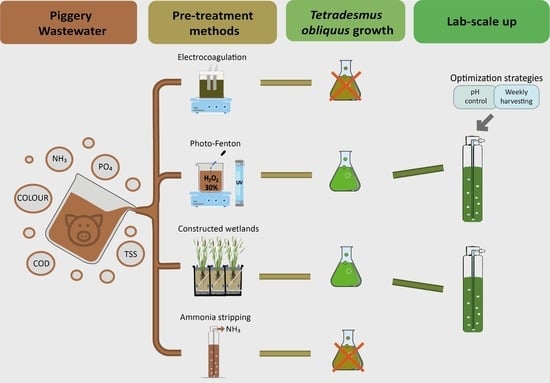
Exploring Different Pretreatment Methodologies for Allowing Microalgae Growth in Undiluted Piggery Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Effluent Feedstock
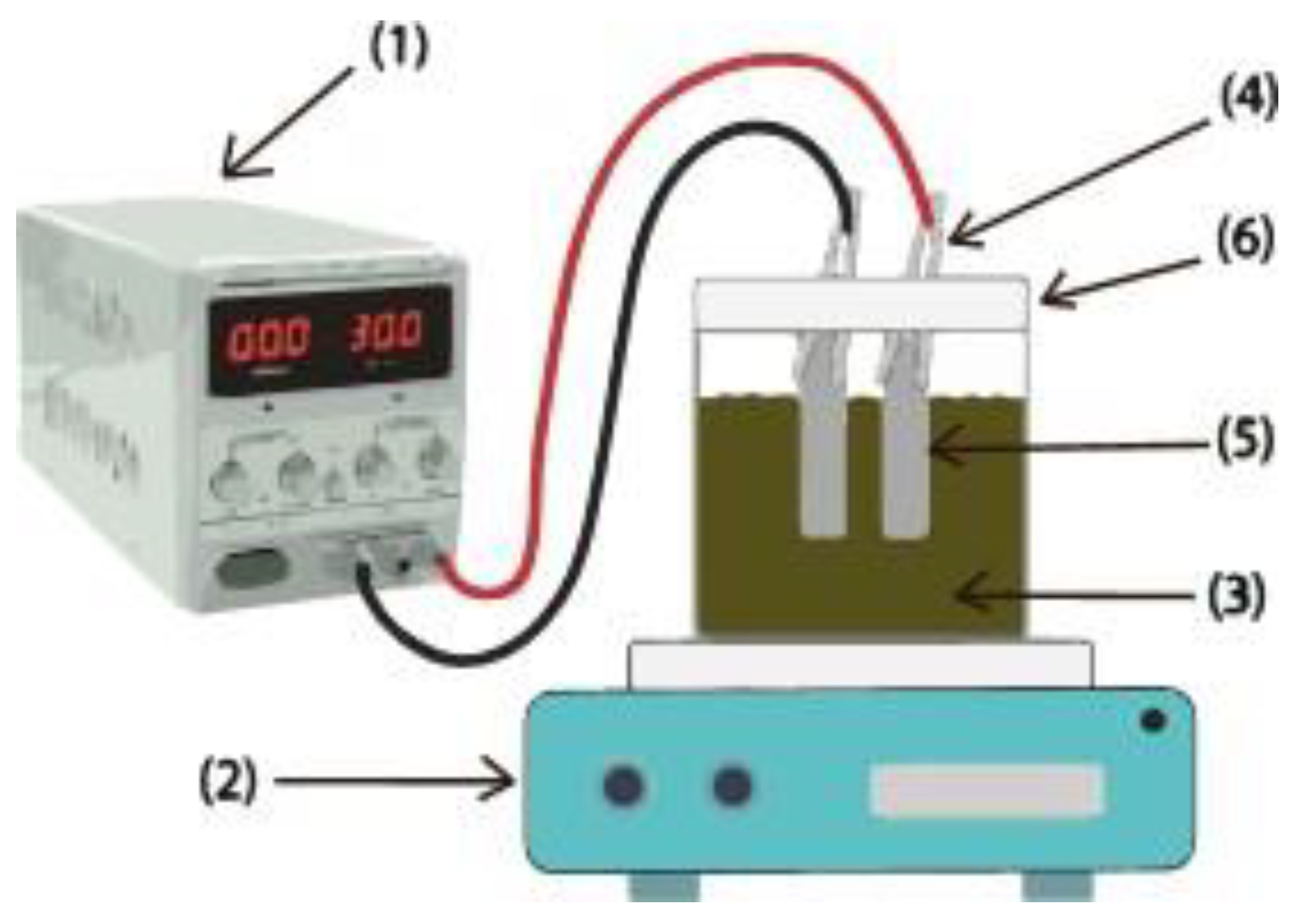
2.2. Electrocoagulation (EC)
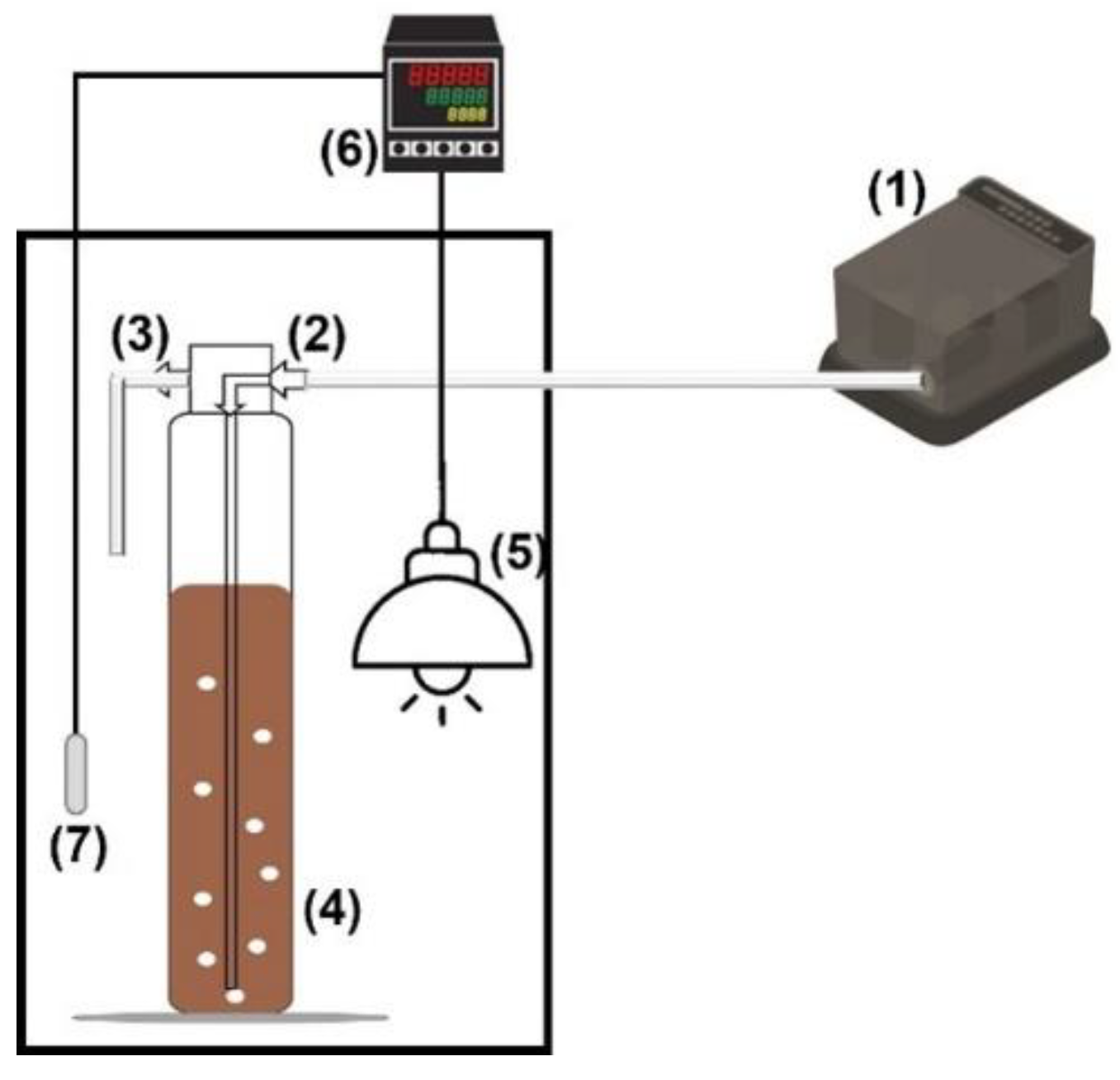
2.3. Ammonia Stripping (AS)
2.4. Photo-Fenton Process (PF)
2.5. Constructed Wetland (CW)
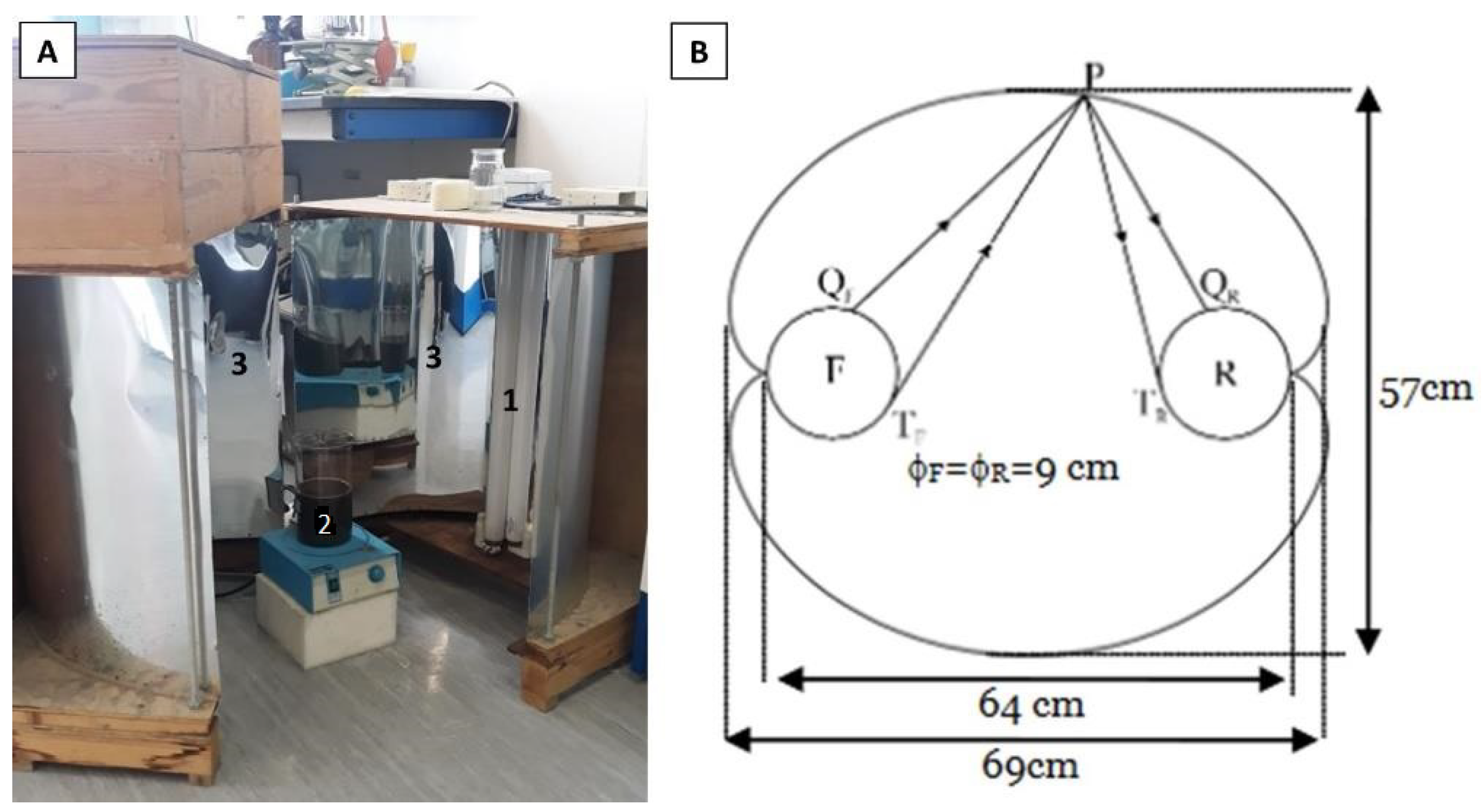
2.6. Microalga Cultivation
2.7. Culture Scale-Up
2.8. Wastewater Characterization
3. Results and Discussion
3.1. Electrocoagulation (EC)
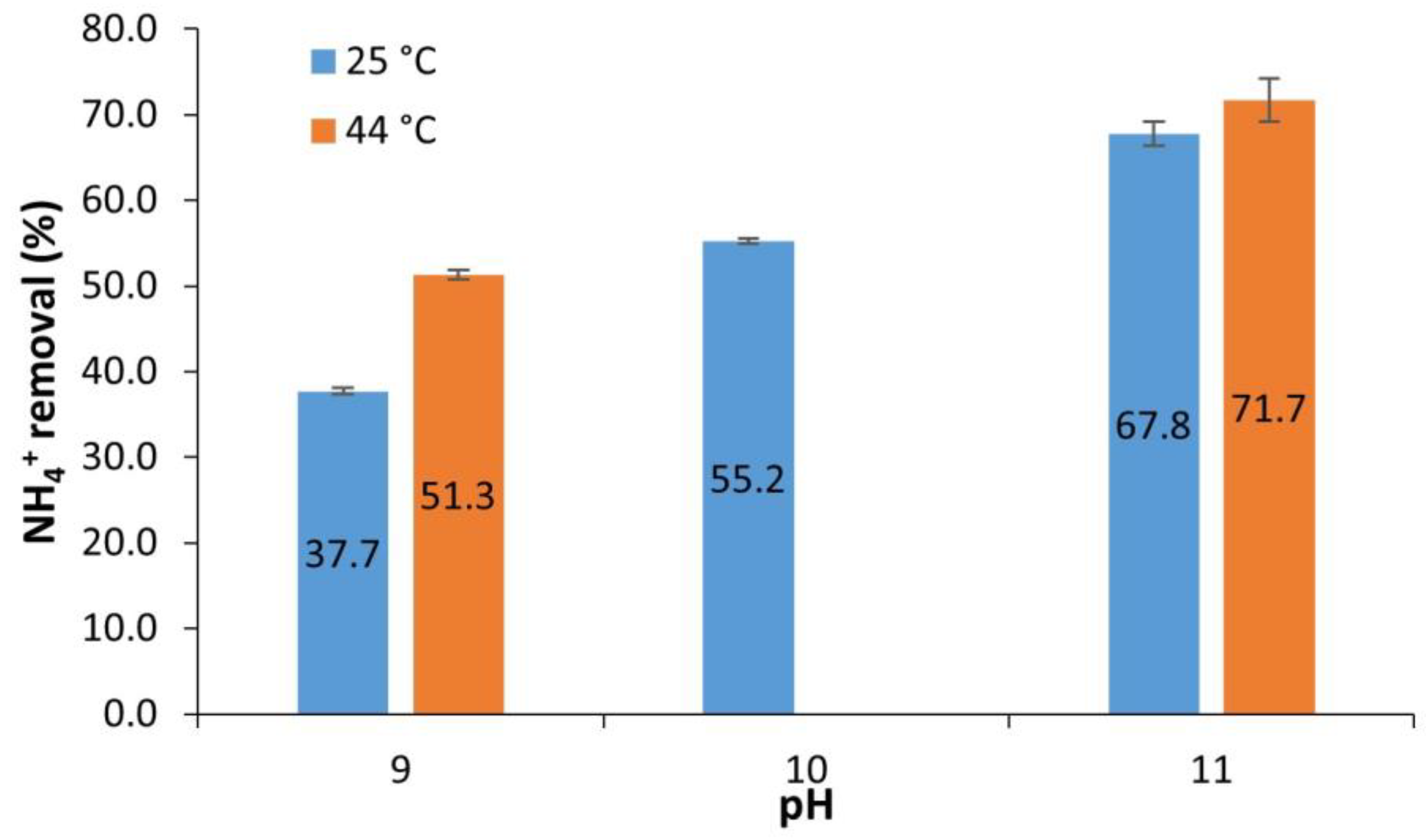
3.2. Ammonia Stripping (AS)
3.3. Photo-Fenton (PF)
3.4. Microalga Growth in the Pretreated Effluents
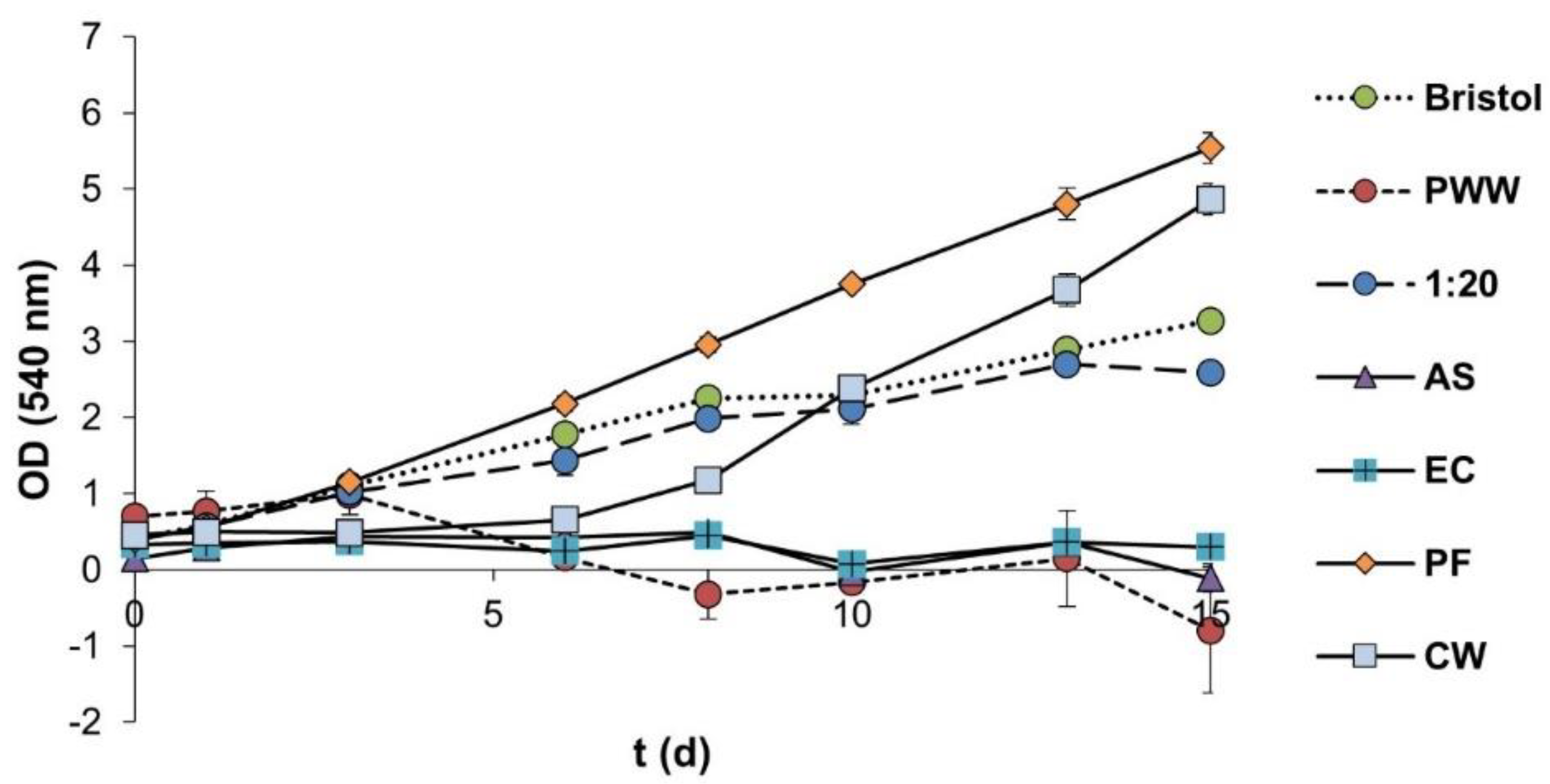
3.4.1. Pretreatment Screening
- (i)
- Electrocoagulation using zinc electrodes and current density of 20 mA·cm−2.
- (ii)
- Ammonia stripping at room temperature (25 °C) and initial pH of 11.
- (iii)
- Photo-Fenton using 1.0 g Fe2+·L−1 and 10.5 g H2O2·L−1.
- (iv)
- Constructed wetlands using expanded clay as substrate and P. australis.
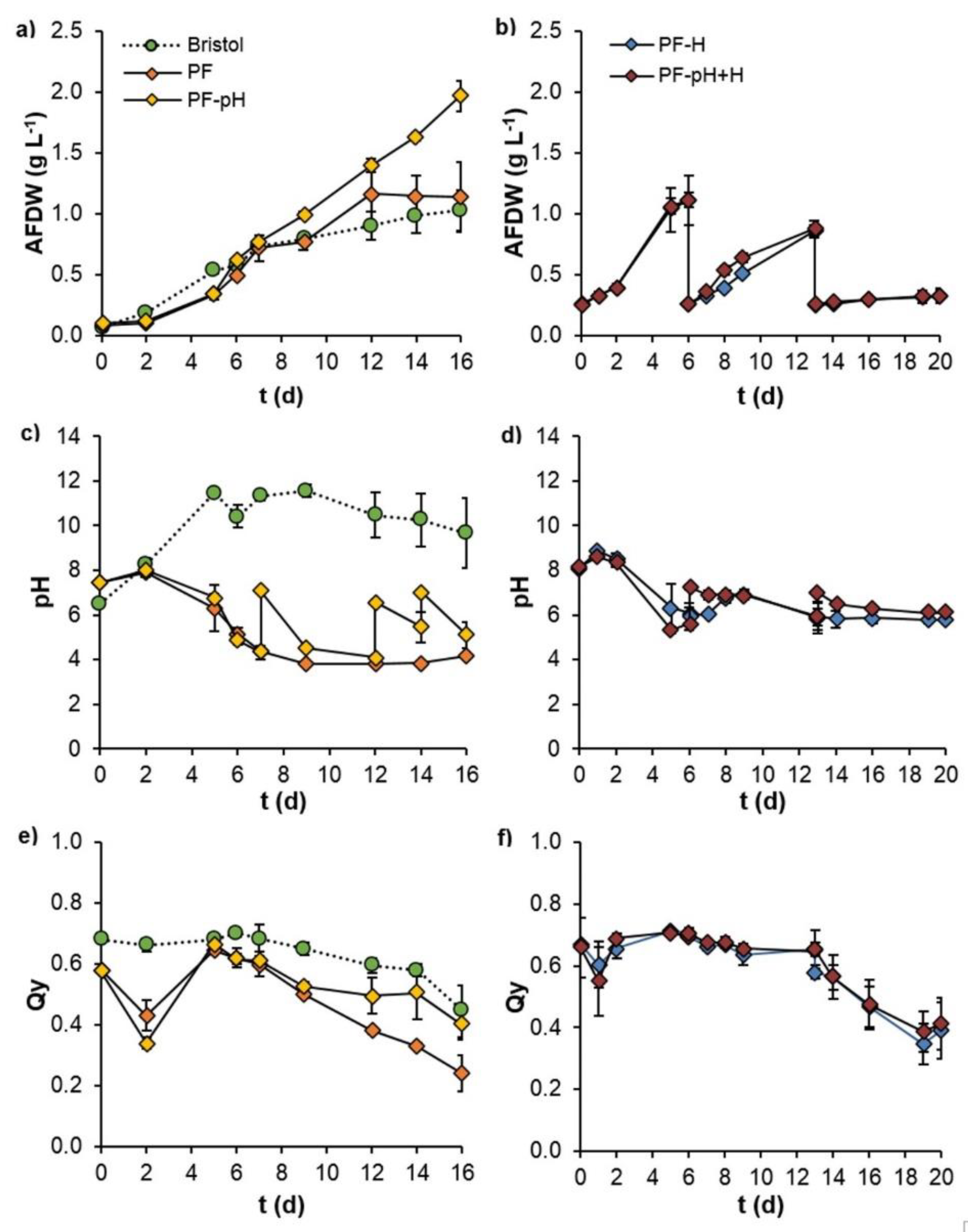
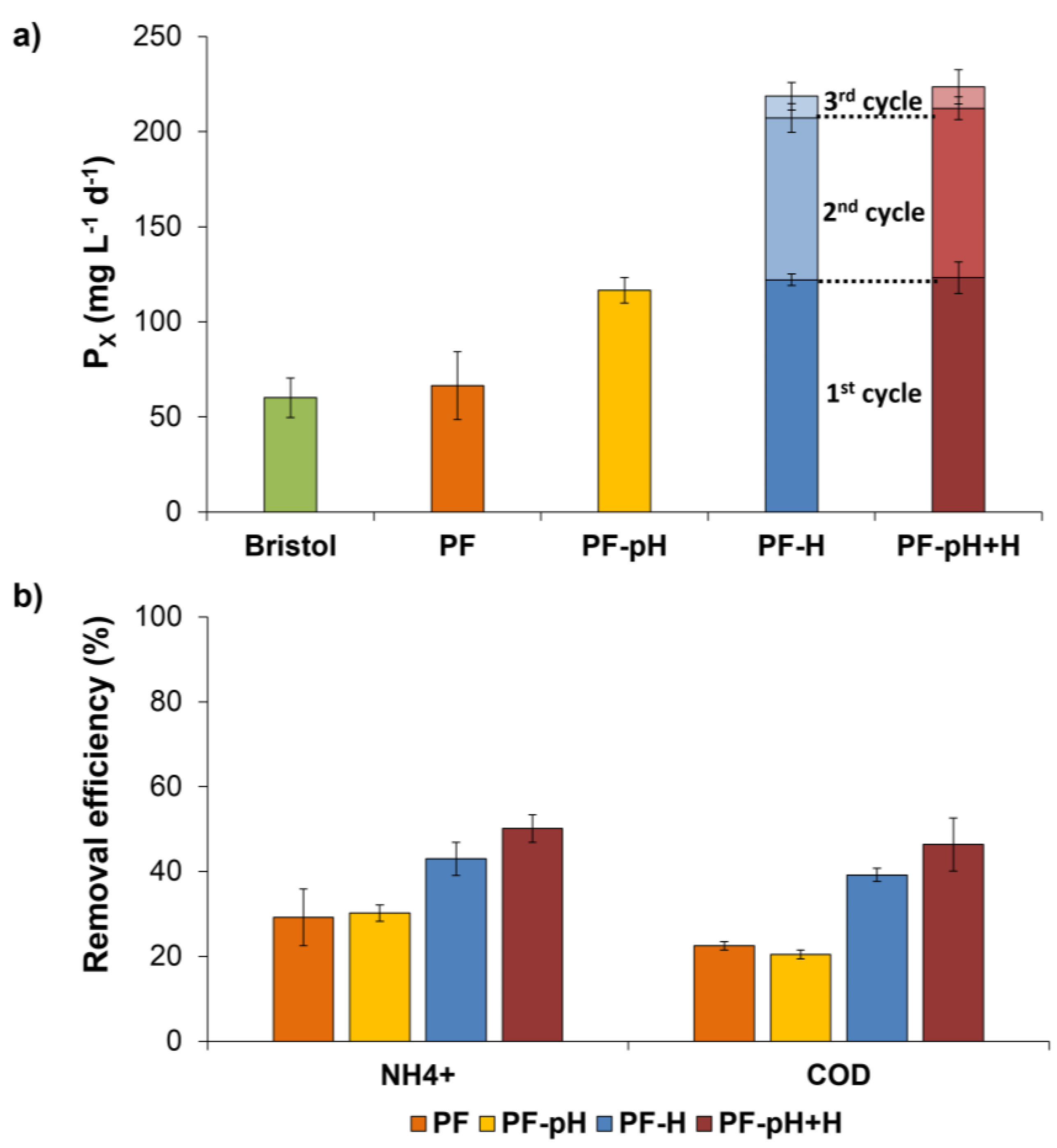
3.4.2. Culture Scale-Up
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eurostat Number of Pigs 2009–2019. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00018/default/table?lang=en (accessed on 2 September 2020).
- García, D.; Posadas, E.; Grajeda, C.; Blanco, S.; Martínez-Páramo, S.; Acién, G.; García-Encina, P.; Bolado, S.; Muñoz, R. Comparative evaluation of piggery wastewater treatment in algal-bacterial photobioreactors under indoor and outdoor conditions. Bioresour. Technol. 2017, 245, 483–490. [Google Scholar] [CrossRef] [PubMed]
- McGahan, E.J.; Phillips, F.A.; Wiedemann, S.G.; Naylor, T.A.; Warren, B.; Murphy, C.M.; Griffith, D.W.T.; Desservettaz, M.; McGahan, E.J.; Phillips, F.A.; et al. Methane, nitrous oxide and ammonia emissions from an Australian piggery with short and long hydraulic retention-time effluent storage. Anim. Prod. Sci. 2016, 56, 1376–1389. [Google Scholar] [CrossRef][Green Version]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.W.; Dong, C.D.; Lee, D.J.; Chang, J.S. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef] [PubMed]
- Ayre, J.M.; Moheimani, N.R.; Borowitzka, M.A. Growth of microalgae on undiluted anaerobic digestate of piggery effluent with high ammonium concentrations. Algal Res. 2017, 24, 218–226. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Azov, Y.; Goldman, J.C. Free Ammonia Inhibition of Algal Photosynthesis in Intensive Cultures. Appl. Environ. Microbiol. 1982, 43, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Cerqueira, A.; Russo, C.; Marques, M.R.C. Electroflocculation for textile wastewater treatment. Brazilian J. Chem. Eng. 2009, 26, 659–668. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Kinidi, L.; Tan, I.A.W.; Abdul Wahab, N.B.; Tamrin, K.F.B.; Hipolito, C.N.; Salleh, S.F. Recent Development in Ammonia Stripping Process for Industrial Wastewater Treatment. Int. J. Chem. Eng. 2018, 2018, 3181087. [Google Scholar] [CrossRef]
- Folino, A.; Calabrò, P.S.; Zema, D.A. Effects of Ammonia Stripping and Other Physico-Chemical Pretreatments on Anaerobic Digestion of Swine Wastewater. Energies 2020, 13, 3413. [Google Scholar] [CrossRef]
- Folino, A.; Zema, D.A.; Calabrò, P.S. Environmental and Economic Sustainability of Swine Wastewater Treatments Using Ammonia Stripping and Anaerobic Digestion: A Short Review. Sustainability 2020, 12, 4971. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, H.; Lee, E. Influence of Ammonia Stripping Parameters on the Efficiency and Mass Transfer Rate of Ammonia Removal. Appl. Sci. 2021, 11, 441. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Ammonia stripping for enhanced biomethanization of piggery wastewater. J. Hazard. Mater. 2012, 199–200, 36–42. [Google Scholar] [CrossRef]
- Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Jain, B.; Singh, A.K.; Kim, H.; Lichtfouse, E.; Treatment, V.S. Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett. 2019, 16, 947–967. [Google Scholar] [CrossRef]
- Sánchez Pérez, J.A.; Román Sánchez, I.M.; Carra, I.; Cabrera Reina, A.; Casas López, J.L.; Malato, S. Economic evaluation of a combined photo-Fenton/MBR process using pesticides as model pollutant. Factors affecting costs. J. Hazard. Mater. 2013, 244–245, 195–203. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Pani, N.; Tejani, V.; Anantha-Singh, T.S.; Kandya, A. Simultaneous removal of COD and Ammoniacal Nitrogen from dye intermediate manufacturing Industrial Wastewater using Fenton oxidation method. Appl. Water Sci. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Rueda-Márquez, J.J.; Levchuck, I.; Manzano, M.; Sillanpää, M. Toxicity Reduction of Industrial and Municipal Wastewater by Advanced Oxidation Processes (Photo-Fenton, UVC/H2O2, Electro-Fenton and Galvanic Fenton): A review. Catalysts 2020, 10, 612. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; Plaza-Bolaños, P.; Lorenzo, A.; Agüera, A.; García Saánchez, J.L.; Malato, S.; Sánchez Pérez, J.A. Assessment of solar raceway pond reactors for removal of contaminants of emerging concern by photo-Fenton at circumneutral pH from very different municipal wastewater effluents. Chem. Eng. J. 2019, 366, 141–149. [Google Scholar] [CrossRef]
- Abbasi, H.N.; Xie, J.; Hussain, S.I.; Lu, X. Nutrient removal in hybrid constructed wetlands: Spatial-seasonal variation and the effect of vegetation. Water Sci. Technol. 2019, 79, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Yadav, A.K.; Garaniya, V.; Abbassi, R. Constructed wetland coupled microbial fuel cell technology: Development and potential applications. In Biomass, Biofuels, Biochemicals: Microbial Electrochemical Technology: Sustainable Platform for Fuels, Chemicals and Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1021–1036. ISBN 9780444640529. [Google Scholar]
- Harrington, C.; Scholz, M. Assessment of pre-digested piggery wastewater treatment operations with surface flow integrated constructed wetland systems. Bioresour. Technol. 2010, 101, 6950–6960. [Google Scholar] [CrossRef] [PubMed]
- Nuamah, L.A.; Li, Y.; Pu, Y.; Nwankwegu, A.S.; Haikuo, Z.; Norgbey, E.; Banahene, P.; Bofah-Buoh, R. Constructed wetlands, status, progress, and challenges. The need for critical operational reassessment for a cleaner productive ecosystem. J. Clean. Prod. 2020, 269, 122340. [Google Scholar] [CrossRef]
- Dias, S.; Mucha, A.P.; Duarte Crespo, R.; Rodrigues, P.; Almeida, C.M.R. Livestock Wastewater Treatment in Constructed Wetlands for Agriculture Reuse. Int. J. Environ. Res. Public Health 2020, 17, 8592. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062. [Google Scholar] [CrossRef]
- APHA Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998; Volume 51, ISBN 0002-9572.
- Ghernaout, D.; Ghernaout, B.; Kellil, A. Natural organic matter removal and enhanced coagulation as a link between coagulation and electrocoagulation. New Pub Balaban 2012, 2, 203–222. [Google Scholar] [CrossRef]
- Chen, R.F.; Wu, L.; Zhong, H.T.; Liu, C.X.; Qiao, W.; Wei, C.H. Evaluation of electrocoagulation process for high-strength swine wastewater pretreatment. Sep. Purif. Technol. 2021, 272, 118900. [Google Scholar] [CrossRef]
- Lei, X.; Maekawa, T. Electrochemical treatment of anaerobic digestion effluent using a Ti/Pt–IrO2 electrode. Bioresour. Technol. 2007, 98, 3521–3525. [Google Scholar] [CrossRef]
- Lourinho, G.; Brito, P.S.D. Electrolytic Treatment of Swine Wastewater: Recent Progress and Challenges. Waste Biomass Valorization 2020, 12, 553–576. [Google Scholar] [CrossRef]
- Yavuz, Y.; Ögütveren, Ü.B. Treatment of industrial estate wastewater by the application of electrocoagulation process using iron electrodes. J. Environ. Manag. 2018, 207, 151–158. [Google Scholar] [CrossRef]
- Holt, P.; Barton, G.; Mitchell, C. Electrocoagulation as a wastewater treatment. Third Annu. Aust. Environ. Eng. Res. Event 1999, 1000, 41–46. [Google Scholar]
- Collos, Y.; Harrison, P.J. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Godos, I.d.; Vargas, V.A.; Blanco, S.; González, M.C.G.; Soto, R.; García-Encina, P.A.; Becares, E.; Muñoz, R. A comparative evaluation of microalgae for the degradation of piggery wastewater under photosynthetic oxygenation. Bioresour. Technol. 2010, 101, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Goi, A.; Trapido, M. Hydrogen peroxide photolysis, Fenton reagent and photo-Fenton for the degradation of nitrophenols: A comparative study. Chemosphere 2002, 46, 913–922. [Google Scholar] [CrossRef]
- Zoh, K.D.; Stenstrom, M.K. Fenton oxidation of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX). Water Res. 2002, 36, 1331–1341. [Google Scholar] [CrossRef]
- Velásquez, M.; Santander, I.P.; Contreras, D.R.; Yáñez, J.; Zaror, C.; Salazar, R.A.; Pérez-Moya, M.; Mansilla, H.D. Oxidative degradation of sulfathiazole by Fenton and photo-Fenton reactions. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2014, 49, 661–670. [Google Scholar] [CrossRef]
- Pulgarin, A.; Giannakis, S.; Pulgarin, C.; Ludwig, C.; Refardt, D. A novel proposition for a citrate-modified photo-Fenton process against bacterial contamination of microalgae cultures. Appl. Catal. B Environ. 2020, 265, 118615. [Google Scholar] [CrossRef]
- Eustance, E.; Gardner, R.D.; Moll, K.M.; Menicucci, J.; Gerlach, R.; Peyton, B.M. Growth, nitrogen utilization and biodiesel potential for two chlorophytes grown on ammonium, nitrate or urea. J. Appl. Phycol. 2013, 25, 1663–1677. [Google Scholar] [CrossRef]
- Mutoti, M.; Gumbo, J.; Jideani, A.I.O. Occurrence of cyanobacteria in water used for food production: A review. Phys Chem Earth 2022, 125, 103101. [Google Scholar] [CrossRef]
- Ji, X.; Verspagen, J.M.H.; Stom, M.; Huisman, J. Competition between cyanobacteria and green algae at low versus elevated CO2: Who will win, and why? J. Exp. Bot. 2017, 68, 3815–3828. [Google Scholar] [CrossRef] [PubMed]
- Decree-Law No 236/98 Decree-Law 236/98 of the Portuguese Ministry of the Environment of 1 August establishing water quality standards. Diário da República I Série-A 1998, 3676–3722.
- Solovchenko, A.; Khozin-Goldberg, I. High-CO2 tolerance in microalgae: Possible mechanisms and implications for biotechnology and bioremediation. Biotechnol. Lett. 2013, 35, 1745–1752. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 1–8. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of Light Intensity and Quality on Growth Rate and Composition of Chlorella vulgaris. Plants 2020, 9, 31. [Google Scholar] [CrossRef]

| Conditions | Removal Efficiency (%) | ||
|---|---|---|---|
| Electrode Material | Current Density (mA·cm−2) | COD | TSS |
| Al | 4 | 18.4 ± 2.6 | −31.6 ± 2.2 |
| 12 | 7.9 ± 2.6 | −56.9 ± 0.0 | |
| 20 | 13.2 ± 2.6 | −56.2 ± 7.9 | |
| Fe | 4 | 6.0 ± 3.2 | −5.4 ± 0.0 |
| 12 | 9.3 ± 6.4 | −19.2 ± 4.3 | |
| 20 | 22.2 ± 6.5 | −33.0 ± 2.6 | |
| Zn | 4 | 34.7± 3.1 | 20.1 ± 2.2 |
| 12 | 37.8 ± 0.0 | 7.1 ± 2.2 | |
| 20 | 40.9 ± 3.1 | 9.3 ± 0.0 | |
| Conditions | Removal Efficiency (%) | Residual H2O2 (g·L−1) | ||||
|---|---|---|---|---|---|---|
| Fe2+ (g·L−1) | H2O2 (g·L−1) | Color | COD | TSS | NH4+ | |
| 0.2 | 1.0 | 81.5 ± 5.0 | 82.3 ± 0.8 | 91.7 ± 0.5 | 7.99 ± 1.76 | 0.26 |
| 4.9 | 83.3 ± 5.3 | 27.4 ± 0.0 | 85.0 ± 1.3 | 9.89 ± 2.57 | 3.21 | |
| 10.5 | 78.3 ± 1.2 | 22.3 ± 0.0 | 90.1 ± 0.8 | 3.76 ± 2.57 | 3.54 | |
| 0.5 | 1.0 | 91.3 ± 1.6 | 84.8 ± 0.0 | 93.1 ± 0.1 | 2.03 ± 0.58 | 0.14 |
| 4.9 | 92.2 ± 4.4 | 89.4 ± 0.4 | 92.7 ± 0.3 | −1.29 ± 0.06 | 1.43 | |
| 10.5 | 88.9 ± 8.3 | 84.4 ± 1.3 | 93.4 ± 0.4 | −4.13 ± 1.70 | 4.17 | |
| 1.0 | 1.0 | 94.5 ± 1.1 | 88.7 ± 0.2 | 99.9 ± 0.1 | −7.07 ± 0.52 | 0.26 |
| 4.9 | 96.9 ± 0.7 | 91.6 ± 0.5 | 100 ± 0 | −1.97 ± 0.29 | 0.30 | |
| 10.5 | 97.2 ± 0.5 | 92.6 ± 0.5 | 98.5 ± 0.1 | 0.69 ± 0.30 | 0.16 | |
| PWW | COD (mg O2·L−1) | NH4+ (mg·L−1) | PO43− (mg·L−1) | TSS (mg·L−1) | Color |
|---|---|---|---|---|---|
| Raw | 3759 ± 71 | 1500 ± 7 | 97.5 | 2575 ± 45 | 6.640 |
| 1:20 | 184.4 ± 7.1 | 64.6 ± 1.05 | 6.40 | 90.0 ± 0.00 | 0.300 |
| AS | 2766 ± 71 | 731.2 ± 1.1 | 81.0 | 1285 ± 125 | 2.830 |
| EC | 1489 ± 71 | 1329 ± 12 | 37.5 | 207.5 ± 27.5 | 1.070 |
| PF | 276.6 ± 14.2 | 1209 ± 11 | 0.560 | 37.5 ± 2.5 | 0.019 |
| CW | 319.1 ±7.1 | 122.6 ± 2.5 | 77.0 | 52.5 ± 2.5 | 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.; Figueiredo, D.; Cardeiras, R.; Nabais, R.; Ferreira, F.; Ribeiro, B.; Cordovil, C.M.d.S.; Acién, F.G.; Gouveia, L. Exploring Different Pretreatment Methodologies for Allowing Microalgae Growth in Undiluted Piggery Wastewater. Agronomy 2022, 12, 580. https://doi.org/10.3390/agronomy12030580
Ferreira A, Figueiredo D, Cardeiras R, Nabais R, Ferreira F, Ribeiro B, Cordovil CMdS, Acién FG, Gouveia L. Exploring Different Pretreatment Methodologies for Allowing Microalgae Growth in Undiluted Piggery Wastewater. Agronomy. 2022; 12(3):580. https://doi.org/10.3390/agronomy12030580
Chicago/Turabian StyleFerreira, Alice, Daniel Figueiredo, Rodrigo Cardeiras, Rui Nabais, Francisca Ferreira, Belina Ribeiro, Cláudia M. d. S. Cordovil, F. Gabriel Acién, and Luisa Gouveia. 2022. "Exploring Different Pretreatment Methodologies for Allowing Microalgae Growth in Undiluted Piggery Wastewater" Agronomy 12, no. 3: 580. https://doi.org/10.3390/agronomy12030580
APA StyleFerreira, A., Figueiredo, D., Cardeiras, R., Nabais, R., Ferreira, F., Ribeiro, B., Cordovil, C. M. d. S., Acién, F. G., & Gouveia, L. (2022). Exploring Different Pretreatment Methodologies for Allowing Microalgae Growth in Undiluted Piggery Wastewater. Agronomy, 12(3), 580. https://doi.org/10.3390/agronomy12030580