Enhancing the Rural Landscape Character: The Low Frequency of Inter-Row Wildflower Meadow Harvest Positively Affects Biodiversity While Maintaining Grape Quantitative and Qualitative Traits in a ‘Sultanina’ Vineyard in Greece
Abstract
:1. Introduction
2. Materials and Methods
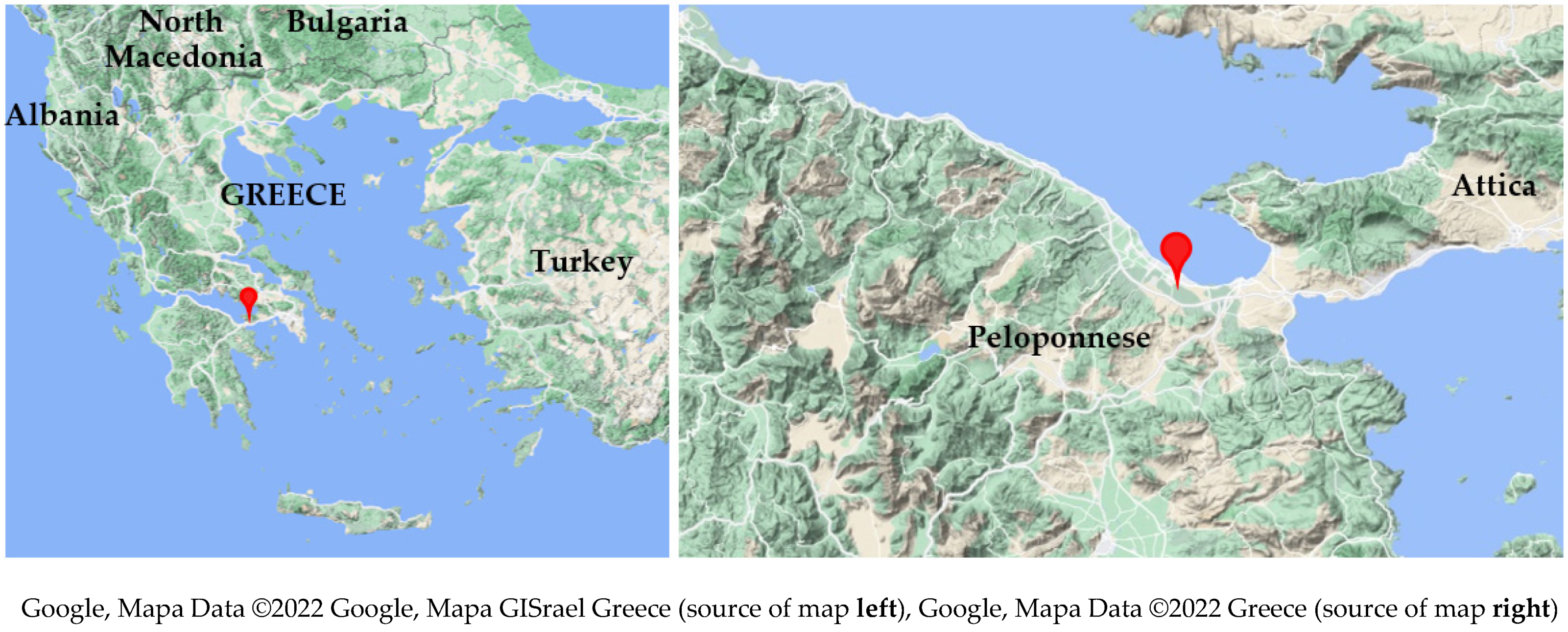
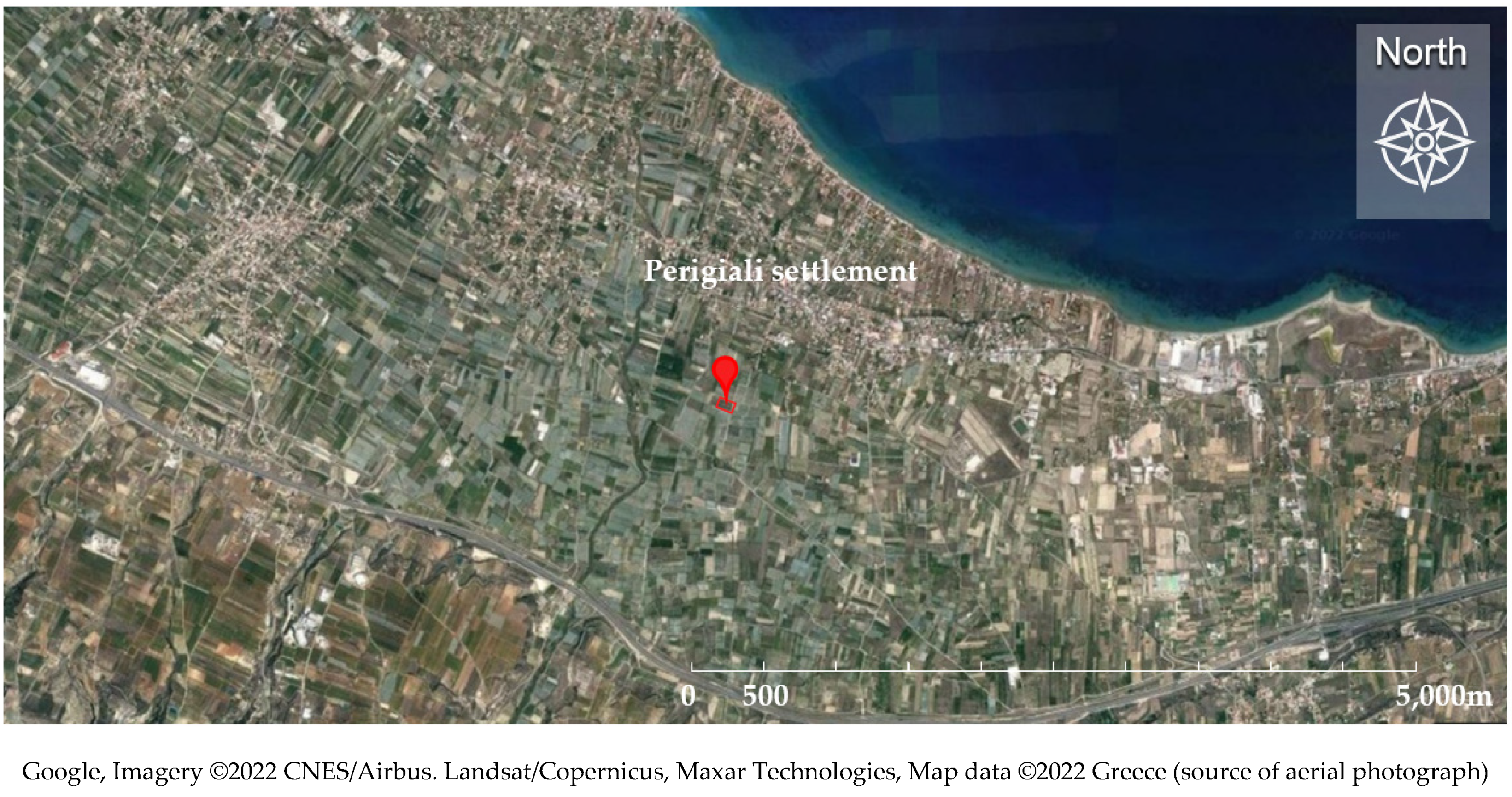
2.1. Experimental Site and Growth Conditions
2.2. Experimental Design and Treatments
2.3. Measurements
2.3.1. Meadow Composition
2.3.2. Arthropod Abundance
2.3.3. Quantitative and Qualitative Characteristics of Grapes
2.4. Statistical Analysis
3. Results
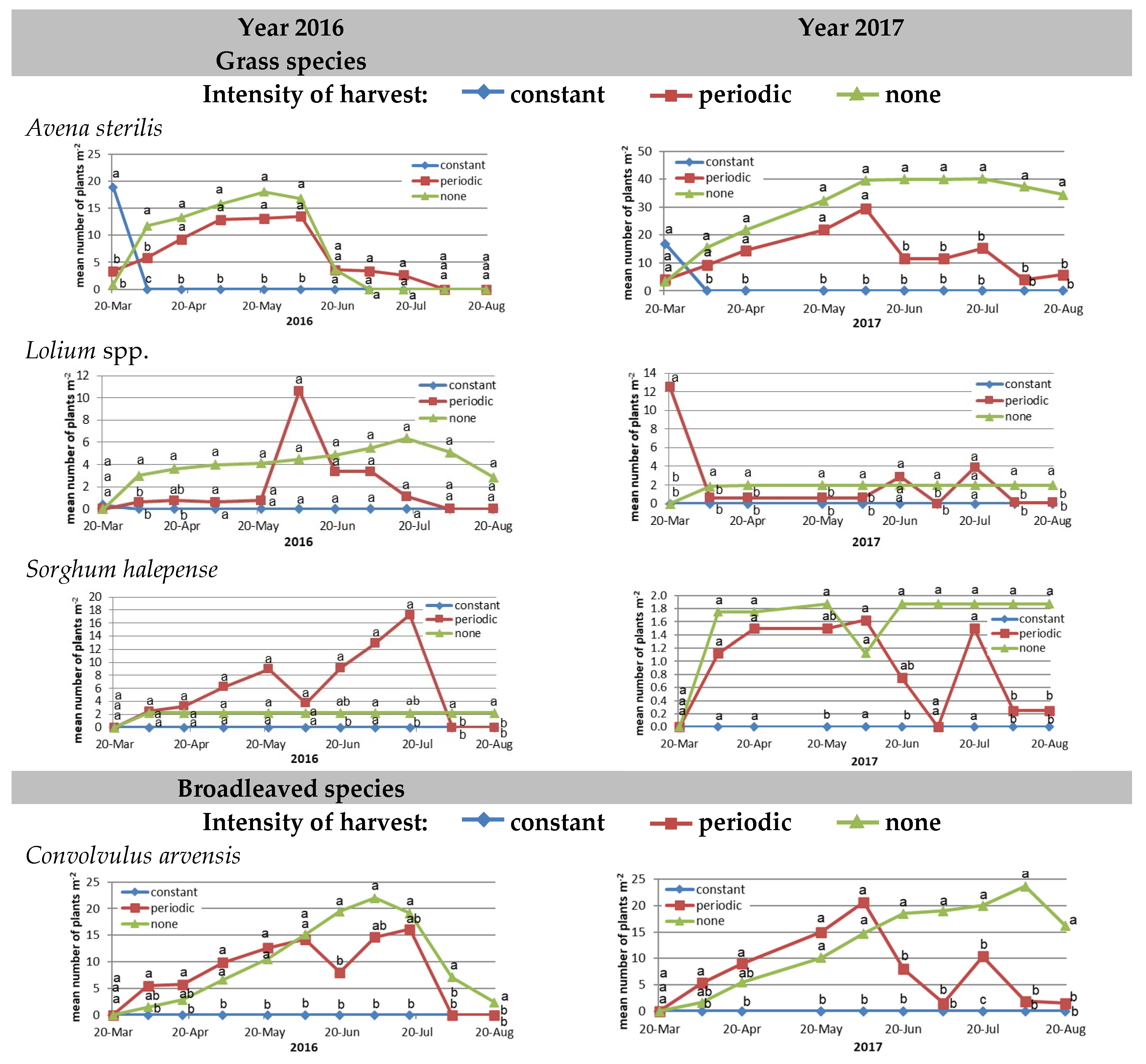
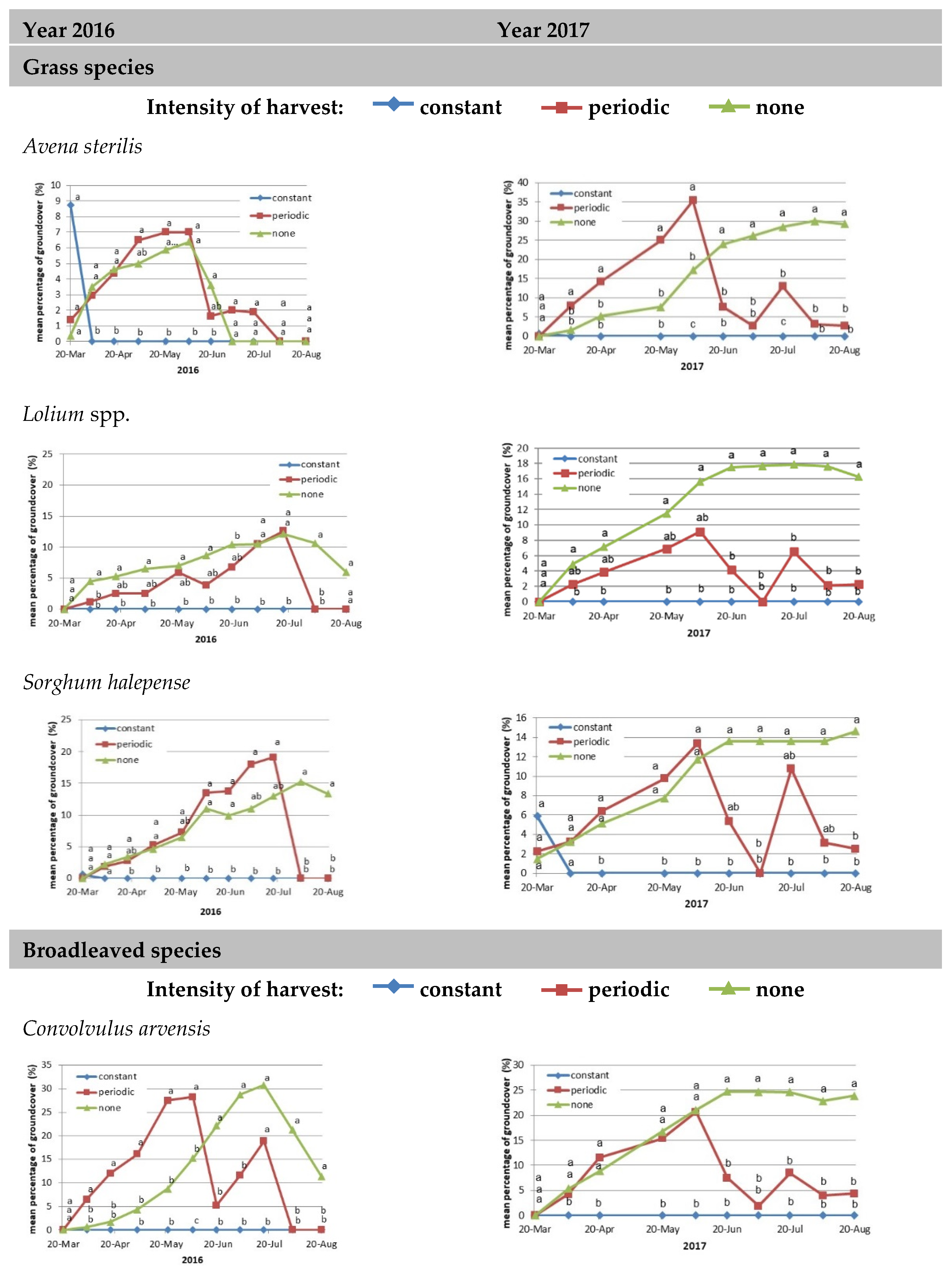
3.1. Meadow Composition
3.2. Arthropod Abundance
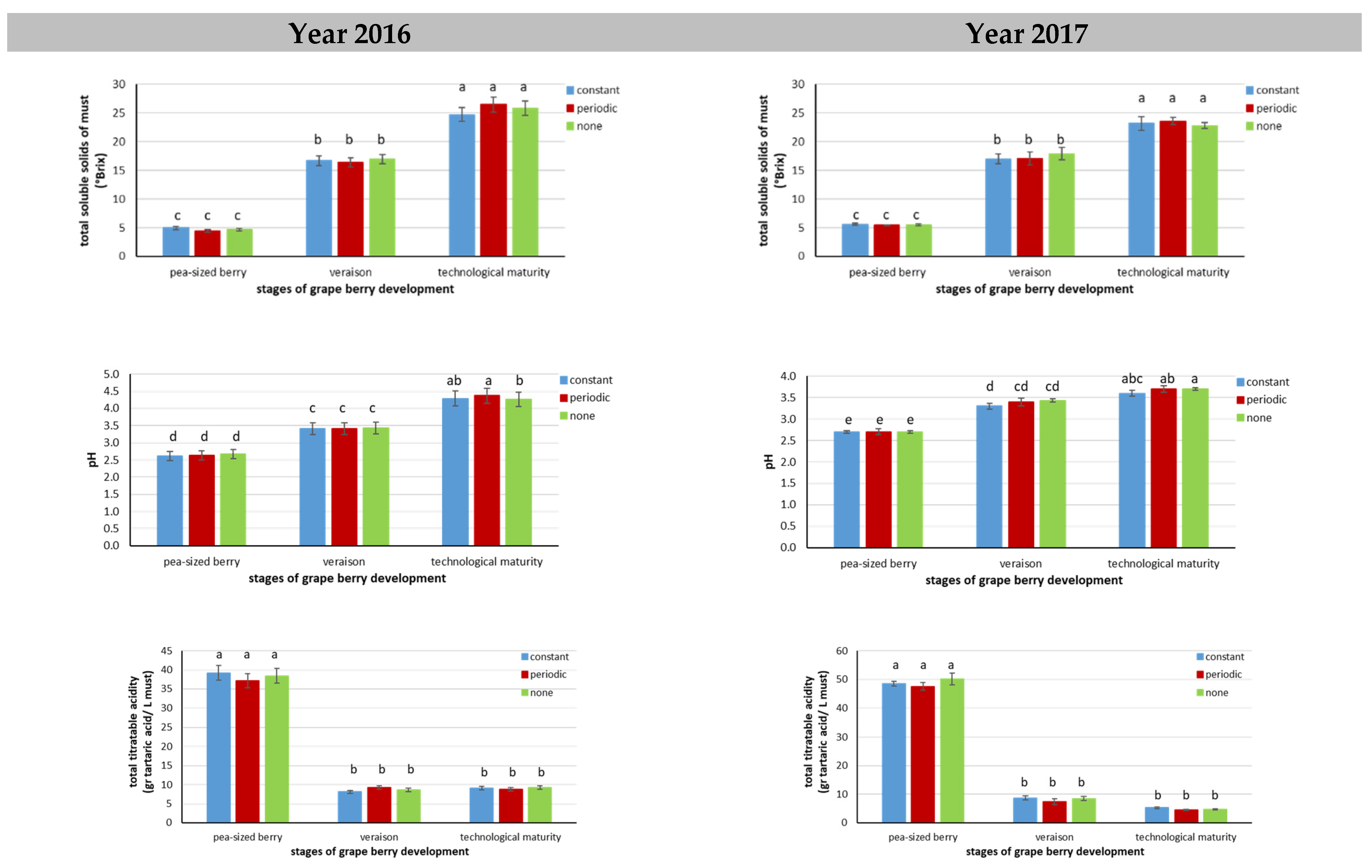
3.3. Quantitative and Qualitative Characteristics of Grapes
4. Discussion
4.1. Meadow Composition
4.2. Arthropod Abundance
4.3. Quantitative and Qualitative Characteristics of Grapes
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Feced, C.; Weissteiner, C.J.; Baraldi, A.; Paracchini, M.L.; Maes, J.; Zulian, G.; Kempen, M.; Elbersen, B.S.; Pérez-Soba, M. Semi-natural vegetation in agricultural land: European map and links to ecosystem service supply. Agron. Sustain. Dev. 2015, 35, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Kefalas, G.; Kalogirou, S.; Poirazidis, K.; Lorilla, R.S. Landscape transition in Mediterranean islands: The case of Ionian islands, Greece 1985–2015. Landsc. Urban Plan. 2019, 191, 103641. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Ministry of Environment, Energy & Climate Change. National Biodiversity Strategy and Action Plan; Hellenic Republic, Ministry of Environment, Energy & Climate Change: Athens, Greece, 2014; p. 128.
- UNESCO. Florence Declaration on Landscape. Final Declaration of the UNESCO International Meeting on “The International Protection of Landscapes”; UNESCO: Florence, Italy, 2012. [Google Scholar]
- Haaland, C.; Naisbit, R.; Bersier, L.-F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2010, 4, 60–80. [Google Scholar] [CrossRef]
- Greenwell, M.P.; Brereton, T.; Day, J.C.; Roy, D.B.; Oliver, T.H. Predicting resilience of ecosystem functioning from co-varying species’ responses to environmental change. Ecol. Evol. 2019, 9, 11775–11790. [Google Scholar] [CrossRef] [Green Version]
- Lindemann-Matthies, P.; Junge, X.; Matthies, D. The influence of plant diversity on people’s perception and aesthetic appreciation of grassland vegetation. Biol. Conserv. 2010, 143, 195–202. [Google Scholar] [CrossRef] [Green Version]
- UN. Transforming Our World: The 2030 Agenda for Sustainable Development; A/RES/70/1; UN General Assembly: New York, NY, USA, 2015. [Google Scholar]
- UN. The Sustainable Development Report, 2021; United Nations Publications: New York, NY, USA, 2021. [Google Scholar]
- European Commission. Sustainable Agriculture for the Future We Want; European Union: Luxembourg, 2012; p. 8. [Google Scholar]
- Dessyllas, M. Organic viticulture in Greece. In Proceedings of the 6th International Congress on Organic Viticulture, Basel, Switzerland, 25–26 August 2000; pp. 58–61. [Google Scholar]
- ELSTAT. 2009 & 2010 Statistical Yearbook of Greece; Hellenic Statistical Authority: Piraeus, Greece, 2011; p. 613. [Google Scholar]
- Banilas, G.; Korkas, E.; Kaldis, P.; Hatzopoulos, P. Olive and Grapevine Biodiversity in Greece and Cyprus—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Springer: Dordrecht, Germany, 2009; pp. 401–428. [Google Scholar] [CrossRef]
- Verešová, M.; Supuka, J. Changes of landscape structure and cultural values of vineyard landscape. Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 1459–1470. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, A.M. The Exile in Search of Refuge: Lines written among the Euganean Hills. In Shelley’s Italian Experience. Studies in Romanticism; Weinberg, A.M., Ed.; Palgrave Macmillan: London, UK, 1991; pp. 21–43. [Google Scholar] [CrossRef]
- Bruwer, J.D.W.; Lesschaeve, I. Wine Tourists’ Destination Region Brand Image Perception and Antecedents: Conceptualization of a Winescape Framework. J. Travel Tour. Mark. 2012, 29, 611–628. [Google Scholar] [CrossRef]
- McNicol, B.J.; Glorioso, R.S. Second home leisure landscapes and retirement in the Canadian Rocky Mountain community of Canmore, Alberta. Ann. Leis. Res. 2014, 17, 27–49. [Google Scholar] [CrossRef]
- Bennett, J. The Artist’ Garden, the Secret Spaces That Inspired Great Art; White Lion Publushing: London, UK, 2019; p. 19. [Google Scholar]
- Sfikas, G. The Botanical Paradises of Greece; Toubis Editions: Athens, Greece, 2001; p. 256. [Google Scholar]
- Paracchini, M.L.; Petersen, J.-E.; Hoogeveen, Y.; Bamps, C.; Burfield, I.; van Swaay, C. High Nature Value Farmland in Europe—An Estimate of the Distribution Patterns on the Basis of Land Cover and Biodiversity Data; EUR—Scientific and Technical Research Series; Office for Official Publications of the European Communities: Luxembourg, 2008; p. 87. [Google Scholar]
- Andersen, E.; Baldock, D.; Bennet, H.; Beaufoy, G.; Bignal, E.; Brower, F.; Elbersen, B.; Eiden, G.; Godeschalk, F.; Jones, G.; et al. Developing a High Nature Value Farming Area Indicator; Internal Report for the European Environment Agency; IEEP: Bruxelles, Belgium, 2007. [Google Scholar]
- Hitchmough, J.D. Semi-Natural Grasslands and Meadows. In Environmental Horticulture, Science and Management of Green Landscapes; Cameron, R.W.F., Hitchmough, J.D., Eds.; CABI: Wallingford, UK, 2016; pp. 175–191. [Google Scholar]
- Hitchmough, J. Naturalistic herbaceous vegetation for urban landscapes. In The Dynamic Landscape; Dunnett, N., Hitchmough, J., Eds.; Spon Press: London, UK, 2004; pp. 130–183. [Google Scholar]
- Council of Europe. European Landscape Convention. European Treaty Series—No. 176; Council of Europe: Florence, Italy, 2000; p. 7. [Google Scholar]
- Bretzel, F.; Pezzarossa, B. Sustainable Management of Urban Landscapes with Wildflowers. Acta Hortic. 2010, 881, 213–219. [Google Scholar] [CrossRef]
- Bullock, J.M.; McCracken, M.E.; Bowes, M.J.; Chapman, R.E.; Graves, A.R.; Hinsley, S.A.; Hutchins, M.G.; Nowakowski, M.; Nicholls, D.J.; Oakley, S.; et al. Does agri-environmental management enhance biodiversity and multiple ecosystem services? A farm-scale experiment. Agric. Ecosyst. Environ. 2021, 320, 107582. [Google Scholar] [CrossRef]
- Hitchmough, J. The plant community: A model for horticultural thought and practice in the 21st century? Acta Hortic. 2017, 1189, 113–118. [Google Scholar] [CrossRef]
- Bretzel, F.; Pezzarossa, B.; Carrai, C.; Malorgio, F. Wildflower plantings to reduce the management costs of urban gardens and roadsides. Acta Hortic. 2009, 813, 263–270. [Google Scholar] [CrossRef]
- Guerra, B.; Steenwerth, K. Influence of Floor Management Technique on Grapevine Growth, Disease Pressure, and Juice and Wine Composition: A Review. Am. J. Enol. Vitic. 2012, 63, 149–164. [Google Scholar] [CrossRef]
- Baumgartner, K.; Steenwerth, K.L.; Veilleux, L. Cover-Crop Systems Affect Weed Communities in a California Vineyard. Weed Sci. 2008, 56, 596–605. [Google Scholar] [CrossRef]
- Madge, D. Organic Farming: Vineyard Weed Management; Department of Primary Industries: Melbourne, VC, Australia, 2007; p. 10.
- Beslic, Z.; Pantelić, M.; Dabic, D.; Todic, S.; Natić, M.; Tesic, Z. Effect of vineyard floor management on water regime, growth response, yield and fruit quality in Cabernet Sauvignon. Sci. Hortic. 2015, 197, 650–656. [Google Scholar] [CrossRef]
- Gómez, J.A. Sustainability using cover crops in Mediterranean tree crops, olives and vines—Challenges and current knowledge. Hung. Geogr. Bull. 2017, 66, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Novara, A.; Cerda, A.; Barone, E.; Gristina, L. Cover crop management and water conservation in vineyard and olive orchards. Soil Tillage Res. 2021, 208, 104896. [Google Scholar] [CrossRef]
- Bekkers, T. Weed Control Options for Commercial Organic Vineyards. Wine Vitic. J. 2011, 26, 62–64. [Google Scholar]
- Carvell, C.; Mitschunas, N.; McDonald, R.; Hulmes, S.; Hulmes, L.; O’Connor, R.S.; Garratt, M.P.; Potts, S.G.; Fountain, M.T.; Sadykova, D.; et al. Establishment and management of wildflower areas for insect pollinators in commercial orchards. Basic Appl. Ecol. 2021, 58, 2–14. [Google Scholar] [CrossRef]
- Koniak, G.; Noy-Meir, I.; Perevolotsky, A. Modelling dynamics of ecosystem services basket in Mediterranean landscapes: A tool for rational management. Landsc. Ecol. 2011, 26, 109–124. [Google Scholar] [CrossRef]
- Muscas, E.; Cocco, A.; Mercenaro, L.; Cabras, M.; Lentini, A.; Porqueddu, C.; Nieddu, G. Effects of vineyard floor cover crops on grapevine vigor, yield, and fruit quality, and the development of the vine mealybug under a Mediterranean climate. Agric. Ecosyst. Environ. 2017, 237, 203–212. [Google Scholar] [CrossRef]
- Power, A.G. Ecosystem services and agriculture: Tradeoffs and synergies. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Romo, M.G.; Veas-Bernal, A.; Martínez-García, H.; Campos-Herrera, R.; Ibáñez-Pascual, S.; Martínez-Villar, E.; Pérez-Moreno, I.; Marco-Mancebón, V.S. Ground cover management in a Mediterranean vineyard: Impact on insect abundance and diversity. Agric. Ecosyst. Environ. 2019, 283, 106571. [Google Scholar] [CrossRef]
- Fiera, C.; Ulrich, W.; Popescu, D.M.; Bunea, C.-I.; Manu, M.; Nae, I.; Stan, M.; Markó, B.; Urák, I.; Giurginca, A.; et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in Central Romania. J. Insect Conserv. 2020, 24, 175–185. [Google Scholar] [CrossRef] [Green Version]
- ELSTAT. Aπογραφή Πληθυσμού—Κατοικιών 2011. Μόνιμος Πληθυσμός (Census 2011); Hellenic Statistical Authority: Piraeus, Greece, 2011. [Google Scholar]
- IERSD-NOA. Monthly Bulletin; Institute of Environmental Research and Sustainable Development of the National Observatory of Athens: Penteli, Greece, 2016; Available online: https://meteo.gr/Monthly_Bulletins.cfm (accessed on 5 January 2022).
- IERSD-NOA. Monthly Bulletin; Institute of Environmental Research and Sustainable Development of the National Observatory of Athens: Penteli, Greece, 2017; Available online: https://meteo.gr/Monthly_Bulletins.cfm (accessed on 5 January 2022).
- HNMS. Climatic Bulletin; Hellenic National Meteorological Service: Helliniko, Greece, 2016; Available online: http://www.emy.gr/emy/en/climatology/climatology (accessed on 5 January 2022).
- HNMS. Climatic Bulletin; Hellenic National Meteorological Service: Helliniko, Greece, 2017; Available online: http://www.emy.gr/emy/en/climatology/climatology (accessed on 5 January 2022).
- Meléndez, E.; Ortiz, M.; Sarabia, L.; Íñiguez, M.; Puras, P. Modelling phenolic and technological maturities of grapes by means of the multivariate relation between organoleptic and physicochemical properties. Anal. Chim. Acta 2013, 761, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Paraskevopoulou, A.; Pappous, E.; Bertsouklis, K.; Biniari, K.; Daskalakis, I.; Perdikis, D. The potential use of a wildflower meadow in a vineyard to enhance the local rural landscape character. Acta Hortic. 2020, 1278, 29–38. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, H.; Liu, L.; Zhu, B.; Cao, G.; et al. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef] [Green Version]
- Albano, C.M.; McClure, M.L.; Gross, S.E.; Kitlasten, W.; Soulard, C.E.; Morton, C.; Huntington, J. Spatial patterns of meadow sensitivities to interannual climate variability in the Sierra Nevada. Ecohydrology 2019, 12, 2128. [Google Scholar] [CrossRef]
- Stampfli, A. Species composition and standing crop variation in an unfertilized meadow and its relationship to climatic variability during six years. Folia Geobot. 1995, 30, 117–130. [Google Scholar] [CrossRef]
- Wheeler, M.M.; Collins, S.L.; Grimm, N.B.; Cook, E.M.; Clark, C.; Sponseller, R.A.; Hall, S.J. Water and nitrogen shape winter annual plant diversity and community composition in near-urban Sonoran Desert preserves. Ecol. Monogr. 2021, 91, 1450. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Römermann, C.; Sperlich, S.; Schmidt, W. Explaining grassland biomass—The contribution of climate, species and functional diversity depends on fertilization and mowing frequency. J. Appl. Ecol. 2011, 48, 1088–1097. [Google Scholar] [CrossRef] [Green Version]
- Przybysz, A.; Popek, R.; Stankiewicz-Kosyl, M.; Zhu, C.; Małecka-Przybysz, M.; Maulidyawati, T.; Mikowska, K.; Deluga, D.; Griżuk, K.; Sokalski-Wieczorek, J.; et al. Where trees cannot grow—Particulate matter accumulation by urban meadows. Sci. Total Environ. 2021, 785, 147310. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Knapp, A.K.; Briggs, J.M.; Blair, J.M.; Steinauer, E.M. Modulation of Diversity by Grazing and Mowing in Native Tallgrass Prairie. Science 1998, 280, 745–747. [Google Scholar] [CrossRef]
- Klimešová, J.; Janeček, Š.; Bartušková, A.; Lanta, V.; Doležal, J. How is Regeneration of Plants after Mowing Affected by Shoot Size in Two Species-Rich Meadows with Different Water Supply? Folia Geobot. 2010, 45, 225–238. [Google Scholar] [CrossRef]
- Humbert, J.-Y.; Pellet, J.; Buri, P.; Arlettaz, R. Does delaying the first mowing date benefit biodiversity in meadowland? Environ. Évid. 2012, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Sweet, R.M.; Schreiner, R.P. Alleyway Cover Crops Have Little Influence on Pinot noir Grapevines (Vitis vinifera L.) in Two Western Oregon Vineyards. Am. J. Enol. Vitic. 2010, 61, 240–252. [Google Scholar]
- Nicholls, C.I.; Parrella, M.; Altieri, M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Landsc. Ecol. 2001, 16, 133–146. [Google Scholar] [CrossRef]
- Franin, K.; Barić, B.; Kuštera, G. The role of ecological infrastructure on beneficial arthropods in vineyards. Span. J. Agric. Res. 2016, 14, 303. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, H.F.; Gent, D.H.; Fichtner, S.M.; Otto, K.; Boateng, C.O.; Szostek, S.; Cranshaw, W.S.; Mahaffey, L.A. Thrips tabaci (Thysanoptera: Thripidae) and Iris yellow spot virus Associated with Onion Transplants, Onion Volunteers, and Weeds in Colorado. Southwest. Èntomol. 2014, 39, 691–704. [Google Scholar] [CrossRef]
- Wilson, H.; Daane, K.M. Review of Ecologically-Based Pest Management in California Vineyards. Insects 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Steenwerth, K.L. Rootstock and vineyard floor management influence on ‘Cabernet Sauvignon’ grape yeast assimilable nitrogen (YAN). Food Chem. 2011, 127, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Steenwerth, K.L.; McElrone, A.J.; Hanifin, R.C.; Storm, C.; Collatz, W.; Manuck, C.; Calderón-Orellana, A. Cover Crops and Tillage in a Mature Merlot Vineyard Show Few Effects on Grapevines. Am. J. Enol. Vitic. 2013, 64, 515–521. [Google Scholar] [CrossRef]
- Dias, F.A.N.; Torregrosa, L.; Luchaire, N.; Houel, C.; Pellegrino, A. The microvine, a model to study the effect of temperature on grapevine latent bud development and fruitfulness. OENO One 2019, 53, 393–407. [Google Scholar] [CrossRef]
- Stavrakakis, M.N. Aμπελουργία (Viticulture); Tropi Publications: Athens, Greece, 2013; p. 744. [Google Scholar]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clément, C. Sugars and flowering in the grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M. The Science of Grapevines: Anatomy and Physiology; Elsevier Inc. Academic Press: London, UK, 2010; p. 377. [Google Scholar]









| Month | 1 Tmean (°C) | 1 Tmax (°C) | 1 Tmin (°C) | 2 RHmean (%) | 2 RHmax (%) | 2 RHmin (%) | 3 P (mm) | 4 AWS (km/h) | 5 DWD |
|---|---|---|---|---|---|---|---|---|---|
| Year 2016 [44] | |||||||||
| March | 12.8 | 21.9 | 5.8 | 86.9 | 57.5 | 56.6 | 8.4 | NW | |
| April | 17.8 | 28.8 | 9.1 | 77.1 | 44.7 | 0.2 | 8.1 | NW | |
| May | 19.5 | 33.3 | 9.8 | 77.0 | 43.9 | 5.6 | 9.4 | NW | |
| June | 25.5 | 38.3 | 15.4 | 73.5 | 38.5 | 0.4 | 7.8 | NNE | |
| July | 28.1 | 36.8 | 19.8 | 66.3 | 36.2 | 0.0 | 7.1 | E | |
| August | 27.8 | 37.2 | 18.9 | 66.4 | 37.6 | 0.4 | 7.0 | NNE | |
| Year 2017 [45] | |||||||||
| March | 13.6 | 24.5 | 5.8 | 80.0 | 49.2 | 40.4 | 7.4 | SW | |
| April | 16.3 | 27.9 | 8.1 | 76.4 | 40.3 | 4.8 | 7.6 | NNE | |
| May | 20.4 | 33.2 | 13.0 | 76.8 | 41.0 | 15.8 | 8.4 | NW | |
| June | 24.8 | 42.6 | 16.3 | 78.7 | 41.5 | 15.4 | 6.4 | NNE | |
| July | 28.1 | 40.5 | 17.8 | 68.0 | 34.9 | 85.8 | 7.4 | NNE | |
| August | 28.2 | 38.6 | 18.7 | 63.3 | 33.6 | 7.4 | 6.9 | E | |
| Climatic data 1988–2010 [46,47] | |||||||||
| March | 11.8 | 16.5 | 6.5 | 70.9 | 53.7 | 6.8 | E | ||
| April | 15.7 | 20.3 | 9.0 | 65.9 | 26.7 | 7.1 | E | ||
| May | 21.1 | 25.7 | 12.9 | 59.7 | 22.3 | 6.8 | E | ||
| June | 26.1 | 30.7 | 16.8 | 54.0 | 6.4 | 6.9 | E | ||
| July | 28.7 | 33.2 | 19.5 | 53.3 | 5.0 | 6.9 | E | ||
| August | 28.1 | 32.9 | 19.8 | 56.7 | 11.9 | 6.2 | E | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevopoulou, A.T.; Pappous, E.; Biniari, K.; Bertsouklis, K.F.; Daskalakis, I.; Perdikis, D. Enhancing the Rural Landscape Character: The Low Frequency of Inter-Row Wildflower Meadow Harvest Positively Affects Biodiversity While Maintaining Grape Quantitative and Qualitative Traits in a ‘Sultanina’ Vineyard in Greece. Agronomy 2022, 12, 550. https://doi.org/10.3390/agronomy12030550
Paraskevopoulou AT, Pappous E, Biniari K, Bertsouklis KF, Daskalakis I, Perdikis D. Enhancing the Rural Landscape Character: The Low Frequency of Inter-Row Wildflower Meadow Harvest Positively Affects Biodiversity While Maintaining Grape Quantitative and Qualitative Traits in a ‘Sultanina’ Vineyard in Greece. Agronomy. 2022; 12(3):550. https://doi.org/10.3390/agronomy12030550
Chicago/Turabian StyleParaskevopoulou, Angeliki T., Euaggelos Pappous, Katerina Biniari, Konstantinos F. Bertsouklis, Ioannis Daskalakis, and Dionysios Perdikis. 2022. "Enhancing the Rural Landscape Character: The Low Frequency of Inter-Row Wildflower Meadow Harvest Positively Affects Biodiversity While Maintaining Grape Quantitative and Qualitative Traits in a ‘Sultanina’ Vineyard in Greece" Agronomy 12, no. 3: 550. https://doi.org/10.3390/agronomy12030550
APA StyleParaskevopoulou, A. T., Pappous, E., Biniari, K., Bertsouklis, K. F., Daskalakis, I., & Perdikis, D. (2022). Enhancing the Rural Landscape Character: The Low Frequency of Inter-Row Wildflower Meadow Harvest Positively Affects Biodiversity While Maintaining Grape Quantitative and Qualitative Traits in a ‘Sultanina’ Vineyard in Greece. Agronomy, 12(3), 550. https://doi.org/10.3390/agronomy12030550









