Abstract
Background: The conventional fertilization regime ignores the allometric characteristics of Quercus nuttallii seedlings, challenging them to meet the nutritional needs at each growth stage. This study was conducted to determine the impact of exponential fertilization on the growth and nutrient status of Q. nuttallii container seedlings. Methods: Two fertilization regimes (average/exponential) were performed on Q. nuttallii container seedlings, and varied gradients (0, 300, 500, 700, and 900 mg/seedling) of N supply were set for the two regimes. Result: N application promoted the seedling height, root collar diameter, total biomass, and N/P/K accumulation of Q. nuttallii, and the exponential fertilization obtained better effects in general. An appropriate amount of exponentially N application was beneficial to the growth of root system, whereas excessive dosages inhibited it. Judging from seedlings growth status and nutrient accumulation, 900 mg/seedling under exponential fertilization was adequate for Q. nuttallii seedlings. However, 500 and 700 mg/seedling under exponential fertilization may have advantages in improving seedlings’ stress resistance. Conclusions: Exponential fertilization did not only meet the nutrient requirements and promote the growth of Q. nuttallii seedlings, but also facilitated the root growth to indirectly accelerate the assimilation of N/P/K, therefore improving the afforestation quality.
1. Introduction
Nutritional elements are the basis of various metabolic activities in plants. Fertilization can supplement the nutrient elements and maintain soil fertility, thereby promoting rapid growth and enhancing the quality of seedlings [1,2]. One purpose of plants nutrition research is to provide sufficient nutrients through a reasonable fertilization strategy, create a favorable nutritional environment, and ultimately increase plant yields. The conventional fertilization regime usually adopts one-time fertilization or applies the same amount of fertilizer from the beginning to the end of the seedling growth stages. However, researchers gradually notice that this regime ignores the allometric characteristics of seedlings, challenging them to meet the nutritional needs at each growth stage, and it may also lead to waste of fertilizer and deterioration of soil physical and chemical properties [3,4]. In 1986, Ingestad et al. [5,6] established the “Exponential nutrient loading theory” based on the research of steady-state mineral nutrition. Subsequently, the “Exponential fertilization regime” was proposed by Timmer [7]. With the increasing fertilizer application, the relationship between plant nutrient status and fertilizer dose began with nutrient deficiency, followed by luxury consumption, and finally poison plants [5,8,9]. Exponential fertilizer loading synchronizes with each exponential growth phase by exponentially increasing nutrient application, which induces steady-state luxury nutrient consumption in plants. The exponential fertilization regime was successively tested and conducted in dozens of tree species, such as Tsuga heterophylla, Picea mariana, P. glauca, Pseudotsuga menziesii, and Pinus monticola [10,11,12,13,14]. Furthermore, studies revealed that exponential fertilization could meet the nutrient requirements of seedlings in different growth stages, especially in the fast-growing stages, directly promoted seedling growth, and improved the stress resistance to adversity; therefore, the survival rate of afforestation and forest productivity has been significantly enhanced.
Quercus nuttallii is a deciduous tree, belonging to the family Fagaceae. It has strong resistance to environmental extremes and high ornamental value [15,16]. In recent years, there have been many studies on the biological and ecological characteristics of Q. nuttallii. For example, Qu et al. [17] reported that planting Q. nuttallii has a good remediation effect on soil contaminated by heavy metals. Tang et al. [18] analyzed the photosynthetic physiology of Q. nuttallii, and the results revealed that Q. nuttallii is a fast-growing tree with high photosynthetic capacity. McCurry et al. [19] demonstrated that the survival rate of Q. nuttallii seedlings after 30 days of flooding reached 89%. Dey et al. [20] found that interplanting Q. nuttallii in young plantations of pioneer tree species, such as Populus deltoides, could simulate natural succession and accelerate the formation of forests. Baietto et al. [21] used varied fungi to test the wood preservative properties of Q. nuttallii, and the results showed that Q. nuttallii sustained high levels of decay, and it might be suitable for paper making. With the deepening of research, the practical value and cultivation technology of Q. nuttallii has been gradually recognized, and the planting number increased rapidly. Nevertheless, the quality of seedlings is uneven, which causes the afforestation effect of Q. nuttallii to benbarely satisfactory. Nutritional management at the seedling stage is a critical technical link that affects nursery stock quality, and high quality out-planted seedlings are the material basis for the cultivation of Q. nuttallii plantations. Consequently, it is urgent to clearly understand the responses of growth and nutritional status of Q. nuttallii to fertilizer loading. At present, there are few reports on the research of seedling fertilization of Q. nuttallii [22].
Nitrogen is one of the three essential nutrient elements for plant growth, as a source of fertilizer, nitrogen is absorbed by roots, transported and assimilated, and then applied to physiological and metabolic activities in the plant [23,24]. A reasonable supply of nitrogen can significantly promote photosynthesis and carbohydrate accumulation, and the level of nitrogen content has a direct impact on nursery stock quality [25]. Therefore, this experiment intends to arrange different regimes (average fertilization and exponential fertilization) and different amounts of nitrogen fertilizer treatments on the 1a container seedlings of Q. nuttallii, and to comprehensively analyze the effects of fertilization regimes and fertilizer gradients on the growth and nutrient status of Q. nuttallii container seedlings. We attempt to provide a theoretical and practical basis for precise fertilization and efficient cultivation of Q. nuttallii container seedlings.
2. Materials and Methods
2.1. Site Conditions and Experimental Material
The site of experiment located in the Shanghai Forestry Germplasm Resource Base (31°23′ N, 121°19′ E), Shanghai, China, which belongs to the northern subtropical monsoon climate. The average temperature, hours of sunshine, and rainfall per year were 16.6 °C, 1900 h, and 1100 mm, respectively.
On 1 April 2018, the seeds of Q. nuttallii imported from North America were sown in white non-woven container (diameter × height: 10 cm × 20 cm) filled with a mixture of peat, perlite, and organic fertilizer (v/v/v: 7:2:1). Container seedlings were placed outdoors. Fertilization experiment started when the Q. nuttallii seedlings height reached about 30 cm (early June).
2.2. Experimental Design
Two fertilization regimes (average/exponential fertilization) were conducted, and varied gradients (300, 500, 700, and 900 mg/seedling) of N supply were set for two regimes, which were denoted as C/E300, C/E500, C/E700, and C/E900. The control group (CK) was treated without fertilizer. Three replicates were set for each fertilizer treatment with 30 Q. nuttallii container seedlings. The average fertilization adopted the equal fertilization strategy, and the exponential fertilization experiment used the exponential fertilization model [7] to calculate the corresponding weekly nutrient amount:
where NT is the total amount of nitrogen applied, NS is the initial seedlings nitrogen reserve, Nt is the nitrogen applied at the time of t-th fertilization, NS is the initial level of N in whole plant, and r is the relative addition rate of nitrogen. NS was 141.83 mg/seedling in this experiment.
NT = NS (erT − 1)
Nt = NS (ert − 1) − Nt−1
The nitrogen fertilizer used in this experiment was urea (N ≥ 46.4%). The fertilization interval was 7 days, and 15 times of fertilizer applications were conducted. With the water-soluble regime, each seedling was applied with 20 mL solution at a time, and the specific nitrogen application amount was shown in Table 1. In order to avoid possible P and K deficiency, each fertilization treatment was given the same amount of phosphate fertilizer and potassium fertilizer (300 mg/seedling, total application amount). Phosphate fertilizer was superphosphate (available phosphorus ≥ 12%), and potassium fertilizer was potassium sulfate (potassium oxide ≥ 50%).

Table 1.
The design of fertilizer treatments.
2.3. Seedling Samplings
When seedlings growth nearly stopped (7 November), six seedlings with good vigor were sampled for each repetition to measure the seedling height and root collar diameter (RCD) with steel tape (0.1 cm accuracy) and vernier calipers (0.01 mm accuracy). After destructively harvesting, we washed the samples with water, cut off the roots, stems, and leaves, and then put them into ovens with envelopes. The oven was initially set at 105 °C for 30 min to kill them and was subsequently set to 70 °C for 3 days to dry them to a constant weight. The root biomass and total biomass were measured with an electronic balance (0.01 g accuracy). WinRHIZO PRO 2007 was used to analyze the root system indexes.
The dried roots, stems, and leaves were crushed separately, sieved by a 0.5 mm mesh sieve, and stored in a desiccator for later use. The plant material was wet digested in a block digester using H2SO4-HClO4 solution. The digest was analyzed for nitrogen by indophenol blue colorimetric regime (LY/T 1269-1999), phosphorus by molybdenum antimony anti-colorimetric regime (NY/T 2421-2013), and potassium by atomic absorption spectrometer and flame spectrophotometer regime (LY/T 1270-1999). The nutrient mass fraction of roots, stems and leaves were obtained by above methods.
Nutrient content of roots, stems, and leaves (mg/seedling) = nutrient mass fraction of roots, stems, and leaves × corresponding biomass.
Total plant nutrient content (mg/seedling) = root nutrient content + stem nutrient content + leaf nutrient content.
2.4. Statistical Analysis
Excel 2016 and Origin 2018 were adopted for processing data and figures. SPSS 23.0 was used for analysis of variance (ANOVA) and Duncan’s multiple range test. p values less than 0.05 were considered to indicate significance between groups.
3. Results
3.1. Seedling Height, Root Collar Diameter, Total Biomass, and Proportion of the Plant Structure
Seedling height directly reflects the growth status of Q. nuttallii. As shown in Figure 1a, treatments all promoted the growth of seedling height, and were significantly different from CK (p < 0.05), the maximum value occurred in E900 (95.94 cm).
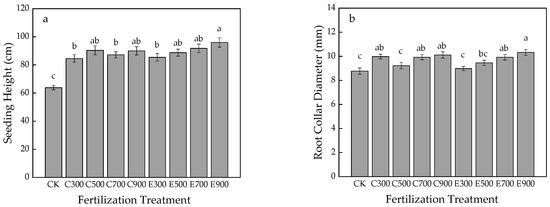
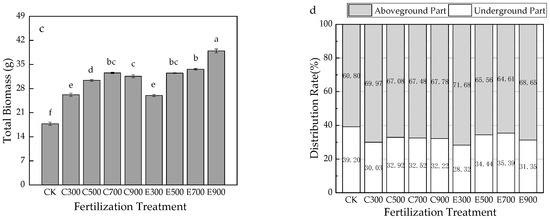
Figure 1.
Effects of fertilization on seedling height (a), root collar diameter (b), total biomass (c), and proportion of the plant structure (d) of Q. nuttallii container seedlings. Lowercase letters represent significance at 0.05 level and the same letter indicates no significant difference in full text.
Figure 1b indicated that all fertilizer amounts can promote root collar diameter indicators. Except for C500 and E300, the discrepancies between other treatments and CK were significant (p < 0.05). Nevertheless, no significant difference occurred in height and RCD indexes between regimes.
As shown in Figure 1c, the total biomass of all treatments was significantly higher than that of CK (p < 0.05). Under the same fertilizer supply (except 300 mg/seedling), the biomass under exponential fertilization was bigger than average fertilization occurred, and the maximum value was presented at E900 (38.95 g).
Developed root system is conducive to seedling stress resistance. Figure 1d displays that CK (39.20%) obtained the largest proportion of underground biomass, and E300 (28.32%) was the smallest. The average proportion of underground biomass between exponential fertilization and average fertilization was 32.34% and 31.92%, respectively.
3.2. Root System
The total root length of E700 was the largest (639.71 cm), which was 3.69 times that of CK. Except for C300, C500, C700, and E900, other treatments had a striking discrepancy with CK (p < 0.05). Fertilization boosted root elongation, and exponential fertilization obtained more prominent effects than average fertilization (Figure 2a).
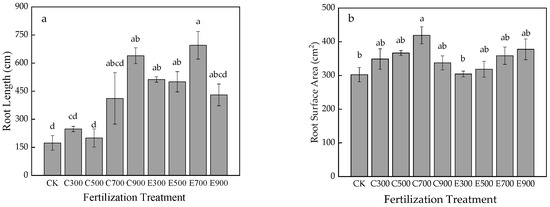
Figure 2.
Effects of fertilization on root system of Q. nuttallii container seedlings (a–c), and root morphology of each treatment (d). Lowercase letters represent significance at 0.05 level and the same letter indicates no significant difference in full text.
As shown in Figure 2b, C700 (419.25 cm2) had the largest root surface area, which was 1.44 times that of CK. The root surface area under fertilization was higher than CK presented. This indicated that the application of N fertilizer had positive impacts on the root surface area.
N application promoted the root biomass accumulation (Figure 2c). The underground biomass of CK was the smallest, and evident differences occurred between treatments and CK with the exception of E300 (p < 0.05). Under the same fertilizer amount (except 300 mg/seedling), exponential fertilization better facilitated the root biomass than average fertilization. Additionally, E900 (12.21 g) reached the maximum value. The root morphology of each treatment is clearly displayed in Figure 2d.
3.3. N, K, and P Mass Fraction
Compared to CK, the N mass fraction under fertilization increased by 35.7–238.8% in roots, 39.4–145.4% in stems, and 32.9–103.8% in leaves. The discrepancies between exponential fertilization treatments and CK were significant in each organ (p < 0.05), and N fertilization had a promoting impact on the N mass fraction of Q. nuttallii. Furthermore, in roots and leaves, the effects of exponential fertilization were obviously better than those of average fertilization (Table 2).

Table 2.
Effects of fertilization on K, P, and K mass fraction of roots, stems, and leaves of Q. nuttallii container seedlings.
The P mass fraction under fertilization reduced by 29.1–58.6% in roots compared to CK. In stems, each treatment was slightly lower than CK but not obvious. In leaves, C500 reduced by 19.5% compared to CK; nonetheless, the other fertilization treatments increased by 7.5–66.0%. The P mass fraction decreased by N fertilizer application in the roots and stems while it increased in leaves.
The K mass fraction in roots of treatment groups was 18.7–35.1% lower than that of CK (p < 0.05). In stems, treatments (except C300) decreased by 7.7–57.3% compared to CK. In leaves, C300 and C500 groups were 29.3% and 5.3% higher than that of CK, respectively, and the rest of the treatments decreased by 1.0–11.8%. To conclude, N application reduced the K mass fraction in each organ.
3.4. N, P, and K Contents
Table 3 shows the N, P, and K contents of seedlings. Regardless of the whole seedling or in the roots, the N content of E900 was the largest, and exponential fertilization treatments were varied greatly from CK (p < 0.05). N accumulated in each organ with the increase of fertilizer application. In addition, under the same fertilizer amount, exponential fertilization treatments obtained preferable effects with the exception of stems (p < 0.05).

Table 3.
Effects of fertilization on K, P, and K contents of roots, stems, and leaves of Q. nuttallii container seedlings.
In the whole seedling and leaves, the maximum value of P contents occurred in E900. On the contrary, the P content of CK was the largest in the roots. N fertilization facilitated P accumulation in the whole seedling and leaves of Q. nuttallii, and exponential fertilization was the better regime. N application had an inhibitory impact on P accumulation in roots and stems.
The K content in the whole seedling showed an upward trend with the fertilizer application, but there was no considerable difference in the effects caused by average and exponential fertilization.
4. Discussion
The purpose of fertilization is to improve the quality of seedlings, and seedling height and root collar diameter are vital indicators to measure the quality of seedlings, which can reflect the impacts of fertilization from an indirect perspective [26,27]. Chen et al. [28] found that the root collar diameter and the shoot height of Betula alnoides rapidly increased with time after exponential fertilization. Pokhareal et al. [29] pointed out that both exponential fertilization and nursery weeding could significantly promote the growth of plant height and ground diameter of P. banksiana, as well as the accumulation and utilization of nutrients. The conclusions obtained in this research are similar to the above findings. With the increase of fertilizer dosage, the seedling height and root collar diameter of Q. nuttallii rose remarkably and reached the maximum value in E900. The results indicated that fertilization could promote the growth of seedling height and root collar diameter significantly. When the fertilizer amount was large, the growth of seedling height and root collar diameter showed a trend of slowing down or even decreasing under the average fertilization regime. However, the exponential fertilization regime did not act like this, which may be the result of the nitrogen nutrient supply rate of the exponential fertilization regime, which was synchronized with the seedling growth and met the nutrient demand of seedling in each growth stages [30,31].
Biomass could reflect the productivity of seedlings, and N fertilizer could affect the accumulation and distribution of biomass in plants [32,33]. The application of nitrogen fertilizer promoted the biomass accumulation in Q. coccifera [34]. The seedling biomass of Carya illinoensis firstly increased and then decreased (nitrogen application ≥ 600 mg/seedling) with increasing nitrogen fertilizer application [35]. Tim [36] also got similar results in the study of Panicum virgatum. In the current study, the total biomass of treatments was significantly increased when compared to CK, disclosing that nitrogen application could evidently enhance seedling biomass accumulation. This might be connected to the increase of leaf chlorophyl content due to nitrogen fertilization, which accelerated the production and operation of photosynthetic products [2,37]. Furthermore, exponential fertilization improved the utilization efficiency of fertilizer nutrients, and the biomass indicators obtained were higher than those of average fertilization. However, there was no obvious process of biomass increase and then decrease in both the average and exponential fertilization regimes, considering it may be related to the discrepancies of experiment materials, or the fertilizer amount did not exceed the nutrient demand of seedlings. Seedling robustness is a vital indicator related to stress resistance, and seedlings with developed root system are more likely to survive [38,39]. Though N fertilization increase the biomass of Q. nuttallii, it reduced the proportion of underground biomass in this study. However, it is worth noting that the average proportion of underground biomass of exponential fertilization was bigger than average fertilization, and the medium concentration of N application (E500 and E700) obtained preferable impact. Therefore, an appropriate N fertilizer application may increase the total biomass while minimizing the sacrifice of plant stress resistance of Q. nuttallii seedlings.
As an essential organ to absorb nutrients, the root is closely correlated with the growth, development, and yield of the aboveground part of plants. A developed root system is an imperative guarantee for maintaining productivity [40,41,42]. In the present experiment, the root morphology, total root length, and root biomass of Q. nuttallii apparently increased after fertilizer addition. However, some indexes showed a decreasing trend exceeding 700 mg/seedling, especially under the average fertilization regime. These results indicated that appropriate nitrogen fertilization could promote root growth and morphogenesis of the root system, and that excessive nitrogen supply restrained root growth. A similar result was also reported in the study of Larix kampferi by Qu et al. [43]. On the other hand, nitrogen fertilization also directly inhibited the root growth of some plants such as Populus deltoides [44] and Fraxinus mandshuric [45] without the process of promotion. The phenomenon of promoting first and then inhibiting did not occur under exponential fertilization, which might be because the nutrient supply of the exponential fertilization was more in accordance with the fertilizer demand of roots, and no excessive nutrients poisoned the roots.
Nitrogen is a mineral element that has the most significant impact on plant growth. Absorption and utilization of nitrogen is a complex physiological and biochemical reaction in plants, reflecting different morphological and physiological characteristics [46,47,48]. Fertilization can promote the accumulation of nutrients in plants and consequently helps to improve the growth and stress resistance [49,50]. Previous studies have shown that nitrogen fertilizer facilitated N concentration and accumulation in organs of P. tabulaeformis [51], and N, P, and K accumulation in organs of Cunninghamia lanceolata [52] and Betula alnoides [28]; furthermore, the effects of exponential nitrogen fertilization were better than those of average nitrogen fertilization. This kind of positive correlation was also detected in this study, specifically, the accumulation of N, P, and K in container seedlings of Q. nuttallii rose with the increase of the nitrogen addition (except P in root). At this time, Q. nuttallii seedlings were in a stage of luxuriant nutrient consumption, and no nutrient toxicity was observed in all treatments. Nevertheless, the research of Timmer et al. [7] and Salifu et al. [53] showed that the nutrient accumulation and biomass of plants under exponential fertilization ascended first then descended with increasing of nitrogen addition. Different varieties or the differences in experiment methods, such as the type of substrate, the action of microorganisms, and the nutrient composition of fertilizers, explained this divergence [54,55,56]. Various nitrogen supplies were capable of affecting the absorption of mineral elements by plants. Just like Dalbergia odorifera [30], the mass fractions of P and K in Q. nuttallii also tended to decrease after nitrogen application; however, it was contrary to the conclusion reached by Populus tomentasa [57] and Podocarpus macrophyllus [58]. This diversity is notable, considering that the biomass of Q. nuttallii increased too fast under fertilization, and although nitrogen addition promoted the accumulation of P and K in seedlings, the supply of P and K from the substrate might be insufficient, which led to above results.
Correlation analysis was conducted to determine interactions of growth and nutrient status (Table 4). Total biomass was significantly positively correlated with root length, root surface area, and root biomass (p < 0.01), suggesting the increase of root parameters enhanced the total biomass of Q. nuttallii. Moreover, growth-related indexes were positively correlated with the contents of N, P, and K. Nitrogen addition not only increased the accumulation of N, P, and K of Q. nuttallii, but also facilitated the biomass of different organs, the growth of seedling height, and the root collar diameter. It may be indicated that the content of mineral elements exists a mutually promoting relationship with the growth of Q. nuttallii seedlings.

Table 4.
Correlation analysis of growth and nutrient status.
5. Conclusions
In summary, fertilization promoted the growth of seedling height, root collar diameter, biomass, and the N, P, and K accumulation of the annual container seedlings of Q. nuttallii. Further analysis illustrated that the performance of exponential fertilization regime was better than average fertilization regime.
Judging from growth status and nutrient accumulation, 900 mg/seedling under exponential fertilization (E900) was adequate for Q. nuttallii seedlings. However, E500 and E700 may have advantages in improving seedlings’ stress resistance. The treatment of 900 mg/seedling was also the maximum fertilizer amount in this experiment, so it could not be ascertained that the above fertilizer amount was optimum for the growth of Q. nuttallii, and the setting of nitrogen fertilization gradient has limitations. Questions of how much fertilizer will cause toxic effects, or what kind of toxic effects will be made to seedlings by excessive fertilization, remain to be studied.
Author Contributions
Conceptualization, M.N. and F.Y.; methodology, M.N.; software, M.N.; validation, F.Y.; formal analysis, M.N.; investigation, M.N., Z.G., H.C. and C.C.; resources, F.Y.; data curation, M.N.; writing—original draft preparation, M.N.; writing—review and editing, M.N.; visualization, M.N.; supervision, F.Y.; project administration, F.Y.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Special Innovative Project of Forest Science and Technology in Jiangxi Province, grant number: (2021)16; Joint Research Project based on Cooperative Program for Bachelor of Science in Forestry by Nanjing Forestry University and University of British Columbia; And A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Haque, M.M.; Biswas, J.C.; Islam, M.R.; Islam, A.; Kabir, M.S. Effect of long-term chemical and organic fertilization on rice productivity, nutrient use-efficiency, and balance under a rice-fallow-rice system. J. Plant Nutr. 2019, 42, 2901–2914. [Google Scholar] [CrossRef]
- Duan, M.; Chang, S.X. Nitrogen fertilization improves the growth of lodgepole pine and white spruce seedlings under low salt stress through enhancing photosynthesis and plant nutrition. For. Ecol. Manag. 2017, 404, 197–204. [Google Scholar] [CrossRef]
- Xue, C.X.; Zhang, T.T.; Yao, S.B.; Guo, Y.J. Effects of households’ fertilization knowledge and technologies on over-fertilization: A case study of grape growers in Shaanxi, China. Land 2020, 9, 321. [Google Scholar] [CrossRef]
- Ingestad, T.; Lund, A.B. Theory and techniques for steady state mineral nutrition and growth of plants. Scand. J. For. Res. 1986, 1, 439–453. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, F.; RöMheld, V.; Horlacher, D.; Schulz, R.; Böning-Zilkens, M.; Wang, P.; Claupein, W. Synchronizing N supply from soil and fertilizer and n demand of winter wheat by an improved Nmin regime. Nutr. Cycl. Agroecosys. 2006, 74, 91–98. [Google Scholar] [CrossRef]
- Ingestad, T. New concepts on soil fertility and plant nutrition as illustrated by research on forest trees and stands. Geoderma 1987, 40, 237–252. [Google Scholar] [CrossRef]
- Timmer, V.R.; Miller, B.D. Effects of contrasting fertilization and moisture regimes on biomass, nutrients, and water relations of container grown red pine seedlings. New For. 1991, 5, 335–348. [Google Scholar] [CrossRef]
- Timmer, V.R. Exponential nutrient loading: A new fertilization technique to improve seedling performance on competitive sites. New For. 1996, 13, 275–295. [Google Scholar]
- Salifu, K.F.; Timmer, V.R. Optimizing nitrogen loading of Picea mariana seedlings during nursery culture. Can. J. For. Res. 2003, 33, 1287–1294. [Google Scholar] [CrossRef]
- Hawkins, B.J.; Burgess, D.; Mitchell, A.K. Growth and nutrient dynamics of western hemlock with conventional or exponential greenhouse fertilization and planting in different fertility conditions. Can. J. For. Res. 2005, 35, 1002–1016. [Google Scholar] [CrossRef]
- Quoreshi, A.M.; Timmer, V.R. Exponential fertilization increases nutrient uptake and ectomycorrhizal development of black spruce seedlings. Can. J. For. Res. 1998, 28, 674–682. [Google Scholar] [CrossRef]
- Mcalister, J.A.; Timmer, V.R. Nutrient enrichment of white spruce seedlings during nursery culture and initial plantation establishment. Tree Physiol. 1998, 18, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, D. Western hemlock and Douglas-fir seedling development with exponential rates of nutrient addition. For. Sci. 1991, 37, 54–67. [Google Scholar]
- Dumroese, R.K.; Page-Dumroese, D.S.; Salifu, K.F.; Jacobs, D.F. Exponential fertilization of Pinus monticola seedlings: Nutrient uptake efficiency, leaching fractions, and early outplanting performance. Can. J. For. Res. 2005, 35, 2961–2967. [Google Scholar] [CrossRef] [Green Version]
- Stanturf, J.A.; Conner, W.H.; Gardiner, E.S.; Schweitzer, C.J.; Ezell, A.W. Recognizing and overcoming difficult site conditions for afforestation of bottomland hardwoods. Ecol. Restor. 2004, 22, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Larsen, D.R.; Dey, D.C.; Faust, T. A stocking diagram for midwestern eastern cottonwood-silver maple-american sycamore bottomland forests. North. J. Appl. For. 2010, 27, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Ma, C.; Xiao, J.; Li, X.G.; Wang, S.F.; Chen, G.C. Co-planting of Quercus nuttallii, Quercus pagoda with Solanum nigrum enhanced their phytoremediation potential to multi-metal contaminated soil. Int. J. Phytoremediat. 2021, 23, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Zhou, B.Z.; Zhou, Y. Photo-physiological and photo-biochemical characteristics of several herbaceous and woody species based on FvCB model. J. Appl. Ecol. 2017, 28, 1482–1488. (In Chinese) [Google Scholar]
- Mccurry, J.R.; Gray, M.J.; Mercker, D.C. Early growing season flooding influence on seedlings of three common bottomland hardwood species in western tennessee. J. Fish Wildl. Manag. 2010, 1, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Dey, D.C.; Gardiner, E.S.; Kabrick, J.M.; Stanturf, J.A.; Jacobs, D.F. Innovations in afforestation of agricultural bottomlands to restore native forests in the eastern USA. Scand. J. For. Res. 2010, 25, 31–42. [Google Scholar] [CrossRef]
- Baietto, M.; Wilson, A.D. Relative in vitro wood decay resistance of sapwood from landscape trees of southern temperate regions. Hortic. Sci. 2010, 45, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Taylor, T.S.; Loewenstein, E.F.; Chappelka, A.H. Effect of animal browse protection and fertilizer application on the establishment of planted Nuttall oak seedlings. New For. 2006, 32, 133–143. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Juliette, A.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105–116. [Google Scholar] [CrossRef]
- Thilakarathna, S.K.; Hernandez-Ramirez, G.; Puurveen, D.; Kryzanowski, L.; Lohstraeter, G.; Powers, L.A.; Quan, N.Y.; Tenuta, M. Nitrous oxide emissions and nitrogen use efficiency in wheat: Nitrogen fertilization timing and formulation, soil nitrogen, and weather effects. Soil Sci. Soc. Am. J. 2020, 84, 1910–1927. [Google Scholar] [CrossRef]
- Högberg, P.; Näsholm, T.; Franklin, O.; Högberg, M.N. Tamm Review: On the nature of the nitrogen limitation to plant growth in fennoscandian boreal forests. For. Ecol. Manag. 2017, 403, 161–185. [Google Scholar] [CrossRef] [Green Version]
- Valinger, E.; Sjögren, H.; Nord, G.; Cedergren, J. Effects on stem growth of Scots pine 33 years after thinning and/or fertilization in northern Sweden. Scand. J. For. Res. 2019, 34, 33–38. [Google Scholar] [CrossRef]
- Castro-Garibay, S.L.; Aldrete, A.; López-Upton, J.; Ordáz-Chaparro, V.M. Effect of container, substrate and fertilization on Pinus Greggii Var. australis growth in the Nursery. Agrociencia 2018, 52, 115–127. [Google Scholar]
- Chen, L.; Wang, C.S.; Dell, B.; Zhao, Z.G.; Guo, J.J.; Xu, D.P.; Zeng, J. Growth and nutrient dynamics of Betula alnoides seedlings under exponential fertilization. J. For. Res. 2018, 29, 111–119. [Google Scholar] [CrossRef]
- Pokharel, P.; Kwak, J.H.; Chang, S.X. Growth and nitrogen uptake of jack pine seedlings in response to exponential fertilization and weed control in reclaimed soil. Biol. Fert. Soils 2017, 53, 701–713. [Google Scholar] [CrossRef]
- Li, X.W.; Gao, Y.; Wei, H.Y.; Xia, H.T.; Chen, Q.X. Growth, biomass accumulation and foliar nutrient status in fragrant rosewood (Dalbergia odorifera T.C. Chen) seedlings cultured with conventional and exponential fertilizations under different photoperiod regimes. Soil Sci. Plant Nutr. 2017, 63, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.T.; Chu, M.Y.; Lin, Y.S.; Kung, K.N.; Lin, W.C.; Lee, M.J. Root traits and biomechanical properties of three tropical pioneer tree species for forest restoration in landslide areas. Forests 2020, 11, 179. [Google Scholar] [CrossRef] [Green Version]
- Weiner, J. Allocation, plasticity and allometry in plants. Perspect. Plant Ecol. 2004, 6, 207–215. [Google Scholar] [CrossRef]
- Hedwall, P.O.; Gong, P.C.; Ingerslev, M.; Bergh, J. Fertilization in northern forests—Biological, economic and environmental constraints and possibilities. Scand. J. For. Res. 2014, 29, 301–311. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Peñuelas, J.L.; Nicolás-Peragón, J.L.; Benito, L.F.; Domínguez-Lerena, B.S. Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? New For. 2013, 44, 733–751. [Google Scholar] [CrossRef]
- Wang, Y.M.; Li, R.R.; Zhang, H. Effects of exponential fertilization on biomass and nitrogen accumulation of Carya illinoensis seedlings. Chin. J. Ecol. 2018, 37, 2920–2926. (In Chinese) [Google Scholar]
- Tim, L. Effect of nitrogen fertilization and residual nitrogen on biomass yield of switchgrass. BioEnergy Res. 2017, 10, 648–656. [Google Scholar]
- Hu, Y.B.; Li, C.M.; Jiang, L.P.; Liang, D.Y.; Zhao, X.Y. Growth performance and nitrogen allocation within leaves of two poplar clones after exponential and conventional nitrogen applications. Plant Physiol. Biochem. 2020, 154, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.F.; Zhou, D.X.; Zhao, Y. WUSCHEL-related homeobox gene WOX11 increases rice drought resistance by controlling root hairformation and root system development. Plant Signal. Behav. 2016, 11, 1559–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, H.A.; Men, S.N.; Hussain, S.; Zhang, Q.W.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L.C. Maize tolerance against drought and chilling stresses varied with root morphology and antioxidative defense system. Plants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Villordon, A.Q.; Ginzberg, I.; Firon, N. Root architecture and root and tuber crop productivity. Trends Plant Sci. 2014, 19, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Fahey, T.J.; Xue, S.; Liu, F. Root morphology and architecture respond to N addition in Pinus tabuliformis, west China. Oecologia 2013, 171, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Moreira, A. The role of mineral nutrition on root growth of crop plants. Adv. Agron. 2011, 110, 251–331. [Google Scholar]
- Qu, L.; Quoreshi, A.M.; Koike, T. Root growth characteristics, biomass and nutrient dynamics of seedlings of two larch species raised under different fertilization regimes. Plant Soil 2003, 255, 293–302. [Google Scholar] [CrossRef]
- Lee, K.H.; Jose, S. Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. For. Ecol. Manag. 2003, 185, 263–273. [Google Scholar] [CrossRef]
- Jia, S.; Wang, Z.; Li, X.P.; Sun, Y.; Zhang, X.P.; Liang, A.Z. N fertilization affects on soil respiration, microbial biomass and root respiration in Larix gmelinii and Fraxinus mandshurica plantations in China. Plant Soil 2010, 333, 325–336. [Google Scholar] [CrossRef]
- Lucander, K.; Zanchi, G.; Akselsson, C.; Belyazid, S. The effect of nitrogen fertilization on tree growth, soil organic carbon and nitrogen leaching—A modeling study in a steep nitrogen deposition gradient in sweden. Forests 2021, 12, 298. [Google Scholar] [CrossRef]
- Kakabouki, I.P.; Roussis, I.; Hela, D.; Papastylianou, P.; Folina, A.; Bilalis, D. Root growth dynamics and productivity of quinoa (Chenopodium quinoa Willd.) in response to fertilization and soil tillage. Folia Hortic. 2019, 31, 277–291. [Google Scholar] [CrossRef] [Green Version]
- Razaq, M.; Zhang, P.; Shen, H.L. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar]
- Wright, S.J.; Yavitt, J.B.; Wurzburger, N.; Turner, B.L.; Tanner, E.V.J.; Sayer, E.J.; Santiago, L.S.; Kaspari, M.; Hedin, L.O.; Harms, K.E.; et al. Potassium, phosphorus, or nitrogen limit root allocation, tree growth, or litter production in a lowland tropical forest. Ecology 2011, 92, 1616–1625. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J. Plant responses to nutrient addition experiments conducted in tropical forests. Ecol. Monogr. 2019, 89, 1382–1399. [Google Scholar] [CrossRef]
- Li, G.L.; Wang, J.X.; Oliet, J.A.; Jacobs, D.F. Combined pre-hardening and fall fertilization facilitates N storage and field performance of Pinus tabulaeformis seedlings. iForest 2016, 9, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.L.; Fan, H.H.; Xuan, H.F.; Mgelwa, A.S.; Chen, S.P. Distinct growth and nutrient status responses to fertilization regimes in two generations of Chinese fir seedlings. Forests 2019, 10, 719. [Google Scholar] [CrossRef] [Green Version]
- Salifu, K.F.; Timmer, V.R. Nitrogen retranslocation response of young Picea mariana to nitrogen-15 supply. Soil Sci. Soc. Am. J. 2003, 67, 309–317. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 36–47. [Google Scholar] [CrossRef]
- Hassan, M.K.; Mcinroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Li, J.Y.; Dong, W.Y.; Wei, H.X.; He, C.X. Late-season fluxes of ammonium and nitrate in roots of two poplar clones pretreated with nutrient addition. Int. J. Agric. Biol. 2017, 19, 1525–1534. [Google Scholar]
- Wei, H.X.; Ren, J.; Zhou, J.H. Effect of exponential fertilization on growth and nutritional status in Buddhist pine (Podocarpus macrophyllus [Thunb.] D. Don) seedlings cultured in natural and prolonged photoperiods. Soil Sci. Plant Nutr. 2013, 59, 933–941. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).