Crop Residue Management Strategies to Reduce Nitrogen Losses during the Winter Leaching Period after Autumn Spinach Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Sites and Experimental Set-Up
2.2. Treatments
2.3. Data Collection and Measurements
2.4. Nitrogen Balance Sheet Calculations
2.5. Statistical Analysis
3. Results
3.1. Soil Temperature and Precipitation
3.2. Effects of the Maximum Tillage Depth and Frequency
3.3. Effects of the Season of Tillage
3.4. Effects of the Nitrification Inhibitor DMPP
4. Discussion
4.1. Effects of the Tillage Depth and Frequency on Potential N Losses
4.2. Effects of the Tillage Season on Potential N Losses
4.3. Effects of DMPP on Soil N Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament; Council of European Union Council. Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Union 1991, L 375, 1–8. [Google Scholar]
- European Commission. Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Commun. 2000, L 327, 1–73. [Google Scholar]
- Tei, F.; De Neve, S.; De Haan, J.; Kristensen, H.L. Nitrogen management of vegetable crops. Agric. Water Manag. 2020, 240, 106316. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Botrini, L.; Carmassi, G.; Diara, C.; Paoli, P.D.; Incrocci, G.; Maggini, R.; Pardossi, A. Modelling plant yield and quality response of fresh-market spinach (Spinacia oleracea L.) to mineral nitrogen availability in the root zone. Ital. J. Agron. 2018, 13, 248–259. [Google Scholar] [CrossRef]
- Nett, L.; Fuss, R.; Flessa, H.; Fink, M. Emissions of nitrous oxide and ammonia from a sandy soil following surface application and incorporation of cauliflower leaf residues. J. Agric. Sci. 2015, 153, 1341–1352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Haan, J.J.; Zwart, K.B.; Smit, A.L.; Van Geel, W.C.A. Can intensive arable farming systems on sandy soils in the Netherlands meet the targets in the nitrate directive? In Connecting Different Scales of Nitrogen Use in Agriculture, Proceedings of the 16th Nitrogen Workshop, Turin, Italy, 28 June–1 July 2009; Grignani, C., Acutis, M., Zavattaro, L., Bechini, L., Bertora, C., Gallina, P.M., Sacco, D., Eds.; Tipografia Fiordo: Turin, Italy, 2009; pp. 471–472. [Google Scholar]
- Agostini, F.; Tei, F.; Silgram, M.; Farneselli, M.; Benincasa, P.; Aller, M.F. Decreasing nitrate leaching in vegetable crops with better N management. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming. Sustainable Agriculture Reviews 4; Lichtfouse, E., Ed.; Springer Science & Business Media B.V.: Dordrecht, The Netherlands, 2010; pp. 147–200. [Google Scholar]
- Benincasa, P.; Tosti, G.; Guiducci, M.; Farneselli, M.; Tei, F. Crop rotation as a system approach for soil fertility management in vegetables. In Advances in Research on Fertilization Management in Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 115–148. [Google Scholar]
- Congreves, K.A.; Van Eerd, L.L. Nitrogen cycling and management in intensive horticultural systems. Nutr. Cycl. Agroecosyst. 2015, 102, 299–318. [Google Scholar] [CrossRef]
- Frerichs, C.; Key, G.; Broll, G.; Daum, D. Nitrogen fertilization strategies to reduce the risk of nitrate leaching in open field cultivation of spinach (Spinacia oleracea L.). J. Plant Nutr. Soil Sci. 2022, 185, 1–18. [Google Scholar] [CrossRef]
- Frerichs, C.; Daum, D. Field-grown spinach production-fertilization strategies to reduce risk of nitrate leaching. Acta Hortic. 2021, 1327, 155–160. [Google Scholar] [CrossRef]
- Feller, C.; Fink, M.; Laber, H.; Maync, A.; Paschold, P.; Scharpf, H.C.; Schlaghecken, J.; Strohmeyer, K.; Weier, U.; Ziegler, J. Düngung im Freilandgemüsebau, 3rd ed.; Schriftenreihe des Leibniz-Instituts für Gemüse-und Zierpflanzenbau (IGZ): Großbeeren, Germany, 2011; pp. 1–265. [Google Scholar]
- Vandecasteele, B.; Canali, S.; Carranca, C.; Coopman, F.; De Haan, J.; De Neve, S.; Garming, H.; Hajdu, Z.; Javier, B.; Malusa, E.; et al. EIP-AGRI Focus Group. Fertilizer Efficiency–Horticulture in Open Field. Final Report. Available online: https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-fertiliser-efficiency-focus (accessed on 8 February 2022).
- Berbel, J.; Martínez-Dalmau, J. A Simple Agro-Economic Model for Optimal Farm Nitrogen Application under Yield Uncertainty. Agronomy 2021, 11, 1107. [Google Scholar] [CrossRef]
- Gent, M.P. Effect of irradiance and temperature on composition of spinach. HortScience 2016, 51, 133–140. [Google Scholar] [CrossRef]
- Breimer, T. Environmental factors and cultural measures affecting the nitrate content in spinach. Fert. Res. 1982, 3, 191–292. [Google Scholar] [CrossRef]
- De Neve, S. Organic matter mineralization as a source of nitrogen. In Advances in Research on Fertilization Management in Vegetable Crops; Tei, F., Nicola, S., Benincasa, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 65–83. [Google Scholar]
- Heins, B. Bedeutung von Wurzeleigenschaften für die Nutzung des Nitratangebotes Durch Spinat und Kohlrabi. Ph.D. Thesis, Universität Hannover, Hannover, Germany, 26 June 1989. [Google Scholar]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messéan, A.; Aubertot, J.N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Choudhury, R.A.; McRoberts, N. Spinach Growers Value Host Resistance and Synthetic Fungicides in the Fight against Downy Mildew. BioRxiv 2020. [Google Scholar] [CrossRef]
- De Neve, S.; Hofman, G. N mineralization and nitrate leaching from vegetable crop residues under field conditions: A model evaluation. Soil Biol. Biochem. 1998, 30, 2067–2075. [Google Scholar] [CrossRef]
- Whitmore, A.P. Modelling the release and loss of nitrogen after vegetable crops. Neth. J. Agric. Sci. 1996, 44, 73–86. [Google Scholar] [CrossRef]
- De Neve, S.; Pannier, J.; Hofman, G. Fractionation of vegetable crop residues in relation to in situ N mineralization. Eur. J. Agron. 1994, 3, 267–272. [Google Scholar] [CrossRef]
- Agneessens, L.; De Waele, J.; De Neve, S. Review of alternative management options of vegetable crop residues to reduce nitrate leaching in intensive vegetable rotations. Agronomy 2014, 4, 529–555. [Google Scholar] [CrossRef]
- Van Dijk, W.; Smit, A.L. How to Meet the EC-Nitrate Directive in Dutch Vegetable Growing? Acta Hortic. 2006, 700, 191–198. [Google Scholar] [CrossRef]
- VLM (Vlaamse Landmaatschappij). Evaluatie van de Metingen van Het Nitraatresidu 2008. Available online: https://www.vlm.be/nl/SiteCollectionDocuments/Mestbank/Studies/Rapport%20Nitraatresidu.pdf (accessed on 8 February 2022).
- VLM (Vlaamse Landmaatschappij). Evaluatie van de Metingen van Het Nitraatresidu 2009. Available online: https://www.vlm.be/nl/SiteCollectionDocuments/Mestbank/Studies/Evaluatie_van_de_metingen_van_het_nitraatresidu_2009.pdf (accessed on 8 February 2022).
- VLM (Vlaamse Landmaatschappij). Nitraatresidurapport 2010. Resultaten van de Nitraatresidumetingen in Vlaanderen tot en met de Staalnamecampagne van 2009. Available online: https://www.vlm.be/nl/SiteCollectionDocuments/Mestbank/Studies/Nitraatresidurapport_2010.pdf (accessed on 8 February 2022).
- Rather, K. Emission control of soil nitrogen content in water protection areas in baden-württemberg, Germany. In Proceedings of the Nutrihort: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality, Ghent, Belgium, 16–18 September 2013; D’Haene, K., Vandecasteele, B., De Vis, R., Crappé, S., Callens, D., Mechant, E., Hofman, G., De Neve, S., Eds.; ILVO: Ghent, Belgium, 2013; pp. 235–240. [Google Scholar]
- Van de Sande, T.; Callens, D.; Coopman, F.; De Nies, J.; De Rooster, L.; De Reycke, L.; Verhaeghe, M. KNS—Based advisory system proves to be a useful tool in reducing residual nitrate content of horticultural soils in the fall. In Proceedings of the Nutrihort: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality, Ghent, Belgium, 16–18 September 2013; D’Haene, K., Vandecasteele, B., De Vis, R., Crappé, S., Callens, D., Mechant, E., Hofman, G., De Neve, S., Eds.; ILVO: Ghent, Belgium, 2013; pp. 156–161. [Google Scholar]
- Zemek, O.; Neuweiler, R.; Spiess, E.; Stüssi, M.; Richner, W. Nitratauswaschungspotenzial im Freilandgemüsebau–eine Literaturstudie. Agroscope Sci. 2020, 95, 1–117. [Google Scholar]
- Kage, H. Simulation Modelling for Improving Nitrogen Use Efficiency in Intensive Cropping Systems; Habilitation, Universität Hannover: Hannover, Germany, 2000. [Google Scholar]
- Thorup-Kristensen, K. Utilising differences in rooting depth to design vegetable crop rotations with high nitrogen use efficiency (NUE). Acta Hortic. 2002, 571, 249–254. [Google Scholar] [CrossRef]
- Van den Bossche, A.; De Bolle, S.; De Neve, S.; Hofman, G. Effect of tillage intensity on N mineralization of different crop residues in a temperate climate. Soil Tillage Res. 2009, 103, 316–324. [Google Scholar] [CrossRef]
- Guerette, V.; Desjardins, Y.; Belec, C.; Tremblay, N.; Weier, U.; Scharpf, H.C. Nitrogen contribution from mineralization of vegetable crop residues. Acta Hortic. 2000, 571, 95–102. [Google Scholar] [CrossRef]
- Rahn, C.R. Management strategies to reduce nutrient losses from vegetable crops. Acta Hortic. 2002, 571, 19–29. [Google Scholar] [CrossRef]
- Schwarz, A.; Pfenning, J.; Bischoff, W.A.; Liebig, H.P. Effects of N Fertilization Strategy and Fixed Ploughing Date on Nitrate Leaching on Field Vegetable Cultivation. Acta Hortic. 2008, 852, 115–122. [Google Scholar] [CrossRef]
- Mitchell, R.D.J.; Harrison, R.; Russell, K.J.; Webb, J. The effect of crop residue incorporation date on soil inorganic nitrogen, nitrate leaching and nitrogen mineralization. Biol. Fertil. Soils 2000, 32, 294–301. [Google Scholar] [CrossRef]
- De Neve, S.; Pannier, J.; Hofman, G. Temperature effects on C-and N-mineralization from vegetable crop residues. Plant Soil 1996, 181, 25–30. [Google Scholar] [CrossRef]
- Heumann, S.; Böttcher, J. Temperature functions of the rate coefficients of net N mineralization in sandy arable soils. Part I. Derivation from laboratory incubations. J. Plant Nutr. Soil Sci. 2004, 167, 381–389. [Google Scholar] [CrossRef]
- Suzuki, T.; Kamada, E.; Ishii, T. Effects of ratoon harvesting on the root systems of processing spinach. Plant Root 2019, 13, 23–28. [Google Scholar] [CrossRef]
- Chaves, B.; De Neve, S.; Boeckx, P.; Van Cleemput, O.; Hofman, G. Manipulating nitrogen release from nitrogen-rich crop residues using organic wastes under field conditions. Soil Sci. Soc. Am. J. 2007, 71, 1240–1250. [Google Scholar] [CrossRef]
- Viaene, J.; Agneessens, L.; Capito, C.; Ameloot, N.; Reubens, B.; Willekens, K.; Vandecasteele, B.; De Neve, S. Co-ensiling, co-composting and anaerobic co-digestion of vegetable crop residues: Product stability and effect on soil carbon and nitrogen dynamics. Sci. Hortic. 2017, 220, 214–225. [Google Scholar] [CrossRef]
- Agneessens, L.; Vandecasteele, B.; Van De Sande, T.; Goovaerts, E.; Crappé, S.; Elsen, A.; Willekens, K.; De Neve, S. Onderzoek Naar Het Beheer van Oogstresten Bij Vollegrondsgroenten en Mogelijkheden van Vanggewassen en Teeltrotaties met het oog op de Waterkwaliteitsdoelstellingen van het Actieprogramma 2011–2014 (MAP4); Hoofdrapport, Vlaamse Landmaatschappij (VLM): Gent, Belgium, 2014; pp. 1–149. [Google Scholar]
- Chaves, B.; Opoku, A.; De Neve, S.; Boeckx, P.; Van Cleemput, O.; Hofman, G. Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol. Fertil. Soils 2006, 43, 62–68. [Google Scholar] [CrossRef]
- Chaves, B.; De Neve, S.; Boeckx, P.; Van Cleemput, O.; Hofman, G. Influence of nitrification inhibitors on soil N dynamics after incorporation of vegetable crop residues: A field study. Cereal Res. Commun. 2006, 34, 115–118. [Google Scholar] [CrossRef]
- Scheurer, M.; Brauch, H.J.; Schmidt, C.K.; Sacher, F. Occurrence and fate of nitrification and urease inhibitors in the aquatic environment. Environ. Sci. Process. Impacts 2016, 18, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Baral, K.R.; Abalos, D.; Strobel, B.W.; Petersen, S.O. Nitrate leaching and nitrous oxide emissions from maize after grass-clover on a coarse sandy soil: Mitigation potentials of 3, 4-dimethylpyrazole phosphate (DMPP). J. Environ. Manag. 2020, 260, 110165. [Google Scholar] [CrossRef] [PubMed]
- VDLUFA. Handbuch der Landwirtschaftlichen Versuchs-und Untersuchungsmethodik (VDLUFA-Methodenbuch). Band 1: Die Untersuchung von Böden. Teillieferungen 1–7, 4th ed.; VDLUFA-Verlag: Darmstadt, Germany, 2016. [Google Scholar]
- Umweltbundesamt. Hintergrundbelastungsdaten Stickstoff. Available online: https://gis.uba.de/website/depo1/ (accessed on 8 February 2022).
- Hoffmann, M.; Schwartengräber, R.; Wessolek, G.; Peters, A. Comparison of simple rain gauge measurements with precision lysimeter data. Atmos. Res. 2016, 174, 120–123. [Google Scholar] [CrossRef]
- DIN EN 16168:2012; Sludge, Treated Biowaste and Soil—Determination of Total Nitrogen Using Dry Combustion Method. German Version EN 16168:2012. Beuth Verlag: Berlin, Germany, 2012.
- Heumann, S.; Böttcher, J. Temperature functions of the rate coefficients of net N mineralization in sandy arable soils. Part II. Evaluation via field mineralization measurements. J. Plant Nutr. Soil Sci. 2004, 167, 390–396. [Google Scholar] [CrossRef]
- DIN EN 15936:2012; Sludge, Treated Biowaste, Soil and Waste—Determination of Total Organic Carbon (TOC) by Dry Combustion; German Version EN 15936:2012. Beuth Verlag: Berlin, Germany, 2012.
- Doran, G.S.; Condon, J.R.; Kaveney, B.F. Rapid analysis of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate in soil using LC-MS/MS. Int. J. Environ. Anal. Chem. 2018, 98, 606–621. [Google Scholar] [CrossRef]
- Larsson, M.; Gerhardson, B. Isolates of Phytophthora cryptogea pathogenic to wheat and some other crop plants. J. Phytopathol. 1990, 129, 303–315. [Google Scholar] [CrossRef]
- De Vries, W.; Schulte-Uebbing, L.; Kros, H.; Voogd, J.C.; Louwagie, G. Spatially explicit boundaries for agricultural nitrogen inputs in the European Union to meet air and water quality targets. Sci. Total Environ. 2021, 786, 147283. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tong, Y.P.; Zhu, Y.G.; Ding, H.; Smith, F.A. Leaf chlorophyll readings as an indicator for spinach yield and nutritional quality with different nitrogen fertilizer applications. J. Plant. Nutr. 2006, 29, 1207–1217. [Google Scholar] [CrossRef]
- D’Haene, K.; Salomez, J.; Verhaeghe, M.; Van de Sande, T.; De Nies, J.; De Neve, S.; Hofman, G. Can optimum yield and quality of vegetables be reconciled with low residual soil mineral nitrogen at harvest? Sci. Hortic. 2018, 233, 78–89. [Google Scholar] [CrossRef]
- Sheng, Q.; Hunt, L.A. Shoot and root dry weight and soil water in wheat, triticale and rye. Can. J. Agric. Sci. 1991, 71, 41–49. [Google Scholar] [CrossRef]
- McLenaghen, R.D.; Cameron, K.C.; Lampkin, N.H.; Daly, M.L.; Deo, B. Nitrate leaching from ploughed pasture and the effectiveness of winter catch crops in reducing leaching losses. N. Z. J. Agric. Res. 1996, 39, 413–420. [Google Scholar] [CrossRef]
- Evans, P.S. Root growth of Lolium perenne L. 1. Effect of plant age, seed weight, and nutrient concentration on root weight, length, and number of apices. N. Z. J. Bot. 1970, 8, 344–356. [Google Scholar] [CrossRef][Green Version]
- Vos, J.; Van der Putten, P.E.L. Field observations on nitrogen catch crops. I. Potential and actual growth and nitrogen accumulation in relation to sowing date and crop species. Plant Soil 1997, 195, 299–309. [Google Scholar] [CrossRef]
- Wahlström, E.M.; Hansen, E.M.; Mandel, A.; Garbout, A.; Kristensen, H.L.; Munkholm, L.J. Root development of fodder radish and winter wheat before winter in relation to uptake of nitrogen. Eur. J. Agron. 2015, 71, 1–9. [Google Scholar] [CrossRef]
- Neeteson, J.J.; Carton, O.T. The environmental impact of nitrogen in field vegetable production. Acta Hortic. 2001, 563, 21–28. [Google Scholar] [CrossRef]
- Niers, H. N-Opname en Gewaseigenschappen bij Spinazie (Spinacia oleracea L.) in de Vollegrond. DLO Instituut voor Agrobiologisch en Bodemvruchtbaarheidsonderzoek. Available online: https://library.wur.nl/WebQuery/wurpubs/fulltext/333105 (accessed on 8 February 2022).
- Chaves, B.; De Neve, S.; Hofman, G.; Boeckx, P.; Van Cleemput, O. Nitrogen mineralization of vegetable root residues and green manures as related to their (bio) chemical composition. Eur. J. Agron. 2004, 21, 161–170. [Google Scholar] [CrossRef]
- Congreves, K.A.; Voroney, R.P.; Van Eerd, L.L. Amending soil with used cooking oil to reduce nitrogen losses after cole crop harvest: A 15 N study. Nutr. Cycl. Agroecosyst. 2014, 100, 257–271. [Google Scholar] [CrossRef]
- Heumann, S.; Böttcher, J.; Springob, G. Pedotransfer functions for the pool size of slowly mineralizable organic N in sandy arable soils. J. Plant Nutr. Soil Sci. 2003, 166, 308–318. [Google Scholar] [CrossRef]
- Heumann, S.; Schlichting, A.; Böttcher, J.; Leinweber, P. Sterols in soil organic matter in relation to nitrogen mineralization in sandy arable soils. J. Plant Nutr. Soil Sci. 2011, 174, 576–586. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Scott, C.L.; Hernandez-Ramirez, G.; Meenken, E.D. Mineralization of soil carbon and nitrogen following physical disturbance: A laboratory assessment. Soil Sci. Soc. Am. J. 2014, 78, 925–935. [Google Scholar] [CrossRef]
- Hansen, E.M.; Djurhuus, J. Nitrate leaching as influenced by soil tillage and catch crop. Soil Tillage Res. 1997, 41, 203–219. [Google Scholar] [CrossRef]
- Nett, L.; Sradnick, A.; Fuß, R.; Flessa, H.; Fink, M. Emissions of nitrous oxide and ammonia after cauliflower harvest are influenced by soil type and crop residue management. Nutr. Cycl. Agroecosyst. 2016, 106, 217–231. [Google Scholar] [CrossRef]
- Cookson, W.R.; Cornforth, I.S.; Rowarth, J.S. Winter soil temperature (2–15 °C) effects on nitrogen transformations in clover green manure amended or unamended soils; a laboratory and field study. Soil Biol. Biochem. 2002, 34, 1401–1415. [Google Scholar] [CrossRef]
- Magid, J.; Henriksen, O.; Thorup-Kristensen, K.; Mueller, T. Disproportionately high N-mineralisation rates from green manures at low temperatures–implications for modeling and management in cool temperate agro-ecosystems. Plant Soil 2001, 228, 73–82. [Google Scholar] [CrossRef]
- Van Schöll, L.; Van Dam, A.M.; Leffelaar, P.A. Mineralisation of nitrogen from an incorporated catch crop at low temperatures: Experiment and simulation. Plant Soil 1997, 188, 211–219. [Google Scholar] [CrossRef]
- Feaga, J.B.; Selker, J.S.; Dick, R.P.; Hemphill, D.D. Long-term nitrate leaching under vegetable production with cover crops in the Pacific Northwest. Soil Sci. Soc. Am. J. 2010, 74, 186–195. [Google Scholar] [CrossRef]
- Thapa, R.; Mirsky, S.B.; Tully, K.L. Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. J. Environ. Qual. 2018, 47, 1400–1411. [Google Scholar] [CrossRef]
- Myrbeck, Å.; Stenberg, M.; Rydberg, T. Establishment of winter wheat—Strategies for reducing the risk of nitrogen leaching in a cool-temperate region. Soil Tillage Res. 2012, 120, 25–31. [Google Scholar] [CrossRef]
- Weinert, T.L.; Pan, W.L.; Moneymaker, M.R.; Santo, G.S.; Stevens, R.G. Nitrogen recycling by nonleguminous winter cover crops to reduce leaching in potato rotations. Agron. J. 2002, 94, 365–372. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. The effect of nitrogen catch crop species on the nitrogen nutrition of succeeding crops. Fert. Res. 1994, 37, 227–234. [Google Scholar] [CrossRef]
- Vos, J.; Van der Putten, P.E.L. Field observations on nitrogen catch crops. III. Transfer of nitrogen to the succeeding main crop. Plant Soil 2001, 236, 263–273. [Google Scholar] [CrossRef]
- Armbruster, M.; Laun, N.; Heger, A.; Wiesler, F. Integrated nitrogen management—A strategy to improve nitrogen efficiency in intensive field vegetable production. In Proceedings of the NUTRIHORT: Nutrient Management, Innovative Techniques and Nutrient Legislation in Intensive Horticulture for an Improved Water Quality, Ghent, Belgium, 16–18 September 2013; D’Haene, K., Vandecasteele, B., De Vis, R., Crappé, S., Callens, D., Mechant, E., Hofman, G., De Neve, S., Eds.; Nutrihort: Ghent, Belgium, 2013; pp. 149–156. [Google Scholar]
- De Ruijter, F.J.; Ten Berge, H.F.M.; Smit, A.L. The fate of nitrogen from crop residues of broccoli, leek and sugar beet. Acta Hortic. 2010, 852, 157–161. [Google Scholar] [CrossRef]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from soils amended with crop residues. Nutr. Cycl. Agroecosyst. 2002, 62, 249–261. [Google Scholar] [CrossRef]
- Pfab, H.; Palmer, I.; Buegger, F.; Fiedler, S.; Müller, T.; Ruser, R. N2O fluxes from a Haplic Luvisol under intensive production of lettuce and cauliflower as affected by different N-fertilization strategies. J. Plant Nutr. Soil Sci. 2011, 174, 545–553. [Google Scholar] [CrossRef]
- Seiz, P.; Guzman-Bustamante, I.; Schulz, R.; Müller, T.; Ruser, R. Effect of crop residue removal and straw addition on nitrous oxide emissions from a horticulturally used soil in South Germany. Soil Sci. Soc. Am. J. 2019, 83, 1399–1409. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, J.; Yang, W.; Cai, Z. Effects of organic material amendment and water content on NO, N2O, and N2 emissions in a nitrate-rich vegetable soil. Biol. Fertil. Soils 2013, 49, 153–163. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Chang. Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef]
- Essich, L.; Nkebiwe, P.M.; Schneider, M.; Ruser, R. Is Crop Residue Removal to Reduce N2O Emissions Driven by Quality or Quantity? A Field Study and Meta-Analysis. Agriculture 2020, 10, 546. [Google Scholar] [CrossRef]
- Baggs, E.M.; Rees, R.M.; Smith, K.A.; Vinten, A.J.A. Nitrous oxide emission from soils after incorporating crop residues. Soil Use Manag. 2000, 16, 82–87. [Google Scholar] [CrossRef]
- De Ruijter, F.J.; Huijsmans, J.F.M.; Rutgers, B. Ammonia volatilization from crop residues and frozen green manure crops. Atmos. Environ. 2010, 44, 3362–3368. [Google Scholar] [CrossRef]
- Constantin, J.; Mary, B.; Laurent, F.; Aubrion, G.; Fontaine, A.; Kerveillant, P.; Beaudoin, N. Effects of catch crops, no till and reduced nitrogen fertilization on nitrogen leaching and balance in three long-term experiments. Agric. Ecosyst. Environ. 2010, 135, 268–278. [Google Scholar] [CrossRef]
- Nett, L.; Feller, C.; George, E.; Fink, M. Effect of winter catch crops on nitrogen surplus in intensive vegetable crop rotations. Nutr. Cycl. Agroecosyst. 2011, 91, 327–337. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. Adv. Agron. 2003, 79, 227–302. [Google Scholar]
- Neeteson, J.J.; Booij, R.; Whitmore, A.P. A review on sustainable nitrogen management in intensive vegetable production systems. Acta Hortic. 1999, 506, 17–28. [Google Scholar] [CrossRef]
- Pretty, J.; Smith, G.; Goulding, K.W.T.; Groves, S.J.; Henderson, I.; Hine, R.E.; King, V.; van Oostrum, J.; Pendlington, D.J.; Vis, J.K.; et al. Multi-year assessment of Unilever’s progress towards agricultural sustainability II: Outcomes for peas (UK), spinach (Germany, Italy), tomatoes (Australia, Brazil, Greece, USA), tea (Kenya, Tanzania, India) and oil palm (Ghana). Int. J. Agric. Sustain. 2008, 6, 63–88. [Google Scholar] [CrossRef]
- Larsson, M.; Gerhardson, B. Disease progression and yield losses from root diseases caused by soilborne pathogens of spinach. Phytopathology 1992, 82, 403–406. [Google Scholar] [CrossRef]
- Rashti, M.R.; Wang, W.J.; Chen, C.R.; Reeves, S.H.; Scheer, C. Assessment of N2O emissions from a fertilised vegetable cropping soil under different plant residue management strategies using 15N tracing techniques. Sci. Total Environ. 2017, 598, 479–487. [Google Scholar] [CrossRef]
- Marsden, K.A.; Marín-Martínez, A.J.; Vallejo, A.; Hill, P.W.; Jones, D.L.; Chadwick, D.R. The mobility of nitrification inhibitors under simulated ruminant urine deposition and rainfall: A comparison between DCD and DMPP. Biol. Fertil. Soils 2016, 52, 491–503. [Google Scholar] [CrossRef]
- Vilas, M.P.; Verburg, K.; Thorburn, P.J.; Probert, M.E.; Bonnett, G.D. A framework for analysing nitrification inhibition: A case study on 3,4-dimethylpyrazole phosphate (DMPP). Sci. Total Environ. 2019, 672, 846–854. [Google Scholar] [CrossRef]
- Li, C.; Hu, H.W.; Chen, Q.L.; Chen, D.; He, J.Z. Growth of comammox Nitrospira is inhibited by nitrification inhibitors in agricultural soils. J. Soils Sediments 2020, 20, 621–628. [Google Scholar] [CrossRef]
- Kleineidam, K.; Košmrlj, K.; Kublik, S.; Palmer, I.; Pfab, H.; Ruser, R.; Fiedler, S.; Schloter, M. Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 2011, 84, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, H.W.; Ye, G.; Fan, J.; Ding, W.; He, Z.Y.; Zheng, Y.; He, J.Z. Ammonia-oxidizing bacteria play an important role in nitrification of acidic soils: A meta-analysis. Geoderma 2021, 404, 115395. [Google Scholar] [CrossRef]
- Aigle, A.; Gubry-Rangin, C.; Thion, C.; Estera-Molina, K.Y.; Richmond, H.; Pett-Ridge, J.; Firestone, M.K.; Nicol, G.W.; Prosser, J.I. Experimental testing of hypotheses for temperature-and pH-based niche specialization of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2020, 22, 4032–4045. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Norton, J.M.; Stark, J.M. Ammonium availability and temperature control contributions of ammonia oxidizing bacteria and archaea to nitrification in an agricultural soil. Soil Biol. Biochem. 2017, 113, 161–172. [Google Scholar] [CrossRef]
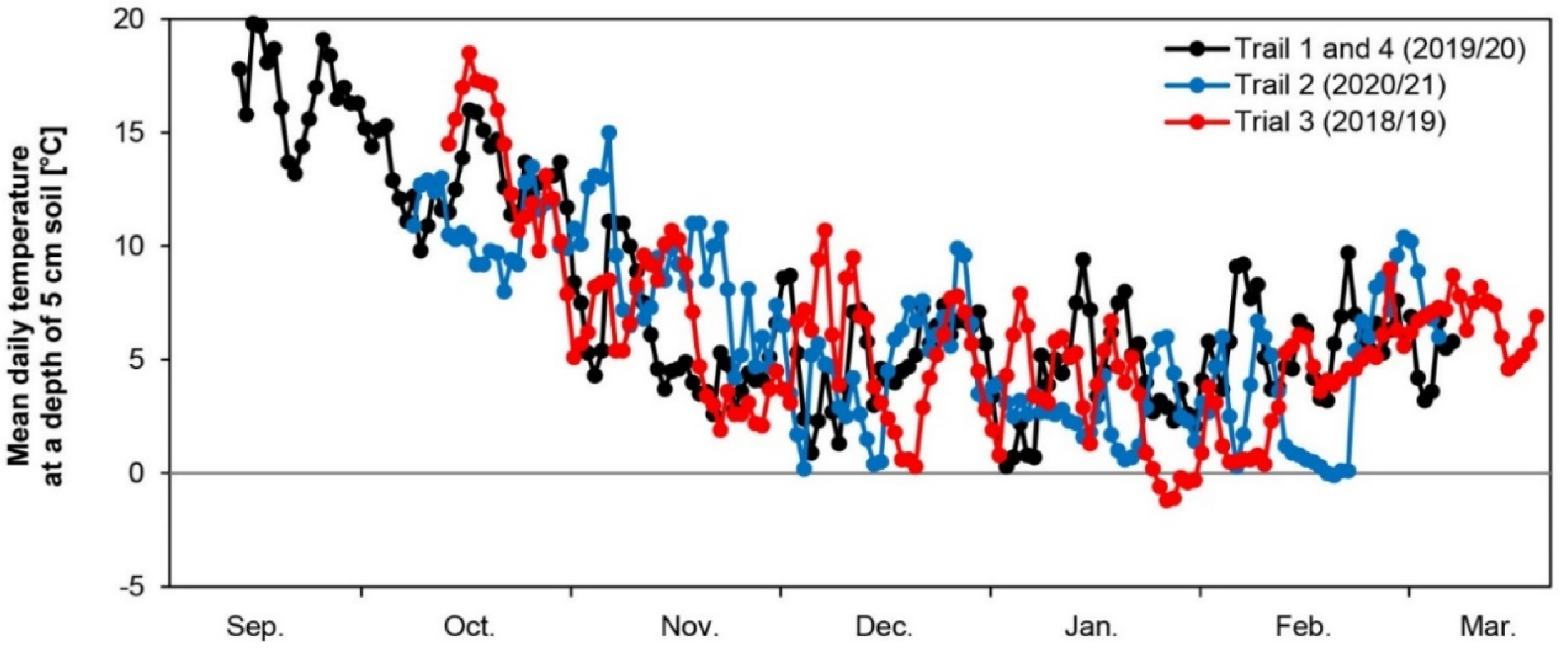





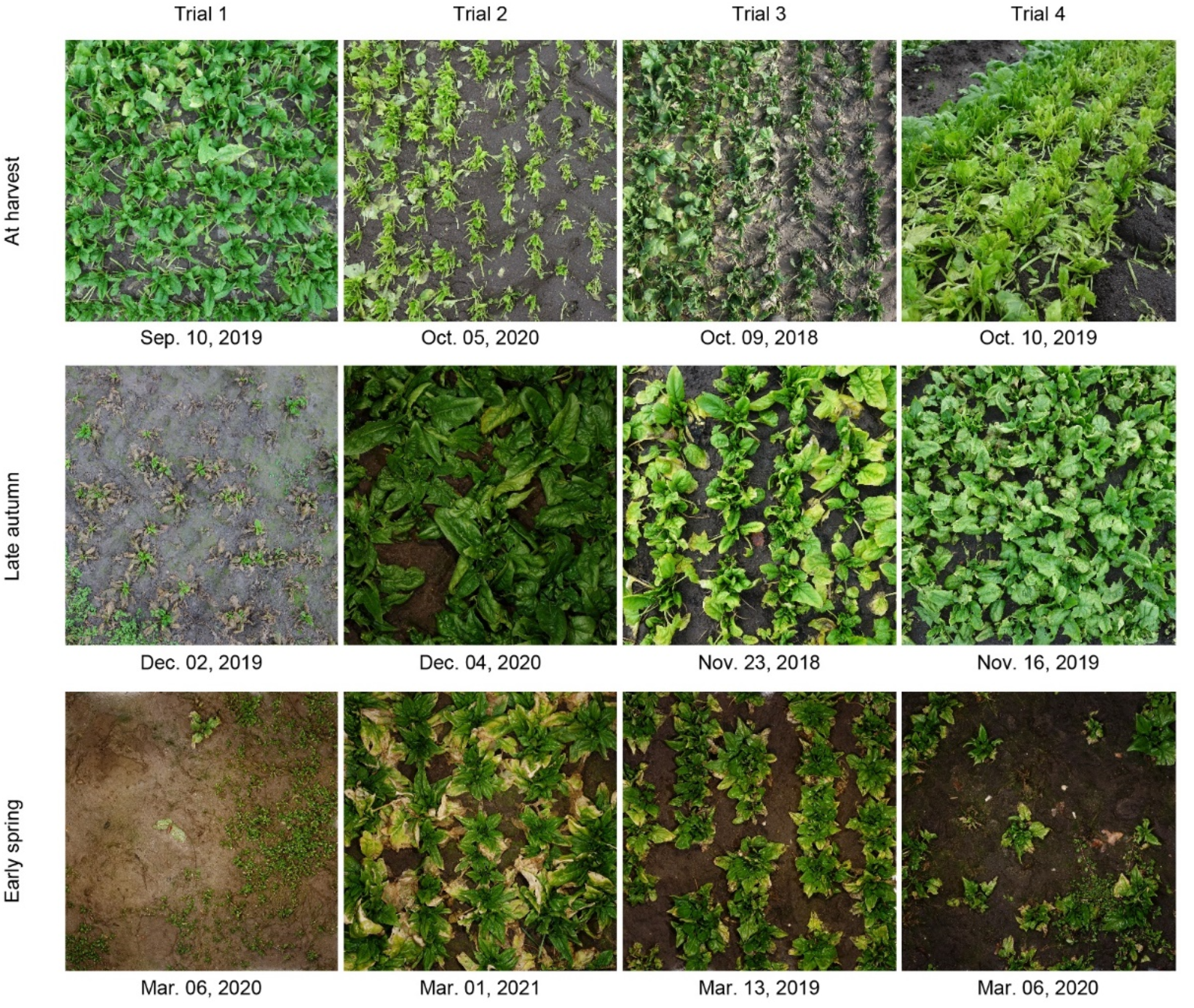

| Trial 1 (10 September 2019–6 March 2020) | Trial 2 (5 October 2020–1 March 2021) | Trial 3 (9 October 2018–13 March 2019) | Trial 4 (10 October 2019–6 March 2020) | ||
|---|---|---|---|---|---|
| Soil parameters (0–30 cm) | Sand [% (w/w)] | 80.5 | 82.4 | 80.2 | 87.3 |
| Silt [% (w/w)] | 12.1 | 11.3 | 13.1 | 06.8 | |
| Clay [% (w/w)] | 07.5 | 06.3 | 06.6 | 05.8 | |
| Organic C [% (w/w)] | 01.1 | 01.5 | 01.2 | 03.6 | |
| C/N ratio | 15.7 | 12.1 | 09.2 | 25.7 | |
| Soil pH | 06.0 | 05.6 | 05.7 | 05.2 | |
| Crop rotation details | Crop rotation | Spinach/Spinach | Triticale/Spinach | Barley/Spinach | Carrots/Spinach |
| Liquid manure [kg N ha−1] | 0 | 170 | 170 | 170 | |
| Mineral fertilization [kg N ha−1] | 162 | 126 | 101 | 122 | |
| Marketable yield autumn-grown spinach [t ha−1] | 17.8 | 20.2 | 7.3 | 17.8 | |
| Aboveground crop residues | Total N [kg ha−1] | 64 | 30 | 44 | 45 |
| N content [% (w/w)] | 5.0 | 4.0 | 3.7 | 5.5 | |
| C/N ratio | 6.6 | 9.0 | 9.0 | 5.9 |
| Trt. | Tillage Depth [cm] (Tillage Implement) | Tillage Season | Nitrification Inhibitor | Catch Crop Sowing Dates | |||
|---|---|---|---|---|---|---|---|
| Trial 1 (Harvest: 10 September 2019) | Trial 2 (Harvest: 5 October 2020) | Trial 3 (Harvest: 9 October 2018) | Trial 4 (Harvest: 10 October 2019) | ||||
| 1. | 10 (Harrow) | Early autumn | n.a. | 16 September 2019 | 16 October 2020 | 13 October 2018 | 19 October 2019 |
| 2. | 10 (Harrow) | Early autumn | DMPP 1 | 16 September 2019 | 16 October 2020 | n.a. | 19 October 2019 |
| 3. | 30 (Plow + harrow) | Early autumn | n.a. | n.a. | n.a. | 13 October 2018 | n.a. |
| 4. | 3–4 (Direct drilling) | Early autumn | n.a. | n.a. | 16 October 2020 | 26 October 2018 | n.a. |
| 5. | 10 (Harrow) | Late autumn | n.a. | 2 December 2019 | n.a. | 23 November 2018 | 16 November 2019 |
| 6. | 10 (Harrow) | Late autumn | DMPP 1 | n.a. | n.a. | n.a. | 16 November 2019 |
| 7. | n.a. 2 | Early spring 2 | n.a. | n.a. | n.a. | n.a. | n.a. |
| Treatment | Tillage Implement | Tillage Depth [cm] | Tillage Season | Potential N Loss [kg ha−1] | |||
|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | ||||
| 1. | Harrow | 10 | Early autumn | 141 a | 64 b | 81 c | 70 ab |
| 3. | Plow + harrow | 30 | Early autumn | n.a. | n.a. | 70 b | n.a. |
| 4. | Direct drilling | 3–4 | Early autumn | n.a. | 48 ab | 48 a | n.a. |
| 5. | Harrow | 10 | Late autumn | 170 b | n.a. | 49 a | 55 a |
| 7. | Without | n.a. | Early spring 1 | 167 b | 17 a | 20 a | 103 b |
| Treatment | Tillage Implement | Tillage Depth [cm] | Tillage Season | Disease Severity Index [%] | ||
|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 4 | ||||
| 1. | Harrow | 10 | Early autumn | 88 ± 8 | 57 ± 14 | 72 ± 10 |
| 3. | Plow + harrow | 30 | Early autumn | n.a. | n.a. | n.a. |
| 4. | Direct drilling | 3–4 | Early autumn | n.a. | 50 ± 6 | n.a. |
| 5. | Harrow | 10 | Late autumn | 72 ± 13 | n.a. | 64 ± 4 |
| 7. | Without | n.a. | Early spring 1 | 74 ± 6 | 40 ± 3 | 67 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frerichs, C.; Glied-Olsen, S.; De Neve, S.; Broll, G.; Daum, D. Crop Residue Management Strategies to Reduce Nitrogen Losses during the Winter Leaching Period after Autumn Spinach Harvest. Agronomy 2022, 12, 653. https://doi.org/10.3390/agronomy12030653
Frerichs C, Glied-Olsen S, De Neve S, Broll G, Daum D. Crop Residue Management Strategies to Reduce Nitrogen Losses during the Winter Leaching Period after Autumn Spinach Harvest. Agronomy. 2022; 12(3):653. https://doi.org/10.3390/agronomy12030653
Chicago/Turabian StyleFrerichs, Christian, Stephan Glied-Olsen, Stefaan De Neve, Gabriele Broll, and Diemo Daum. 2022. "Crop Residue Management Strategies to Reduce Nitrogen Losses during the Winter Leaching Period after Autumn Spinach Harvest" Agronomy 12, no. 3: 653. https://doi.org/10.3390/agronomy12030653
APA StyleFrerichs, C., Glied-Olsen, S., De Neve, S., Broll, G., & Daum, D. (2022). Crop Residue Management Strategies to Reduce Nitrogen Losses during the Winter Leaching Period after Autumn Spinach Harvest. Agronomy, 12(3), 653. https://doi.org/10.3390/agronomy12030653








