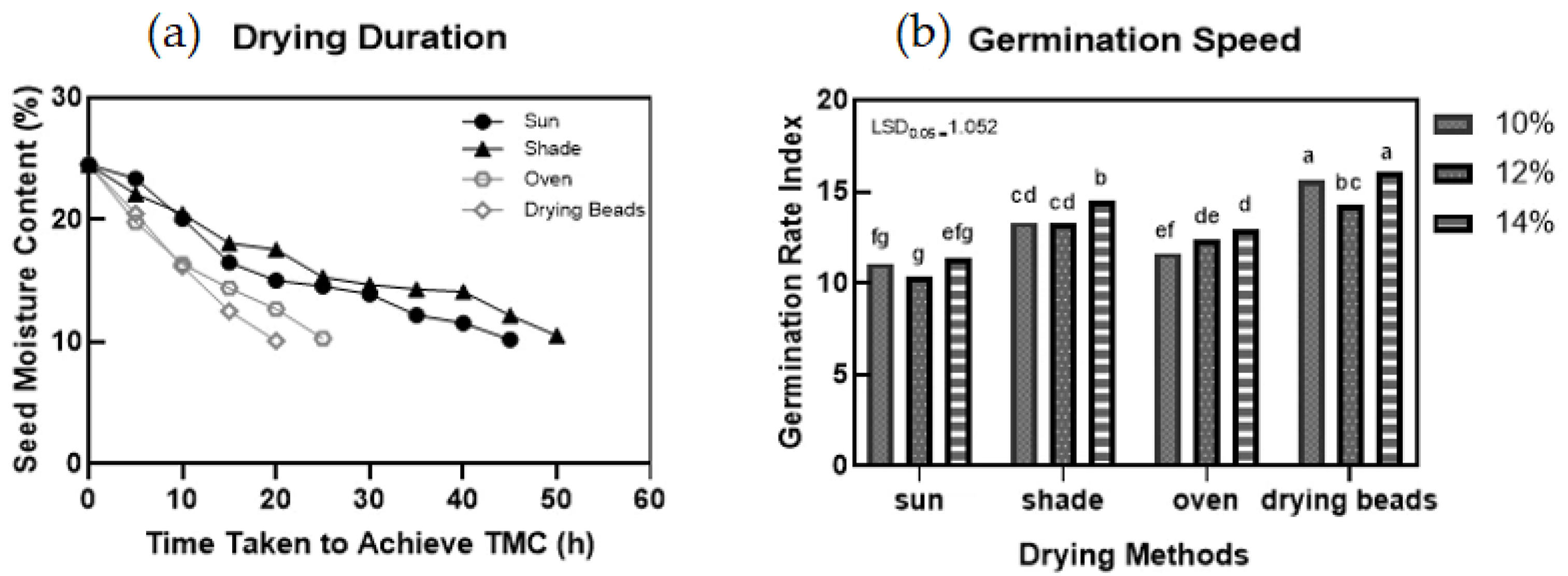
2.2. Seed-Drying Treatments
2.2.1. Seed Moisture
2.2.2. Germination and Vigour
2.2.3. Electrical Conductivity of Leachates
2.3. Post-Drying Storage Temperature
Antioxidant Enzymes
2.4. Data Analysis
3. Results
3.1. Seed and Seedling Quality Based on Different Drying Methods
- I.
- Rapid Drying
- II.
- Slow Drying
3.2. Antioxidant Enzyme
4. Conclusions
5. Future Research Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Snafi, A.E. The pharmacology and medical importance of Dolichos lablab (Lablab purpureus)—A review. IOSR J. Pharm. 2017, 7, 22–30. [Google Scholar] [CrossRef]
- UC Davis. Drying Beads Help Bangladesh Farmers Access Better Seed. 2017. Available online: https://horticulture.ucdavis.edu/blog/drying-beads-help-bangladesh-farmers-access-better-seed (accessed on 27 April 2021).
- Das, S.S.; Fakir, M.S.A. Pod growth and seed composition in two genotypes of Lablab purpureus. Legume Res.-Int. J. 2014, 37, 306–310. [Google Scholar] [CrossRef]
- TeKrony, D.M.; Egli, D.B. Accumulation of seed vigour during development and maturation. Basic Appl. Asp. Seed Biol. 1997, 30, 369–384. [Google Scholar]
- Golezani, G.K.; Mahootchy, H.A. Changes in seed vigour of faba bean (Vicia faba L.) cultivars during development and maturity. Seed Sci. Technol. 2009, 37, 713–720. [Google Scholar] [CrossRef]
- Lassim, M.B.; Chin, H.F. Some trends in the development and maturation of cowpea (Vigna unguiculata L.) seeds. Acta Hort. 1987, 215, 25–30. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Longevity, storage, and deterioration. Seeds 2013, 341–376. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef]
- Krzyzanowski, F.C.; West, S.H.; França Neto, J.D.B. Drying peanut seed using air ambient temperature at low relative humidity. Rev. Bras. Sementes 2006, 28, 1–5. [Google Scholar] [CrossRef][Green Version]
- Justice, O.L.; Bass, L.N. Principles and Practices of Seed Storage. In Agriculture Handbook; No. 506; Department of Agriculture, Science and Education Administration, U.S. Department of Agriculture: Washington, DC, USA, 1978. [Google Scholar]
- Panda, B.B.; Mohapatra, S.; Mallik, S.; Acharya, P. Effect of Tamarind seed mucilage on rheological properties: Evaluation of suspending properties. Int. Res. J. Pharm. Sci. 2010, 1, 8–10. [Google Scholar]
- Nagaveni, P.K. Effect of Storage Conditions, Packing Material and Seed Treatment on Viability and Vigour of Onion Seeds. Ph.D. Thesis, UAS, Dharwad, India, 2005. [Google Scholar]
- Jyoti; Malik, C.P. Seed deterioration. Int. J. Life Sci. Biotechnol. Pharma Res. 2013, 2, 374–385. [Google Scholar]
- Singh, A.; Abhilash, P.C. Varietal dataset of nutritionally important Lablab purpureus L. sweet from Eastern Uttar Pradesh, India. J. Data Brief 2019, 24, 103935. [Google Scholar]
- ISTA. International Rules for Seed Testing; International Seed Testing Association: Bassersdorf, Switzerland, 2016. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Manju, V.; Kumar, S. Seed leachate conductivity and its correlation with the seed viability and germination of Tnau Papaya Cv. Co8 seeds stored under different environmental conditions. Int. J. Agric. Sci. Res. IJASR 2015, 5, 127–130. Available online: http://www.tjprc.org/view-archives.php (accessed on 27 April 2021).
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Methods Enzymol. 1955, 136, 764–775. [Google Scholar]
- Nassari, P.J.; Keshavulu, K.; Manohar, R.K.; Reddy, A.R. Postharvest drying of tomato seeds to ultra low moisture safe for storage using desiccant (zeolite) beads and their effects on seed quality. Am. J. Res. Commun. 2014, 2, 74–83. [Google Scholar]
- Zaitialia, M.; Atilia, H. Evaluation on the effectiveness of rapid drying method and its effects on chilli (Capsicum annuum) seed quality. Trans. Malays. Soc. Plant Physiol. 2016, 23, 68–73. [Google Scholar]
- Ali, M.; Rahman, M.; Rahman, M.; Hossain, M.; Asaduzzaman, M. Effect of Drying Method on Quality of Soybean Seed. Bangladesh Agron. J. 2015, 18, 53–57. [Google Scholar] [CrossRef][Green Version]
- Hor, Y.L. Storage of field crop seeds under Malaysia conditions. In Proceedings of the National Seed Symposium, Serdang, Malaysia, 4–7 February 1976; pp. 124–134. [Google Scholar]
- Muckle, T.B.; Stirling, H.G. Review of the drying of cereals and legumes in the tropics. Trop. Stored Prod. Inform. 1971, 22, 11–30. [Google Scholar]
- Nautiyal, P. Seed and seedling vigor traits in groundnut (Arachis hypogaea L.). Seed Sci. Technol. 2009, 37, 721–735. [Google Scholar] [CrossRef]
- Afonso, P.C., Jr.; Corrêa, P.C. Immediate and latent drying effects of bean seeds harvested with different moisture levels. Ciênc. Agrotecnol. 2000, 24, 33–40. [Google Scholar]
- Hanapiah, N.F.H.; Sinniah, U.R.; Yusoff, M.M. Seed Quality of Lablab Bean (Lablab purpureus) as Influenced by Seed Maturity and Drying Methods. Agronomy 2022, 12, 363. [Google Scholar] [CrossRef]
- Mohammadi, H.; Soltani, A.; Sadeghipour, H.R.; Zeinali, E. Effects of seed aging on subsequent seed reserve utilization and seedling growth in soybean. Int. J. Plant Prod. 2012, 5, 65–70. [Google Scholar]
- Dona, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Carbonera, D. DNA profiling, telomere analysis and antioxidant properties as tools for monitoring ex situ seed longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Badola, H.K. Effect of storage conditions and storage periods on seed germination in eleven populations of Swertia chirayita: A critically endangered medicinal herb in Himalaya. Sci. World J. 2012, 2012, 128105. [Google Scholar] [CrossRef]
- Krishna, S.P.; Gejjalagere, H.C. Advancement of Biodegradable Polysaccharide Nanocarriers for Delivery of Herbal Extracts and Bio-Actives; CRC Press: Boca Raton, FL, USA, 2018; pp. 191–205. [Google Scholar]
- Pukacka, S.; Ratajczak, E. Antioxidative response of ascorbate-glutathione pathway enzymes and metabolites to desiccation of recalcitrant Acer saccharinum seeds. J. Plant Physiol. 2006, 163, 1259–1266. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Come, D.; Bailly, C.; Corbineau, F. Sunflower seed deterioration as related to moisture content during ageing, energy metabolism and active oxygen species scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Lehner, A.; Mamadou, N.; Poels, P.; Come, D.; Bailly, C.; Corbineau, F. Changes in soluble carbohydrates, lipid peroxidation and antioxidant enzyme activities in the embryo during ageing in wheat grains. J. Cereal Sci. 2008, 47, 555–565. [Google Scholar] [CrossRef]
- Xin, X.; Tian, Q.; Yin, G.; Chen, X.; Zhang, J.; Ng, S.; Lu, X. Reduced mitochondrial and ascorbate-glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 2014, 171, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, T.; Kanematsu, S.; Katayama, M.; Tsuji, H. Antioxidative enzymes in seedlings of Nelumbo nucifera germinated under water. Physiol. Plant. 2001, 112, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bogatek-Leszczynska, R.; Côme, D.; Corbineau, F. Changes in activities of antioxidant enzymes and lipoxygenase during growth of sunflower seedlings from seeds of different vigour. Seed Sci. Res. 2002, 12, 47. [Google Scholar] [CrossRef]
- Kandil, A.A.; Sharief, A.E.; Sheteiwy, M.S. Effect of seed storage periods, conditions and materials on germination of some soybean seed cultivars. Am. J. Exp. Agric. 2013, 3, 1020. [Google Scholar]



| Seed Drying Treatments | Seed Drying Process |
|---|---|
| Sun | Lablab bean seeds were sundried by spreading a thin layer of pods on drying mat at temperatures of 29–37 °C; 74% RH and left under the sun until TMC was achieved. Seeds were sun-dried for 5 h/day and kept in the laboratory at night and further dried on the next day. |
| Shade | Lablab bean seeds were placed under 50% shade by spreading a thin layer on drying mat at temperature of 27 °C; 74% RH until TMC was achieved. Seeds were shade dried for 5 h/day and kept in the laboratory at night and further dried the next day. |
| Oven | Lablab bean seeds were dried under low temperature oven drying, 39 °C, until TMC was achieved. |
| Drying beads | Lablab bean seeds were dried using drying beads at 1:1 bead to seed ratio in airtight plastic containers. Seeds were placed in a container with the beads and left to dry. |
| Source of Variation | Germination (%) | Seedling Vigour Index |
|---|---|---|
| Drying methods | ** | ** |
| Sun | 43.89 c | 12.95 c |
| Shade | 67.78 a | 18.08 b |
| Oven | 58.89 b | 12.24 c |
| Drying beads | 70.56 a | 20.46 a |
| Seed moisture content (%) | ** | ** |
| 10 | 70.83 a | 17.76 a |
| 12 | 58.75 b | 15.84 b |
| 14 | 51.25 c | 14.20 b |
| Drying methods × Seed Moisture Content | ns | ns |
| Source of Variation | Germination (%) | Seedling Vigour Index | Coefficient Velocity of Germination |
|---|---|---|---|
| Storage temperature | ** | ** | * |
| Ambient | 50.67 b | 8.67 b | 16.26 b |
| Refrigerator | 68.89 a | 17.49 a | 21.01 a |
| Seed moisture content (%) | ** | ** | ns |
| 10 | 62.67 a | 14.92 a | 19.593 a |
| 12 | 62.66 a | 13.46 a | 18.57 a |
| 14 | 54 b | 10.87 b | 17.75 a |
| Storage temperature × Seed moisture content | ns | ns | ns |
| Storage Temperature | Seed Moisture Content (%) | CAT Activity (μmol/min/mg FW) | POD Activity (μmol/min/mg FW) |
|---|---|---|---|
| Ambient | 10 | 0.38 c | 0.095 c |
| 12 | 0.38 c | 0.097 c | |
| 14 | 0.35 c | 0.091 c | |
| Refrigerator | 10 | 1.76 a | 0.26 b |
| 12 | 1.23 b | 0.31 b | |
| 14 | 1.16 b | 0.50 a | |
| Storage temperature × Seed moisture content | ** | ** | |
| LSD 0.05 | 0.11 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahaya, A.M.; Sinniah, U.R.; Misran, A. Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature. Agronomy 2022, 12, 699. https://doi.org/10.3390/agronomy12030699
Yahaya AM, Sinniah UR, Misran A. Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature. Agronomy. 2022; 12(3):699. https://doi.org/10.3390/agronomy12030699
Chicago/Turabian StyleYahaya, Aliyah Mohd, Uma Rani Sinniah, and Azizah Misran. 2022. "Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature" Agronomy 12, no. 3: 699. https://doi.org/10.3390/agronomy12030699
APA StyleYahaya, A. M., Sinniah, U. R., & Misran, A. (2022). Seed Quality of Lablab Beans (Lablab purpureus L.) as Influenced by Drying Methods and Storage Temperature. Agronomy, 12(3), 699. https://doi.org/10.3390/agronomy12030699






