Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Diversity Analysis
2.3.1. Alpha and Beta Diversity
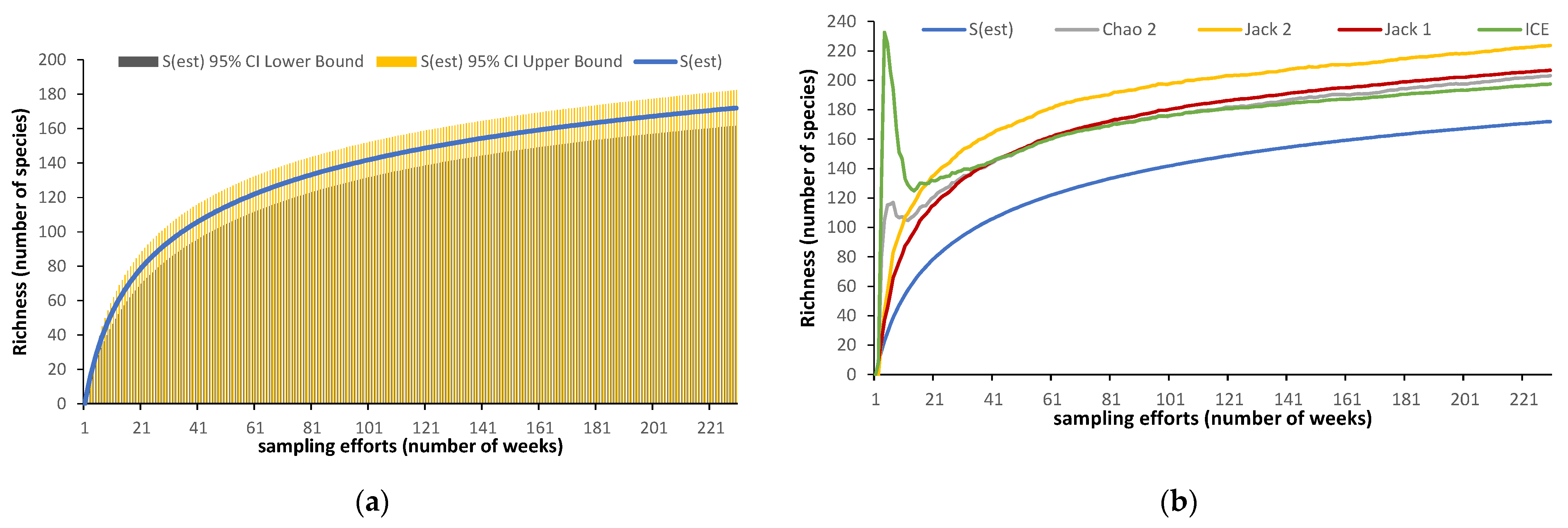
2.3.2. Non-Parametric Estimators
2.4. Statistical Analysis
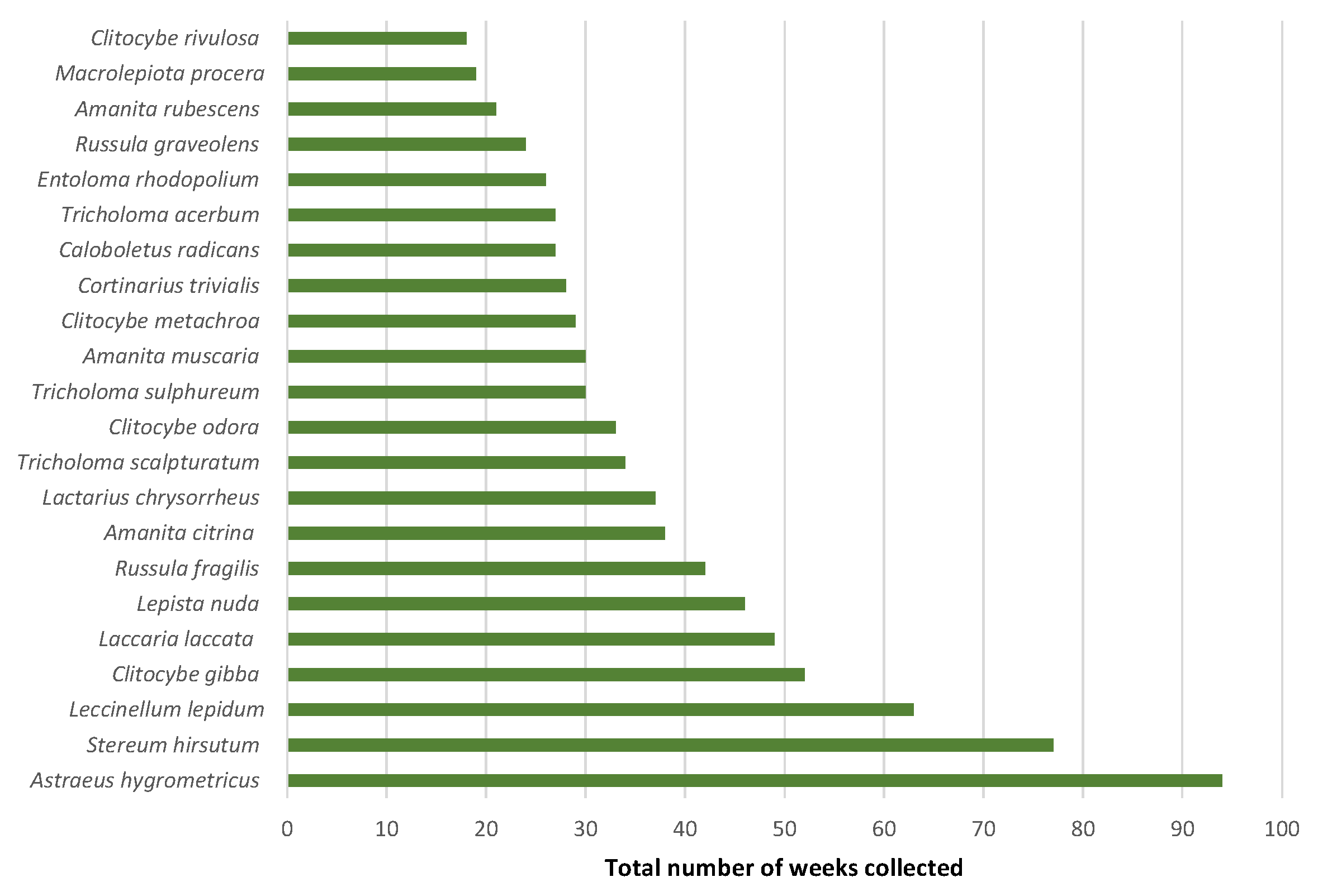
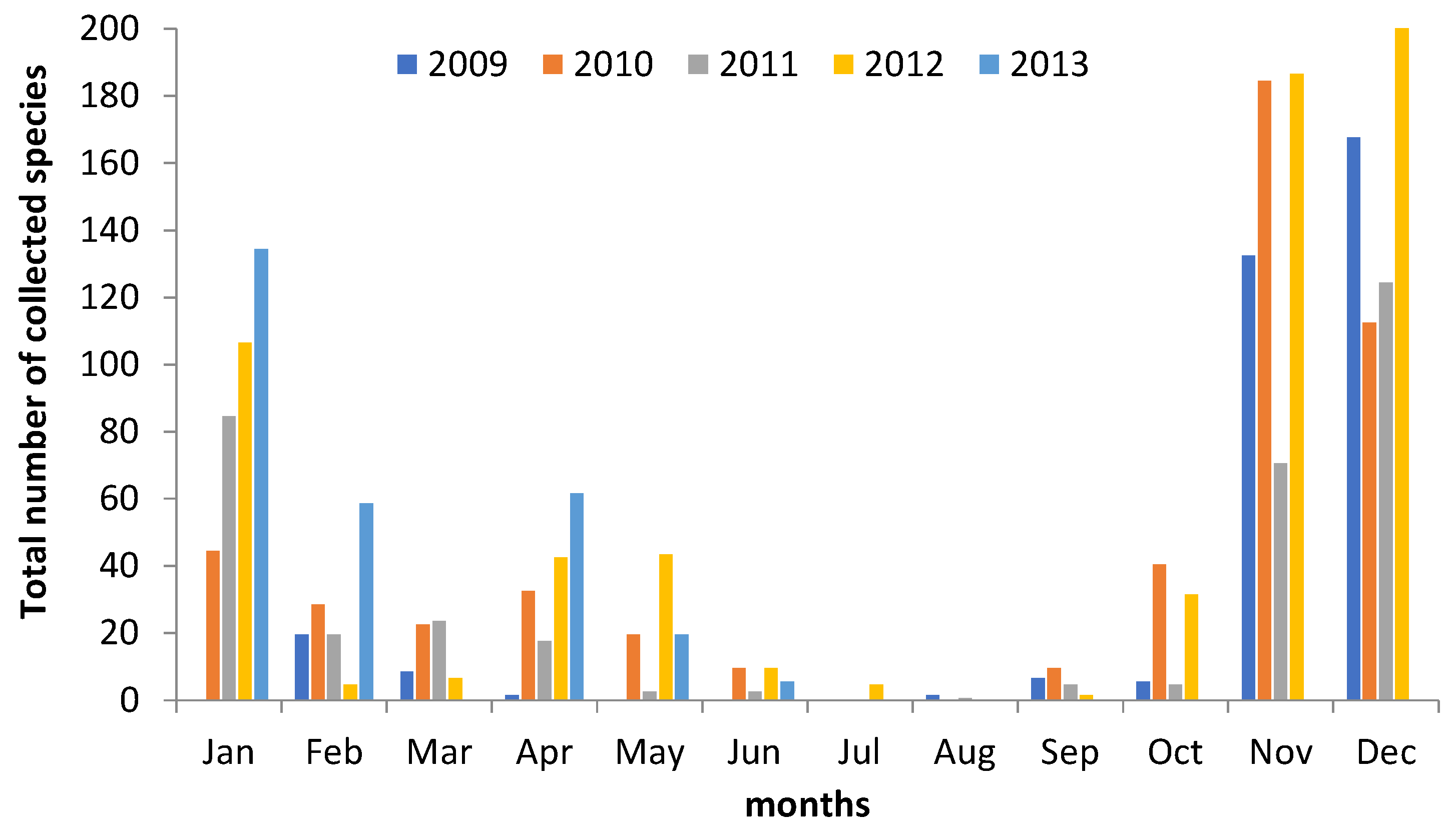
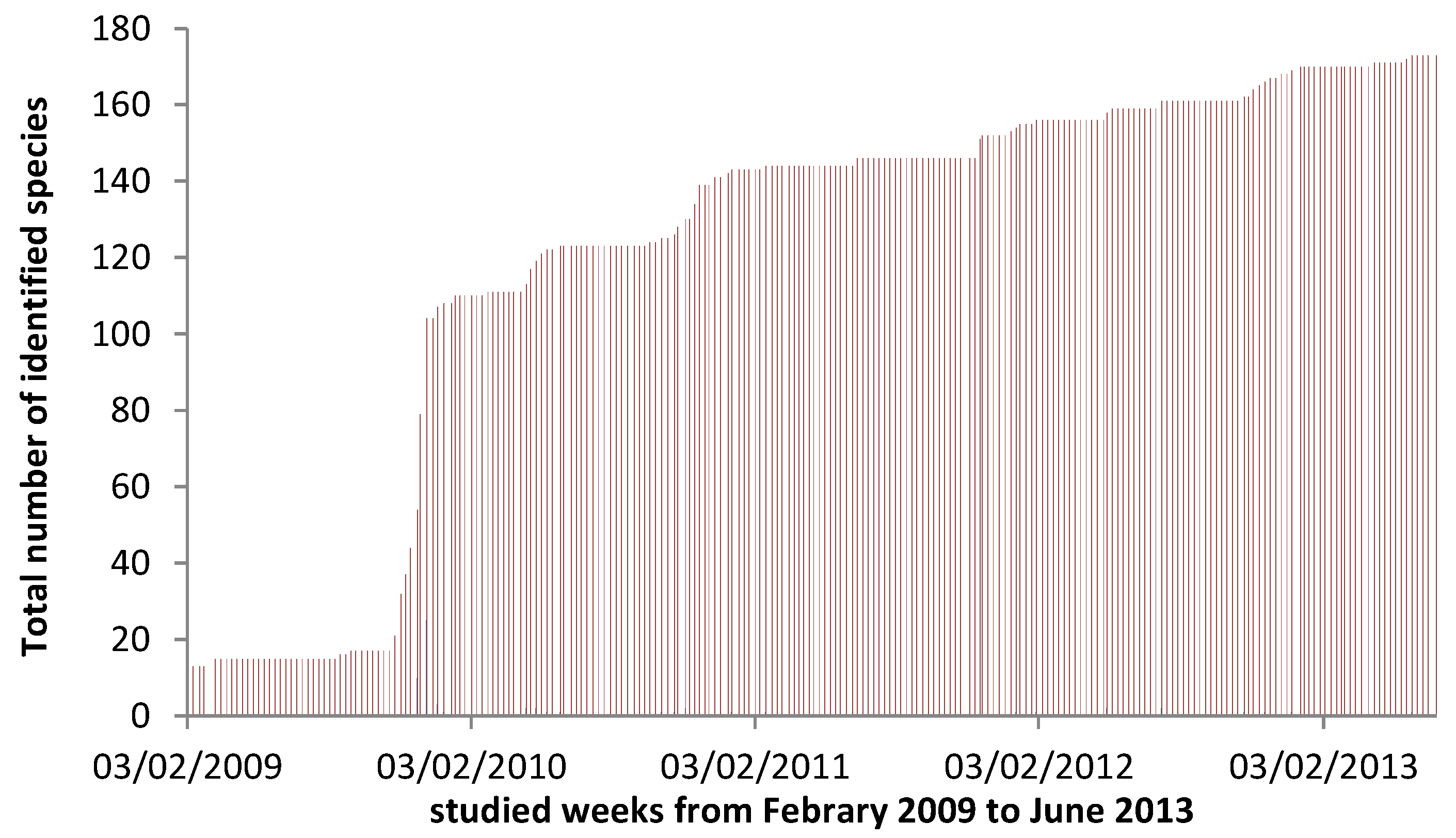
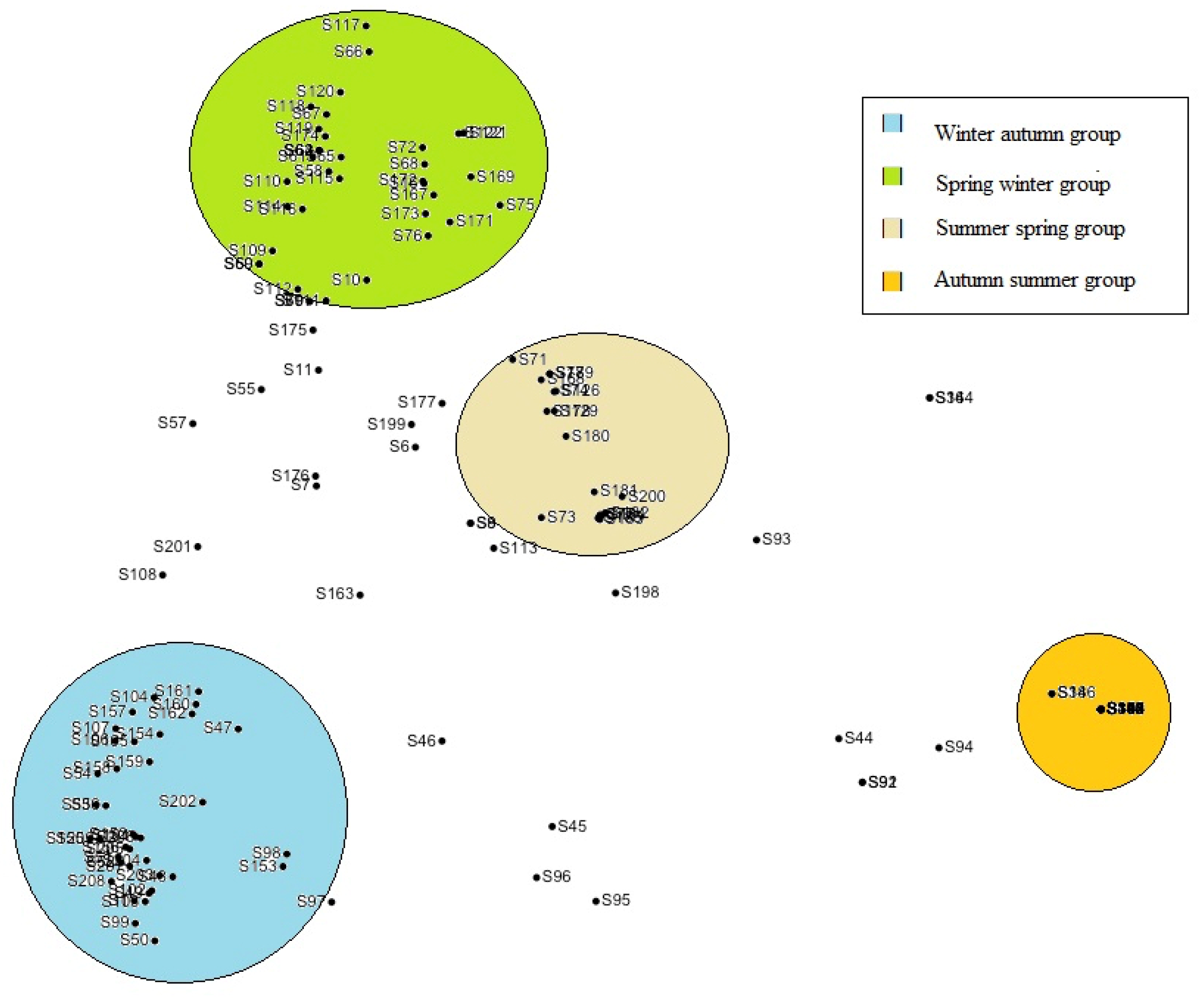
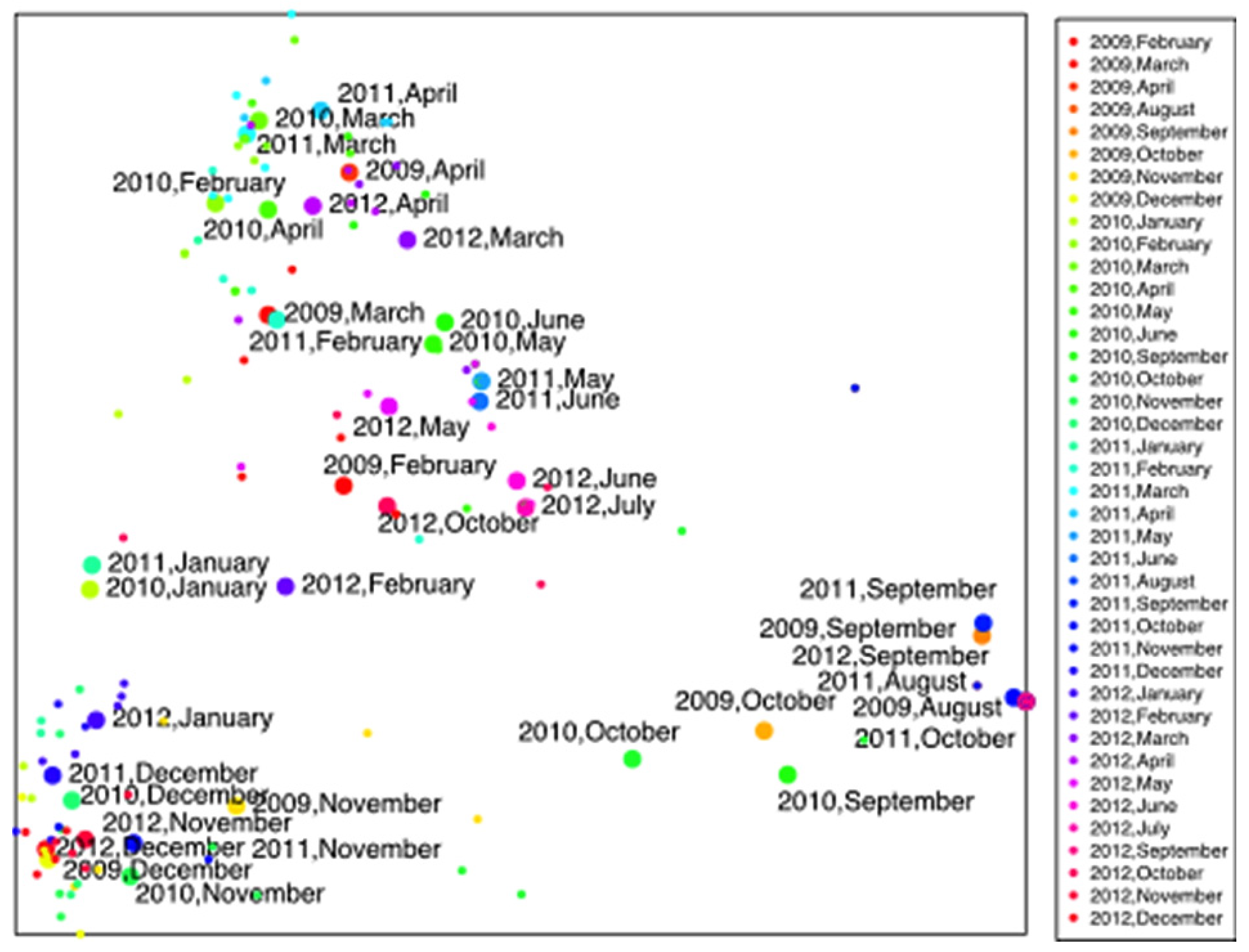
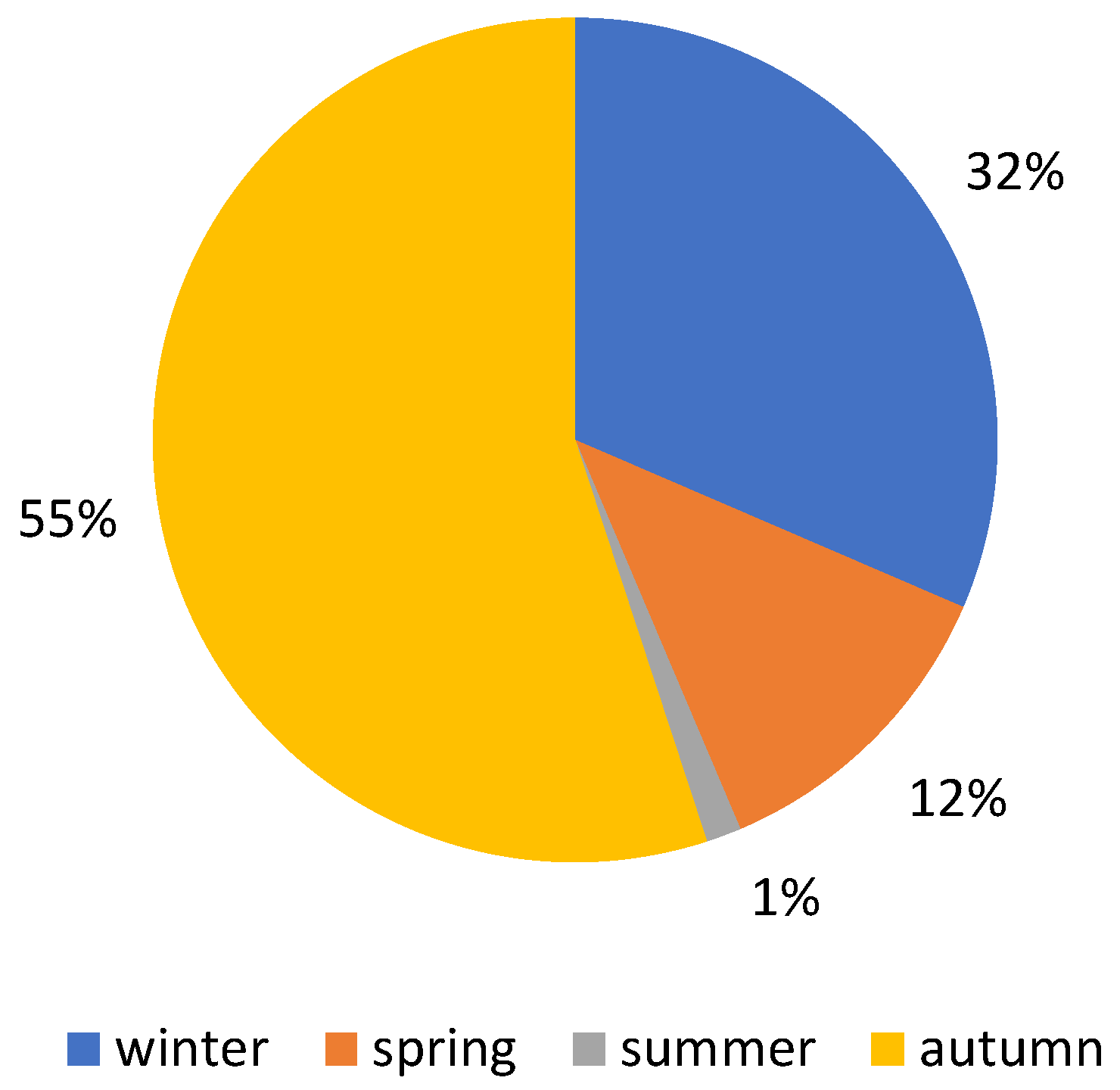
3. Results
4. Discussion
4.1. Macrofungal Fructification
4.2. Climatic and Meteorological Factors
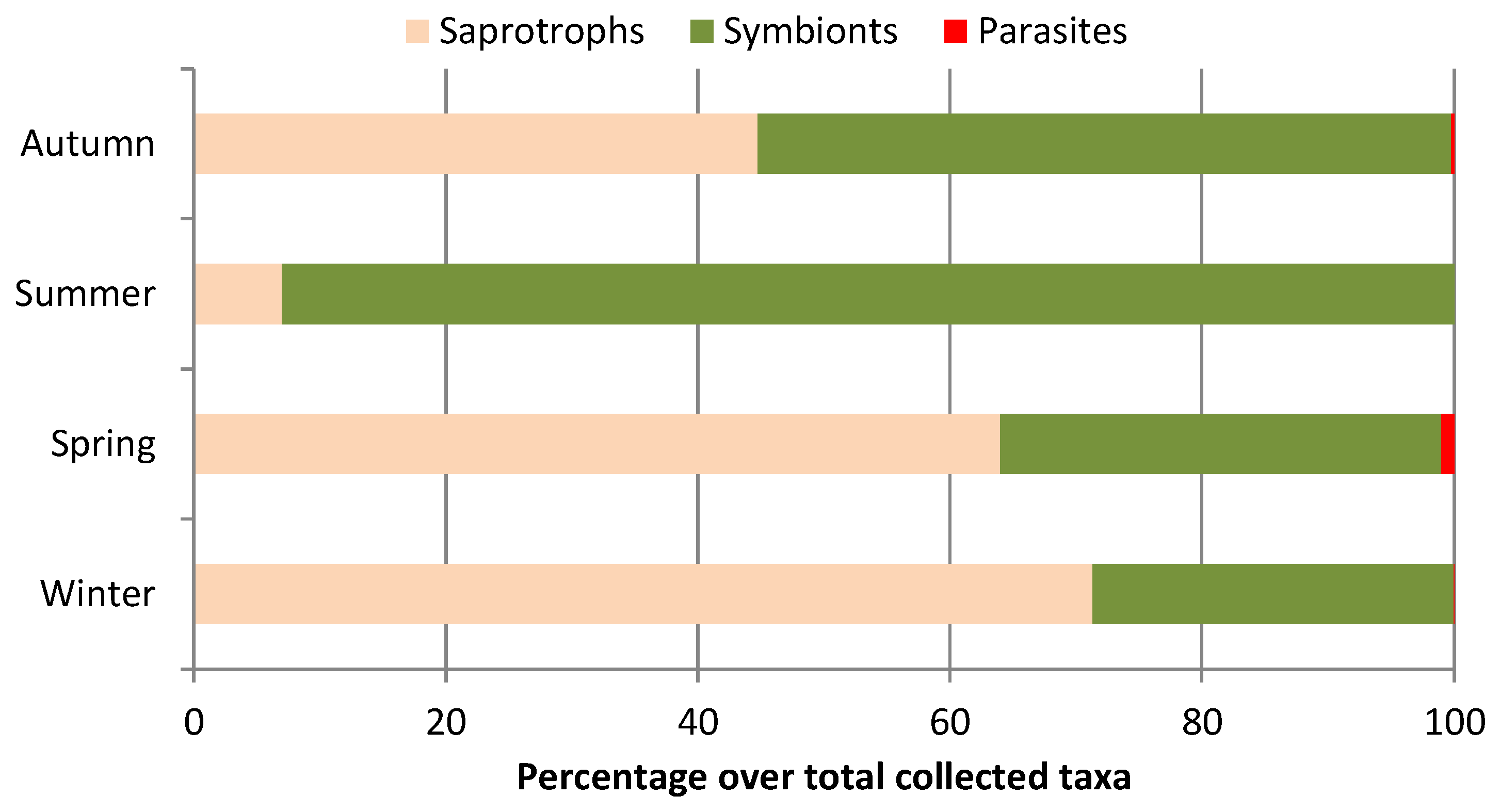
4.3. Macrofungal Lifestyles
4.4. Viable Methodology in Field Studies
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, D.L.; Hollingsworth, T.N.; McFarland, J.W.; Lennon, N.J.; Nusbaum, C.; Ruess, R.W. A first comprehensive census of fungi in soil reveals both hyperdiversity and fine-scale niche partitioning. Ecol. Monogr. 2014, 84, 3–20. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, D.V.; Saxena, R.K.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Perotto, S.; Angelini, P.; Bianciotto, V.; Bonfante, P.; Girlanda, M.; Kull, T. Interaction of fungi with other organisms. Plant Biosyst. 2013, 147, 208–218. [Google Scholar] [CrossRef][Green Version]
- Cannon, P.F.; Sutton, B.C. Microfungi on wood and plant debris. In Biodiversity of Fungi; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2004; pp. 217–239. [Google Scholar]
- Rubino, D.L.; McCarthy, B.C. Composition and ecology of macrofungal and myxomycete communities on oak woody debris in a mixed-oak forest of Ohio. Can. J. For. Res. 2003, 33, 2151–2163. [Google Scholar] [CrossRef]
- Landi, M.; Salerni, E.; Ambrosio, E.; D’Aguanno, M.; Nucci, A.; Saveri, C.; Perini, C.; Angiolini, C. Concordance between vascular plant and macrofungal community composition in broadleaf deciduous forest in central Italy. Iforest 2015, 8, 279–286. [Google Scholar] [CrossRef]
- Kutszegi, G.; Siller, I.; Dima, B.; Merényi, Z.; Varga, T.; Takács, K.; Turcsányi, G.; Bidló, A.; Ódor, P. Revealing hidden drivers of macrofungal species richness by analyzing fungal guilds in temperate forests, West Hungary. Community Ecol. 2021, 22, 13–28. [Google Scholar] [CrossRef]
- Richard, F.; Roy, M.; Shahin, O.; Sthultz, C.; Duchemin, M.; Joffre, R.; Selosse, M.A. Ectomycorrhizal communities in a Mediterranean forest ecosystem dominated by Quercus ilex: Seasonal dynamics and response to drought in the surface organic horizon. Ann. For. Sci. 2011, 68, 57–68. [Google Scholar] [CrossRef]
- Vogiatzakis, I.N.; Mannion, A.M.; Griffiths, G.H. Mediterranean ecosystems: Problems and tools for conservation. Prog. Phys. Geogr. 2006, 30, 175–200. [Google Scholar] [CrossRef]
- Biasi, R.; Brunori, E.; Ferrara, C.; Salvati, L. Towards sustainable rural landscapes? a multivariate analysis of the structure of traditional tree cropping systems along a human pressure gradient in a mediterranean region. Agrofor. Syst. 2017, 91, 1199–1217. [Google Scholar] [CrossRef]
- Bazzato, E.; Lallai, E.; Serra, E.; Mellis, M.T.; Marignani, M. Key role of small woodlots outside forest in a Mediterranean fragmented landscape. For. Ecol. Manag. 2021, 496, 119389. [Google Scholar] [CrossRef]
- Hidalgo, P.J.; Hernández, H.; Sánchez-Almendro, A.J.; López-Tirado, J.; Vessella, F.; Porras, R. Fragmentation and Connectitivy of Island Forests in Agricultural Mediterranean Environments: A Comparative Study between the Guadalquivir Valley (Spain) and the Apulia Region. Forests 2021, 12, 1201. [Google Scholar] [CrossRef]
- Scarascia-Mugnozza, G.; Oswald, H.; Piussi, P.; Radoglou, K. Forests of the Mediterranean region: Gaps in knowledge and research needs. For. Ecol. Manag. 2000, 132, 97–109. [Google Scholar] [CrossRef]
- Rincón, A.M.; Pérez-Izquierdo, L.; de Miguel, S.; Parladé, J. Mycorrhizae in Mediterranean Pine and Mixed Forests. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin; Ne’eman, G., Osem, Y., Eds.; Springer: Cham, Switzerland, 2021; Volume 38. [Google Scholar]
- Büntgen, U.; Kauserud, H.; Egli, S. Linking climate variability to mushroom productivity and phenology. Front. Ecol. Environ. 2012, 10, 14–19. [Google Scholar] [CrossRef]
- Grainger, J. Ecology of the larger fungi. Trans. Br. Mycol. Soc. 1946, 29, 52–63. [Google Scholar] [CrossRef]
- Parker-Rhodes, A.F. The Basidiomycetes of Skokholm Island VII. Some floristic and ecological calculations. New Phytol. 1951, 50, 227–243. [Google Scholar] [CrossRef]
- Richardson, M. Studies of Russula emetica and other agarics in a Scots pine plantation. Trans. Br. Mycol. Soc. 1970, 55, 217–229. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Polemis, E.; Dimou, D.M. Mycodiversity studies in selected ecosystems of Greece: III. Macrofungi recorded in Quercus forests from southern Peloponnese. Mycotaxon 2002, 84, 141–162. [Google Scholar]
- Richard, F.; Moreau, P.A.; Selosse, M.A.; Gardes, M. Diversity and fruiting patterns of ectomycorrhizal and saprobic fungi in an old-growth Mediterranean forest dominated by Quercus ilex L. Can. J. Bot. 2004, 82, 1711–1729. [Google Scholar] [CrossRef]
- Dimou, D.M.; Polemis, E.; Konstantinidis, G.; Kaounas, V.; Zervakis, G.I. Diversity of macrofungi in the Greek islands of Lesvos and Agios Efstratios, NE Aegean Sea. Nova Hedwig. 2016, 102, 439–475. [Google Scholar] [CrossRef]
- Mehus, H. Fruit Body Production of Macrofungi in Some North Norwegian Forest Types. Nord. J. Bot. 1986, 6, 679–702. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar]
- Gange, A.C.; Gange, E.G.; Sparks, T.H.; Boddy, L. Rapid and recent changes in fungal fruiting patterns. Science 2007, 316, 71. [Google Scholar] [CrossRef] [PubMed]
- Kauserud, H.; Heegaard, E.; Büntgen, U.; Halvorsen, R.; Egli, S.; Senn-Irlet, B.; Høiland, K. Warming-induced shift in European mushroom fruiting phenology. Proc. Natl. Acad. Sci. USA 2012, 109, 14488–14493. [Google Scholar] [CrossRef]
- Martiny, J.B.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Buée, M.; De Boer, W.; Martin, F.; Van Overbeek, L.; Jurkevitch, E. The rhizosphere zoo: An overview of plant-associated communities of microorganisms, including phages, bacteria, archaea, and fungi, and of some of their structuring factors. Plant Soil 2009, 321, 189–212. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T.; Gardes, M. Indirect host effect on ectomycorrhizal fungi: Leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks. Soil Biol. Biochem. 2010, 42, 788–796. [Google Scholar] [CrossRef]
- Robertson, G.P.; Crum, J.R.; Ellis, B.G. The spatial variability of soil resources following long-term disturbance. Oecologia 1993, 96, 451–456. [Google Scholar] [CrossRef]
- Hering, T.F. The terricolous higher fungi of four lake district woodlands. Trans. Br. Mycol. Soc. 1966, 49, 369–383. [Google Scholar] [CrossRef]
- Durall, D.M.; Gamiet, S.; Simard, S.W.; Kudrna, L.; Sakakibara, S.M. Effects of clearcut logging and tree species composition on the diversity and community composition of epigeous fruit bodies formed by ectomycorrhizal fungi. Can. J. Bot. 2006, 84, 966–980. [Google Scholar] [CrossRef]
- Halme, P.; Heilmann-Clausen, J.; Rämä, T.; Kosonen, T.; Kunttu, P. Monitoring fungal biodiversity—Towards an integrated approach. Fungal Ecol. 2012, 5, 750–758. [Google Scholar] [CrossRef]
- Langer, E.; Langer, G.; Striegel, M.; Riebesehl, J.; Ordynets, A. Fungal diversity of the Kellerwald-Edersee National Park–indicator species of nature value and conservation. Nova Hedwig. 2014, 99, 129–144. [Google Scholar] [CrossRef]
- Watling, R. Assessment of fungal diversity: Macromycetes, the problems. Can. J. Bot. 1995, 73 (Suppl. S1), S515–S524. [Google Scholar] [CrossRef]
- Angelini, P.; Compagno, R.; Arcangeli, A.; Bistocchi, G.; Gargano, M.L.; Venanzoni, R.; Venturella, G. Macrofungal diversity and ecology in two Mediterranean forest ecosystems. Plant Biosyst. 2016, 150, 540–549. [Google Scholar] [CrossRef]
- Salerni, E.; Laganà, A.; Perini, C.; Loppi, S.; de Dominicis, V. Effects of temperature and rainfall on fruiting of macrofungi in oak forests of the Mediterranean area. Isr.J. Plant Sci. 2002, 50, 189–198. [Google Scholar] [CrossRef]
- García Jiménez, P.; Fernández Ruiz, A.; Sánchez Sánchez, J.; Rodríguez de la Cruz, D. Mycological Indicators in Evaluating Conservation Status: The Case of Quercus spp. Dehesas in the Middle-West of the Iberian Peninsula (Spain). Sustainability 2020, 12, 10442. [Google Scholar] [CrossRef]
- Azul, A.M.; Castro, P.; Sousa, J.P.; Freitas, H. Diversity and fruiting patterns of ectomycorrhizal and saprobic fungi as indicators of land-use severity in managed woodlands dominated by Quercus suber—A case study from southern Portugal. Can. J. For. Res. 2009, 39, 2404–2417. [Google Scholar] [CrossRef]
- Crusafont, M.; Truyols, J. Algunas precisiones sobre la edad y extensión del Paleógeno de las provincias de Salamanca y Zamora. Cursos Conf. Inst. Lucas Mallada 1958, 4, 83–85. [Google Scholar]
- García-Rodríguez, A.; Forteza, J.; Prat-Pérez, L.; Gallardo, J.F.; Lorenzo-Martín, L.F. Suelos. Estudio integrado y multidisciplinario de la dehesa salmantina 1. Estudio fisiográfico descriptivo. Cons. Super. Investig. Científicas Salamanca-Jaca. 1979, 3, 65–100. [Google Scholar]
- Fernández, A.; Sánchez, S.; García, P.; Sánchez, J. Macrofungal diversity in an isolated and fragmented Mediterranean Forest ecosystem. Plant Biosyst. 2020, 154, 139–148. [Google Scholar] [CrossRef]
- Capel Molina, J.J. Los climas de España; Oikos-tau S.A.: Barcelona, Spain, 1981. [Google Scholar]
- Barbancho, A.C.; Tejeda, E.M.; Hernández, M.Q. Evolución de las temperaturas y precipitaciones en las capitales de Castilla y León en el período 1961–2006. Polígonos. Rev. Geogr. 2012, 17, 59–81. [Google Scholar] [CrossRef][Green Version]
- Feest, A. Establishing baseline indices for the quality of the biodiversity of restored habitats using a standardized sampling process. Restor. Ecol. 2006, 14, 112–122. [Google Scholar] [CrossRef]
- CABI Index Fungorum. Available online: http://www.indexfungorum.org/names/names.asp (accessed on 8 December 2021).
- Kirk, P.M.; Cannon, P.; Minter, D.; Stalpers, J. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Sarkar, S. Defining “biodiversity”: Assessing biodiversity. Monist 2002, 85, 131–155. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell: Malden, MA, USA, 2004. [Google Scholar]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Oreja, J.G.; de la Fuente-Díaz-Ordaz, A.A.; Hernández-Santín, L.; Buzo-Franco, D.; Bonache-Regidor, C. Evaluación de estimadores no paramétricos de la riqueza de especies. Un ejemplo con aves en áreas verdes de la ciudad de Puebla, México. Anim. Biodivers. Conserv. 2010, 33, 31–45. [Google Scholar]
- Basualdo, C.V. Choosing the best non-parametric richness estimator for benthic macroinvertebrates databases. Rev. Soc. Entomol. Argent. 2011, 70, 27–38. [Google Scholar]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples, Version 8. User’s Guide and Application. 2006. Available online: http://purl.oclc.org/estimates (accessed on 1 September 2021).
- Gotelli, N.J.; Colwell, R.K. Quantifying bio-diversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Díaz–Frances, E.; Soberón, J. Statistical estimation and model selection of species–accumulation functions. Conserv. Biol. 2005, 19, 569–573. [Google Scholar] [CrossRef]
- Chao, A. Estimating the population size for capture-recapture data with unequal catchability. Biometrics 1987, 43, 783–791. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. R Package Version 2.4-0. Vegan: Community Ecology Package. 2016. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 1 September 2021).
- Vicente-Villardón, J.L. MultBiplot(R): Multivariate Analysis using Biplots. R Package Version 0.3.3. 2016. Available online: https://biplot.usal.es/classicalbiplot/multbiplot-in-r/ (accessed on 1 September 2021).
- Gallego-Álvarez, I.; Vicente-Villardón, J.L. Analysis of environmental indicators in international companies by applying the logistic biplot. Ecol. Indic. 2012, 23, 250–261. [Google Scholar] [CrossRef]
- Shi, L.L.; Mortimer, P.E.; Slik, J.F.; Zou, X.M.; Xu, J.; Feng, W.T.; Qiao, L. Variation in forest soil fungal diversity along a latitudinal gradient. Fungal Divers. 2014, 64, 305–315. [Google Scholar] [CrossRef]
- Ágreda, T.; Águeda, B.; Olano, J.M.; Vicente-Serrano, S.M.; Fernández-Toirán, M. Increase evapotranspiration demand in a Mediterranean climate might cause a decline in fungal yields under global warming. Glob. Chang. Biol. 2015, 21, 3499–3510. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.J.; Van der Gast, C.J.; McNamara, N.P.; Rowe, R.; Bending, G.D. Extreme rainfall affects assembly of the root-associated fungal community. New Phytol. 2018, 220, 1172–1184. [Google Scholar] [CrossRef]
- Jang, S.K.; Kim, S.W. Relationship between Ectomycorrhizal Fruiting Bodies and Climatic and Environmental Factors in Naejangsan National Park. Mycobiology 2015, 43, 122–134. [Google Scholar] [CrossRef][Green Version]
- Sun, Q.; Liu, Y.; Yuan, H.; Lian, B. The effect of environmental contamination on the community structure and fructification of ectomycorrhizal fungi. MicrobiologyOpen 2017, 6, e00396. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.; Martins, A.; Tavares, R.M.; Lino-Nieto, T. Diversity and fruiting pattern of macrofungi associated with chestnut (Castanea sativa) in the Trás-os-Montes region (Northeast Portugal). Fungal Ecol. 2010, 3, 9–19. [Google Scholar] [CrossRef]
- Kauserud, H.; Stige, L.C.; Vik, J.O.; Økland, R.H.; Høiland, K.; Stenseth, N.C. Mushroom fruiting and climate change. Proc. Natl. Acad. Sci. USA 2008, 105, 3811–3814. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.; Polley, H.W.; Daneshgar, P.P.; Harris, M.A.; Wilsey, B.J. Biodiversity, photosynthetic mode, and ecosystem services differ between native and novel ecosystems. Oecologia 2014, 175, 687–697. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Allard, V.; Ourcival, J.M.; Rambal, S.; Joffre, R.; Rocheteau, A. Seasonal and annual variation of carbon exchange in an evergreen Mediterranean forest in southern France. Glob. Chang. Biol. 2008, 14, 1–12. [Google Scholar] [CrossRef]
- Mair, L.; Jönsson, M.; Räty, M.; Bärring, L.; Strandberg, G.; Lämås, T.; Snäll, T. Land use changes could modify future negative effects of climate change on old-growth forest indicator species. Divers. Distrib. 2018, 24, 1416–1425. [Google Scholar] [CrossRef]
- Markkola, A.M.; Saravesi, K.; Aikio, S.; Taulavuori, E.; Taulavuori, K. Light-driven host-symbiont interactions under hosts’ range shifts caused by global warming: A review. Environ. Exp. Bot. 2016, 121, 48–55. [Google Scholar] [CrossRef]
- Thakur, M.P.; Quast, V.; Van Dam, N.M.; Eisenhauer, N.; Roscher, C.; Biere, A.; Martinez-Medina, A. Interactions between functionally diverse fungal mutualists inconsistently affect plant performance and competition. Oikos 2019, 128, 1136–1146. [Google Scholar] [CrossRef]
- Martinová, V.; Van Geel, M.; Lievens, B.; Honnay, O. Strong differences in Quercus robur-associated ectomycorrhizal fungal communities along a forest-city soil sealing gradient. Fungal Ecol. 2016, 20, 88–96. [Google Scholar] [CrossRef]
- Martin, F.; Cullen, D.; Hibbett, D.; Pisabarro, A.; Spatafora, J.W.; Baker, S.E.; Grigoriev, I.V. Sequencing the fungal tree of life. New Phytol. 2011, 190, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Kivlin, S.N.; Rocca, J.D.; Huguet, V.; Thomsen, M.A.; Suttle, K.B. Fungal community responses to precipitation. Glob. Chang. Biol. 2011, 17, 1637–1645. [Google Scholar] [CrossRef]
- Zelnik, Y.R.; Meron, E.; Bel, G. Localized states qualitatively change the response of ecosystems to varying conditions and local disturbances. Ecol. Complex. 2016, 25, 26–34. [Google Scholar] [CrossRef]
- Hui, N.; Liu, X.; Jumpponen, A.; Setälä, H.; Kotze, D.J.; Biktasheva, L.; Romantschuk, M. Over twenty years farmland reforestation decreases fungal diversity of soils, but stimulates the return of ectomycorrhizal fungal communities. Plant Soil. 2018, 427, 231–244. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Evol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Kern, M.; Caldararu, S.; Engel, J.; Rammig, A.; Vicca, S.; Zaehle, S. A novel plant-mycorrhiza interaction model improves representation of plant responses to elevated CO2. In Proceedings of the 20th EGU General Assembly, EGU2018, Vienna, Austria, 4–13 April 2018; p. 7526. [Google Scholar]
- Pickles, B.J.; Egger, K.N.; Massicotte, H.B.; Green, D.S. Ectomycorrhizas and climate change. Fungal Ecol. 2012, 5, 73–84. [Google Scholar] [CrossRef]
- Morgado, L.N.; Semenova, T.A.; Welker, J.M.; Walker, M.D.; Smets, E.; Geml, J. Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Glob. Chang. Biol. 2015, 21, 959–972. [Google Scholar] [CrossRef]
- Akroume, E.; Maillard, F.; Bach, C.; Hossann, C.; Brechet, C.; Angeli, N.; Zeller, B.; Saint-André, L.; Buée, M. First evidences that the ectomycorrhizal fungus Paxillus involutus mobilizes nitrogen and carbon from saprotrophic fungus necromass. Environ. Microbiol. 2018, 21, 197–208. [Google Scholar] [CrossRef]
- Egerton-Warburton, L.M.; Johnson, N.C.; Allen, E.B. Mycorrhizal community dynamics following nitrogen fertilization: A cross-site test in five grasslands. Ecol. Monogr. 2007, 77, 527–544. [Google Scholar] [CrossRef]
- Terrer, C.; Vicca, S.; Stocker, B.D.; Hungate, B.A.; Phillips, R.P.; Reich, P.B.; Finzi, A.C.; Prentice, C.P. Ecosystem responses to elevated CO2 governed by plant-soil interactions and the cost of nitrogen acquisition. New Phytol. 2017, 217, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.C.; Haelewaters, D.; Furci, G.; Mueller, G.M. Include all fungi in biodiversity goals. Science 2021, 373, 403. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.M.; Schmit, J.P.; Leacock, P.R.; Buyck, B.; Cifuentes, J.; Desjardin, D.E.; Halling, R.E.; Hjortstam, K.; Iturriaga, T.; Larsson, K.H.; et al. Global diversity and distribution of macrofungi. Biodivers. Conserv. 2007, 16, 37–48. [Google Scholar] [CrossRef]
- Arnolds, E. The future of fungi in Europe: Threats, conservation and management. Br. Mycol. Symp. Ser. 2001, 22, 64–80. [Google Scholar]
- Cannon, P.F.; Mibey, R.K.; Siboe, G.M. Microfungus diversity and the conservation agenda in Kenya. Br. Mycol. Symp. Ser. 2001, 22, 197–208. [Google Scholar]
- Piazza, G.; Ercoli, L.; Nuti, M.; Pellegrino, E. Interaction between conservation tillage and nitrogen fertilization shapes prokaryotic and fungal diversity at different soil depths: Evidence from a 23-year field experiment in the Mediterranean Area. Front. Microbiol. 2019, 10, 2047. [Google Scholar] [CrossRef]
- Girometta, C.E.; Bernicchia, A.; Baiguera, R.M.; Bracco, F.; Buratti, S.; Cartabia, M.; Picco, A.M.; Savino, E. An Italian research culture collection of wood decay fungi. Diversity 2020, 12, 58. [Google Scholar] [CrossRef]
- De la Varga, H.; Águeda, B.; Ágreda, T.; Martínez-Peña, F.; Parladé, J.; Pera, J. Seasonal dynamics of Boletus edulis and Lactarius deliciosus extraradical mycelium in pine forests of central Spain. Mycorrhiza 2013, 23, 391–402. [Google Scholar] [CrossRef]
- Halme, P.; Kotiaho, J.S. The importance of timing and number of surveys in fungal biodiversity research. Biodivers. Conserv. 2012, 21, 205–209. [Google Scholar] [CrossRef]
- Zamora, J.C.; Svensson, M.; Kirschner, R.; Olariaga, I.; Ryman, S.; Parra, L.A.; Geml, J.; Rosling, A.; Adamčík, S.; Ahti, T.; et al. Considerations and consequences of allowing DNA sequence data as types of fungal taxa. IMA Fungus 2018, 9, 167–175. [Google Scholar] [CrossRef]
- Rey-Benayas, J.M.; Scheiner, S.M. Plant diversity, biogeography and environment in Iberia: Patterns and possible causal factors. J. Veg. Sci. 2002, 13, 245–258. [Google Scholar] [CrossRef]
- Teobaldelli, M.; Cona, F.; Saulino, L.; Migliozzi, A.; D’Urso, G.; Langella, G.; Manna, P.; Saracino, A. Detection of diversity and stand parameters in Mediterranean forests using leaf-off discrete return LiDAR data. Remote Sens. Environ. 2017, 192, 126–138. [Google Scholar] [CrossRef]
- Clavería, V.; de Miguel, A.M. Diversidad ectomicorrícica en una formación natural de carrasca (Quercus ilex L. subsp. ballota (Desf.) Samp.). In Actas del 4º Congreso Forestal Español; Sociedad Española de Ciencias Forestales: Zaragoza, Spain, 2005. [Google Scholar]
- Sarrionandia, E.; Salcedo, I. Macrofungal diversity of holm-oak forests at the northern limit of their distribution range in the Iberian Peninsula. Scand. J. For. Res. 2018, 33, 23–31. [Google Scholar] [CrossRef]
- Carrie, A.; Heegaard, E.; Høiland, K.; Senn-Irlet, B.; Kuyper, T.W.; Krisai-Greilhuber, I.; Kirk, P.M.; Heilmann-Clausen, J.; Gange, A.C.; Egli, S.; et al. Explaining European fungal fruiting phenology with climate variability. Ecology 2018, 99, 1306–1315. [Google Scholar]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlák, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Moreno, G.; Manjón, J.L.; Álvarez-Jiménez, J. Hypogeous desert fungi. In Desert Truffles; Kagan-Zur, V., Sitrit, Y., Roth-Bejerano, N.A., Morte, A., Eds.; Springer: Berlin, Germany, 2013; pp. 129–135. [Google Scholar]
- Abrego, N.; Halme, P.; Purhonen, J.; Ovaskainen, O. Fruit body based inventories in wood-inhabiting fungi: Should we replicate in space or time? Fungal Ecol. 2016, 20, 225–232. [Google Scholar] [CrossRef]
- Ambrosio, E.; Mariotti, M.G.; Zotti, M.; Cecchi, G.; Di Piazza, S.; Feest, A. Measuring macrofungal biodiversity quality using two different survey approaches: A case study in broadleaf Mediterranean forests. Ecol. Indic. 2018, 85, 1210–1230. [Google Scholar] [CrossRef]
- Walther, B.A.; Moore, J.L. The concepts of bias, precision, and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography 2005, 28, 815–829. [Google Scholar] [CrossRef]
- Cannon, P. Options and constraints in rapid diversity analysis of fungi in natural ecosystems. Fungal Divers. 1999, 2, 1–15. [Google Scholar]
- Hyde, K.D. Can we rapidly measure fungal diversity? Mycologist 1997, 11, 176–178. [Google Scholar] [CrossRef]
- Manzo, S. Experimentación, instrumentos científicos y cuantificación en el método de Francis Bacon. Manuscrito 2001, 24, 49–84. [Google Scholar]
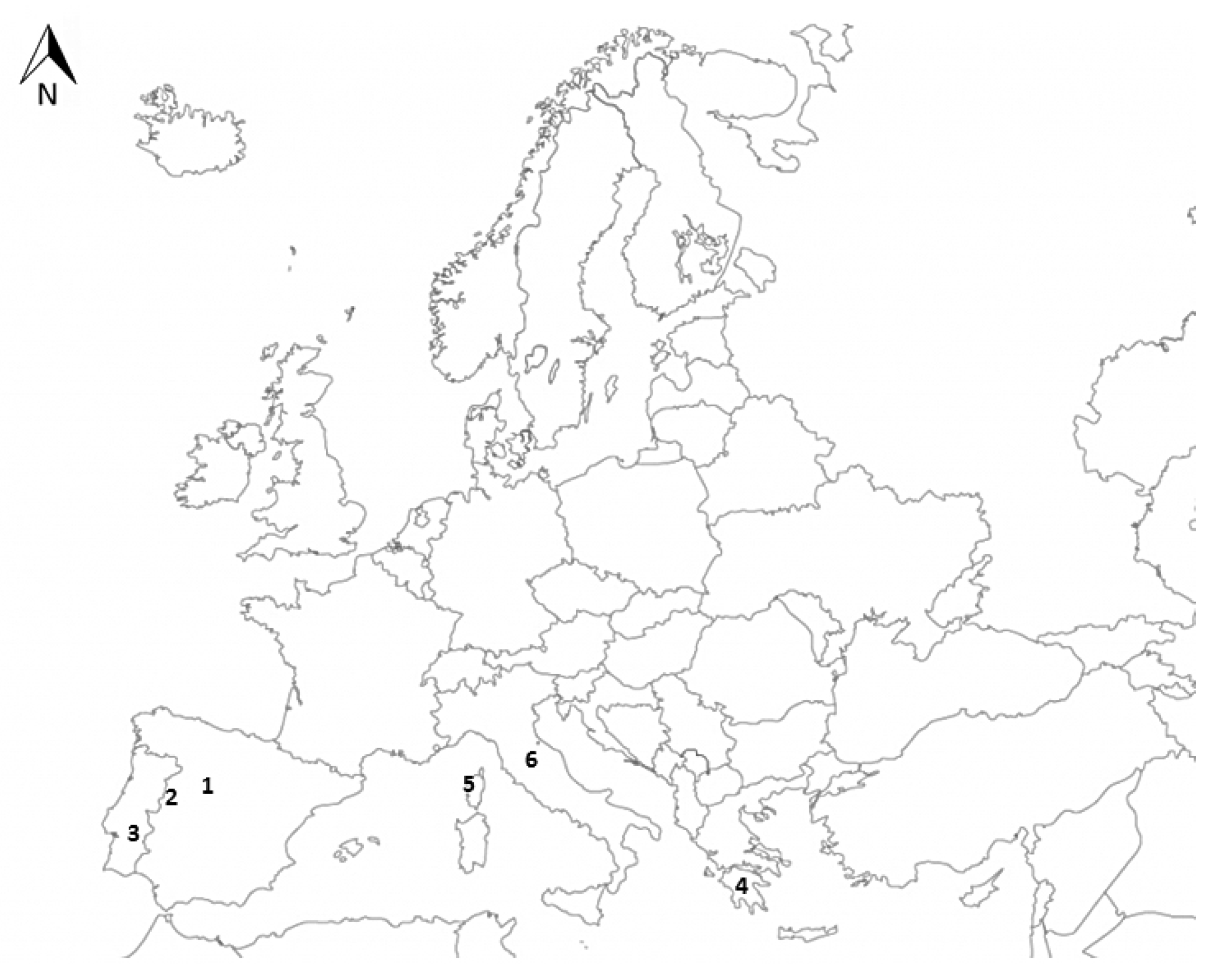




| Sites | S | SC | Is |
|---|---|---|---|
| Campanarios de Azaba BR (Spain) | 148 | 55 | 0.32 |
| Foros “montado” (Portugal) | 172 | 60 | 0.33 |
| Fango forest (Corcega) | 234 | 44 | 0.24 |
| Collestrada (Italy) | 133 | 41 | 0.26 |
| Peloponnese (Greece) | 197 | 42 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Ruiz, A.; Rodríguez de la Cruz, D.; Vicente Villardón, J.L.; Sánchez Durán, S.; García Jiménez, P.; Sánchez Sánchez, J. Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes. Agronomy 2022, 12, 528. https://doi.org/10.3390/agronomy12020528
Fernández Ruiz A, Rodríguez de la Cruz D, Vicente Villardón JL, Sánchez Durán S, García Jiménez P, Sánchez Sánchez J. Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes. Agronomy. 2022; 12(2):528. https://doi.org/10.3390/agronomy12020528
Chicago/Turabian StyleFernández Ruiz, Abel, David Rodríguez de la Cruz, José Luis Vicente Villardón, Sergio Sánchez Durán, Prudencio García Jiménez, and José Sánchez Sánchez. 2022. "Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes" Agronomy 12, no. 2: 528. https://doi.org/10.3390/agronomy12020528
APA StyleFernández Ruiz, A., Rodríguez de la Cruz, D., Vicente Villardón, J. L., Sánchez Durán, S., García Jiménez, P., & Sánchez Sánchez, J. (2022). Considerations on Field Methodology for Macrofungi Studies in Fragmented Forests of Mediterranean Agricultural Landscapes. Agronomy, 12(2), 528. https://doi.org/10.3390/agronomy12020528








