Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
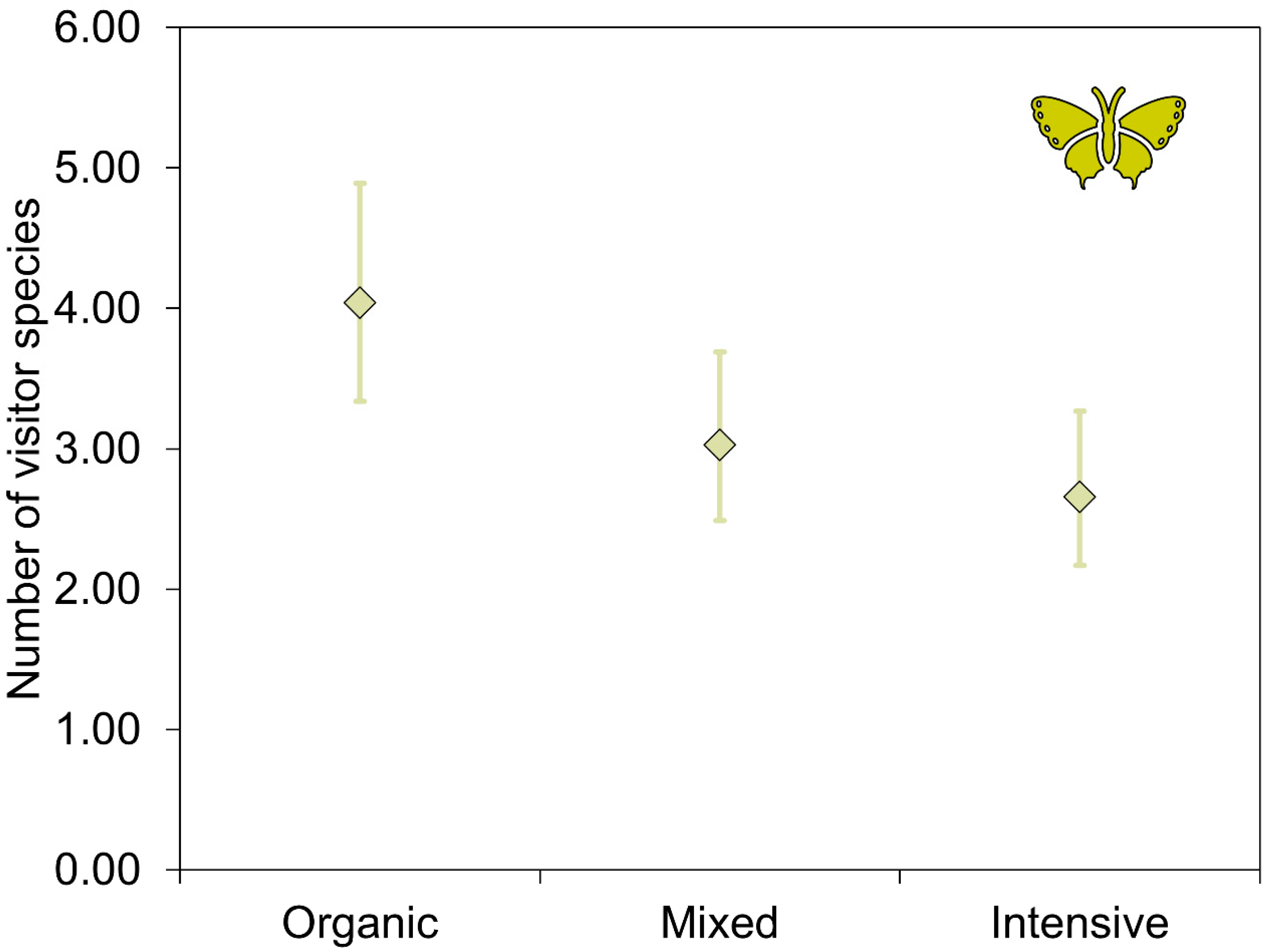
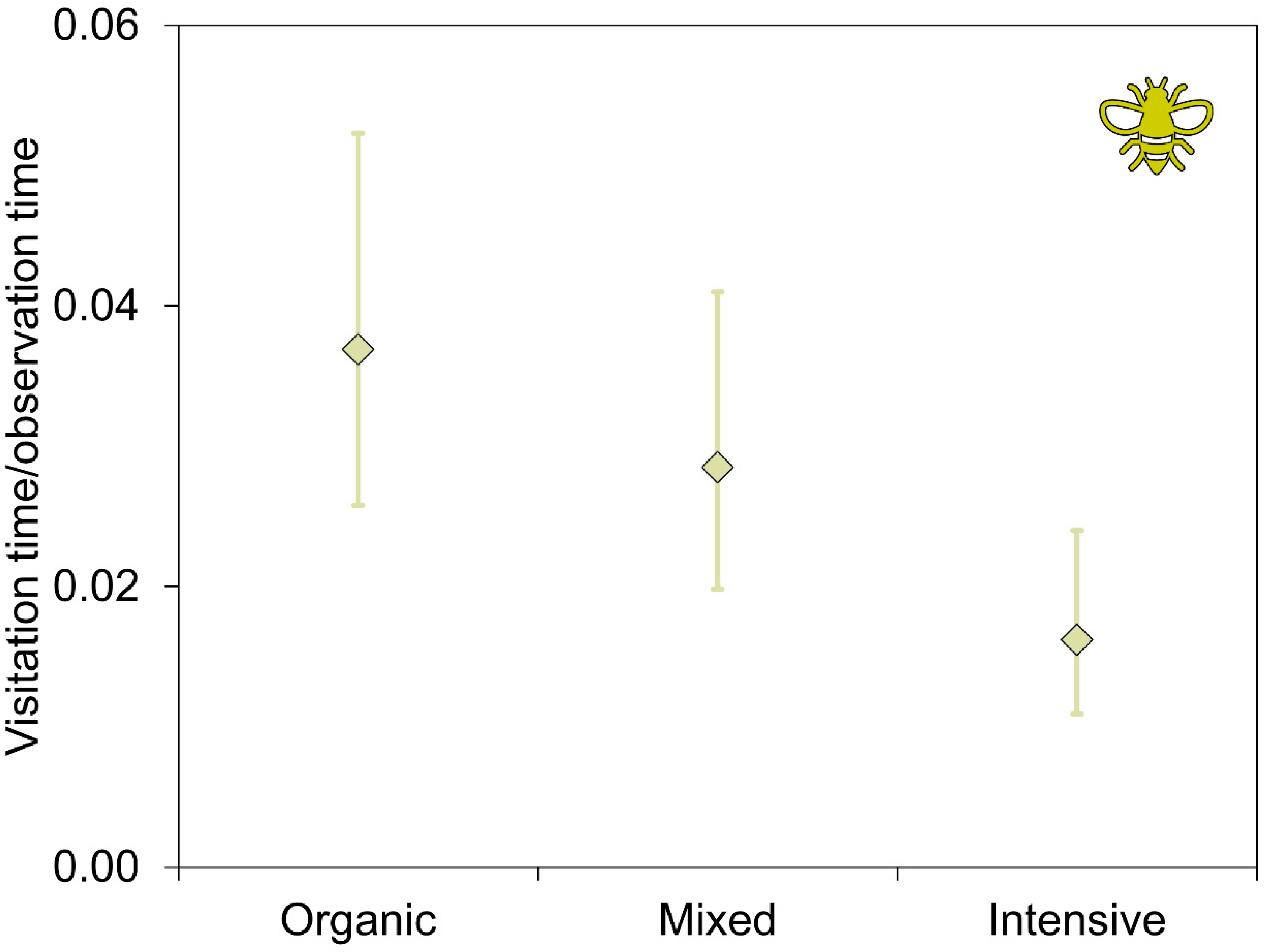
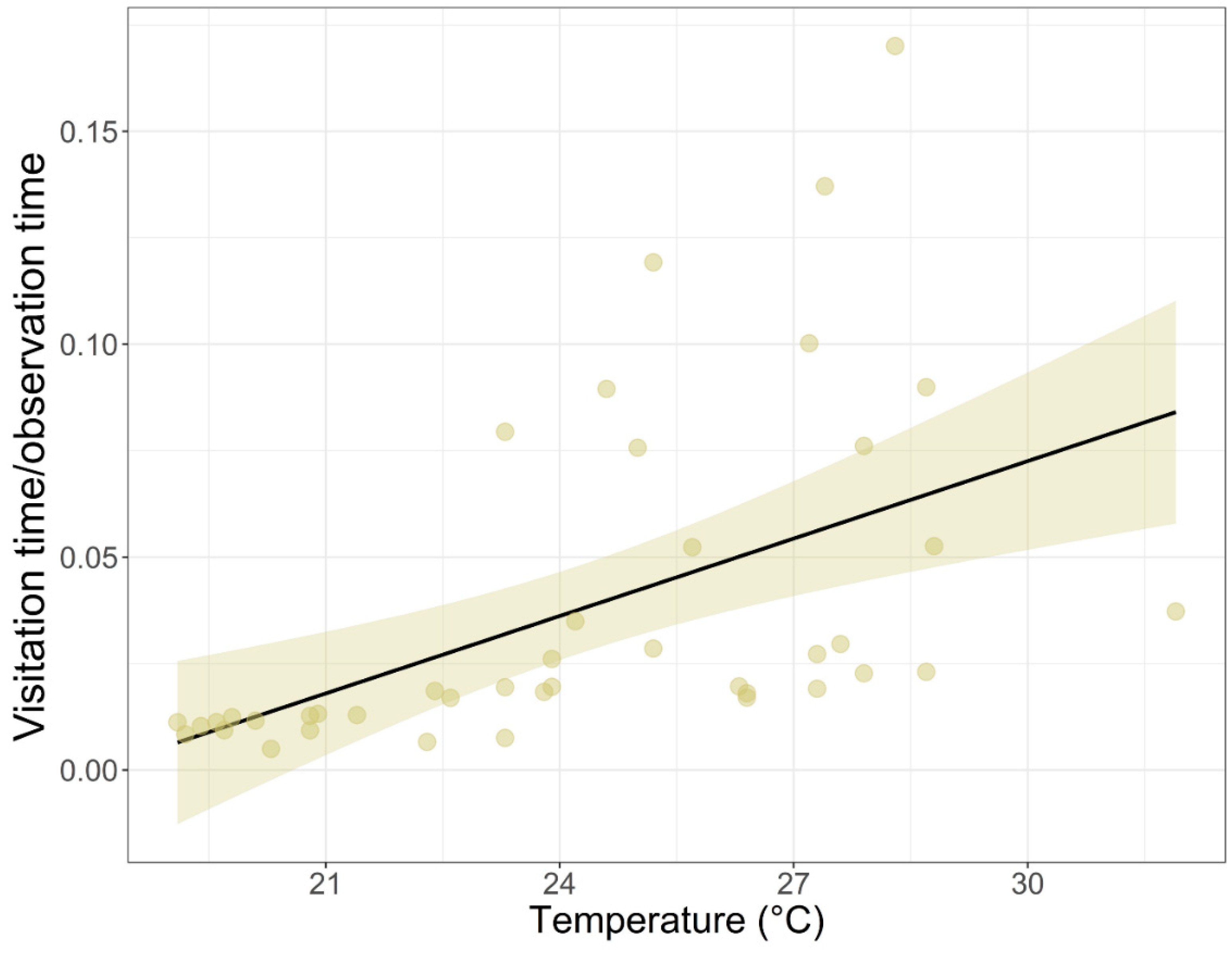
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, A.M.; Vassière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemmerling, L.R.; Griffin, S.R.; Haddad, N.M. Optimizing pollination conservation and crop yield among perennial bioenergy crops. GCB Bioenergy 2021, 13, 1030–1042. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels–The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oka, I.N. Integrated pest management in Indonesia: IPM by Farmers. In Integrated Pest Management in the Global Arena; Maredia, K.M., Dakouo, D., Mota-Sanchez, D., Eds.; CABI Publishing: Wallingford, UK, 2003; pp. 223–237. [Google Scholar]
- Matson, P.A.; Parton, W.J.; Power, A.G.; Swift, M.J. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [Green Version]
- IPBES. Summary for policymakers of the assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. In Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Potts, S.G., Imperatriz-Fonseca, V.L., Ngo, H.T., Biesmeijer, J.C., Breeze, T.D., Dicks, L.V., Garibaldi, L.A., Hill, R., Settele, J., Vanbergen, A.J., et al., Eds.; Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2016; p. 36. [Google Scholar]
- Nicholson, C.C.; Koh, I.; Richardson, L.L.; Beauchemin, A.; Ricketts, T.H. Farm and landscape factors interact to affect the supply of pollination services. Agric. Ecosyst. Environ. 2017, 250, 113–122. [Google Scholar] [CrossRef]
- Schweiger, O.; Biesmeijer, J.C.; Bommarco, R.; Hickler, T.; Hulme, P.E.; Klotz, S.; Kühn, I.; Moora, M.; Nielsen, A.; Ohlemüller, R.; et al. Multiple stressors on biotic interactions: How climate change and alien species interact to affect pollination. Biol. Rev. 2010, 85, 777–795. [Google Scholar] [CrossRef]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [Green Version]
- Millard, J.; Outhwaite, C.L.; Kinnersley, R.; Freeman, R.; Gregory, R.D.; Adedoja, O.; Gavini, S.; Kioko, E.; Kuhlmann, M.; Ollerton, J.; et al. Global effects of land-use intensity on local pollinator biodiversity. Nat. Comm. 2021, 12, 2902. [Google Scholar] [CrossRef]
- Poquet, Y.; Video, C.; Alaux, C. Modulation of pesticide response in honeybees. Apidologie 2016, 47, 412–426. [Google Scholar] [CrossRef] [Green Version]
- Rands, S.A.; Whitney, H.M. Effects of pollinator density-dependent preferences on field margin visitations in the midst of agricultural monocultures: A modelling approach. Ecol. Modell. 2010, 221, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Varah, A.; Jones, H.; Smith, J.; Potts, S.G. Temperate agroforestry systems provide greater pollination service than monoculture. Agric. Ecosyst. Environ. 2020, 301, 107031. [Google Scholar] [CrossRef]
- Özkara, A.; Akyıl, D.; Konuk, M. Pesticides, environmental pollution, and health. In Environmental Health Risk-Hazardous Factors to Living Species; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2016. [Google Scholar]
- Henry, M.; Cerrutti, N.; Aupinel, P.; Decourtye, A.; Gayrard, M.; Odoux, J.F.; Pissard, A.; Rüger, C.; Bretagnolle, V. Reconciling laboratory and field assessments of neonicotinoid toxicity to honeybees. Proc. R. Soc. B 2015, 282, 20152110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- Sponsler, D.B.; Grozinger, C.M.; Hitaj, C.; Rundlöf, M.; Botías, C.; Code, A.; Lonsdorf, E.V.; Melathopoulos, A.P.; Smith, D.J.; Suryanarayanan, S.; et al. Pesticides and pollinators: A socioecological synthesis. Sci. Total Environ. 2019, 662, 1012–1027. [Google Scholar] [CrossRef]
- Liu, X.; Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 67, 65–72. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Challa, G.K.; Firake, D.M.; Behere, G.T. Bio-pesticide applications may impair the pollination services and survival of foragers of honey bee, Apis cerana Fabricius in oilseed brassica. Environ. Poll. 2019, 249, 598–609. [Google Scholar] [CrossRef]
- Tomé, H.V.V.; Barbosa, W.F.; Martins, G.F.; Guedes, R.N.C. Spinosad in the native stingless bee Melipona quadrifasciata: Regrettable non-target toxicity of a bioinsecticide. Chemosphere 2015, 124, 103–109. [Google Scholar] [CrossRef]
- Armbrecht, I.; Gallego, M.C. Testing ant predation on the coffee berry borer in shaded and sun coffee plantations in Colombia. Entomol. Exp. Appl. 2007, 124, 261–267. [Google Scholar] [CrossRef]
- Escobar-Ramírez, S.; Grass, I.; Armbrecht, I.; Tscharntke, T. Biological control of the coffee berry borer: Main natural enemies, control success, and landscape influence. Biol. Control 2019, 136, 103992. [Google Scholar] [CrossRef]
- Mitchell, R.J.; Flanagan, R.J.; Brown, B.J.; Waser, N.M.; Karron, J.D. New frontiers in competition for pollination. Ann. Bot. 2009, 103, 1403–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarakini, G.; Chemura, A.; Tarakini, T.; Mashavakure, N.; Musundire, R. Foraging behaviour of Apis mellifera scutellata and Hypotrigona gribodoi bees in monoculture and polyculture vegetable gardens. Proc. R. Soc. B 2021, 74, 294–304. [Google Scholar] [CrossRef]
- Chèze, B.; David, M.; Martinet, V. Understanding farmers' reluctance to reduce pesticide use: A choice experiment. Ecol. Econ. 2020, 167, 106349. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, O. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Settele, J.; Bishop, J.; Potts, S.G. Climate change impacts on pollination. Nat. Plants 2016, 2, 16092. [Google Scholar] [CrossRef]
- Cohn, A.S.; Newton, P.; Gil, J.D.B.; Samberg, L.; Ricciardi, V.; Manly, J.R.; Northrop, S. Smallholder agriculture and climate change. Annu. Rev. Environ. Resour. 2017, 42, 347–375. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Francisco, H.A. Climate Change Vulnerability Mapping for Southwest Asia; Economy and Environment Program for Southeast Asia (EEPSEA): Singapore, 2009. [Google Scholar]
- Sarirahayu, K.; Aprianingsih, A. Strategy to improving smallholder coffee farmers productivity. Asian J. Technol. Manag. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Wolff, N.H.; Zeppetello, L.R.V.; Parsons, L.A.; Aggraeni, I.; Battisti, D.S.; Ebi, K.L.; Game, E.T.; Kroeger, T.; Masuda, Y.J.; Spector, J.T. The effect of deforestation and climate change on all-cause mortality and unsafe work conditions due to heat exposure in Berau, Indonesia: A modelling study. Lancet Planet. Health 2021, 5, 882–892. [Google Scholar] [CrossRef]
- Buchori, D.; Rizali, A.; Priawandiputra, W.; Raffiudin, R.; Sartiami, D.; Pujiastuti, Y.; Pradana, M.G.; Meilin, A.; Leatemia, J.A.; Sudiarta, I.P.; et al. Beekeeping and managed bee diversity in Indonesia: Perspective and preference of beekeepers. Diversity 2022, 14, 52. [Google Scholar] [CrossRef]
- Kahono, A.; Chantawannakul, P.; Engel, M.S. Social bees and the current status of beekeeping in Indonesia. In Asian Beekeeping in the 21st Century; Chantawannakul, P., Williams, G., Neumann, P., Eds.; Springer: Singapore, 2018; pp. 287–306. [Google Scholar]
- Torné-Noguera, A.; Rodrigo, A.; Osorio, S.; Bosch, J. Collateral effects of beekeeping: Impacts on pollen-nectar resources and wild bee communities. Basic Appl. Ecol. 2016, 17, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Bartomeus, I.; Park, M.G.; Gibbs, J.; Danforth, B.N.; Lakso, A.N.; Winfree, R. Biodiversity ensures plant-pollinator phenological synchrony against climate change. Ecol. Lett. 2013, 16, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Blüthgen, N.; Klein, A.M. Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic Appl. Ecol. 2011, 12, 282–291. [Google Scholar] [CrossRef]
- Nesper, M.; Kueffer, C.; Krishnan, S.; Kushalappa, C.G.; Ghazoul, J. Shade tree diversity enhances coffee production and quality in agroforestry systems in the Western Ghats. Agric. Ecosyst. Environ. 2017, 247, 172–181. [Google Scholar] [CrossRef]
- Gemmill-Herren, B.; Garibaldi, L.A.; Kremen, C.; Ngo, H.T. Building effective policies to conserve pollinators: Translating knowledge into policy. Curr. Opin. Insect Sci. 2021, 46, 64–71. [Google Scholar] [CrossRef]
- Westphal, C.; Steffan-Dewenter, I.; Tscharntke, T. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 2003, 6, 961–965. [Google Scholar] [CrossRef]
- Jahroh, S. Organic farming development in Indonesia: Lesson learned from organic farming in west Java and North Sumatera. In Proceedings of the Innovation and Sustainable Development in Agriculture and Food, Montpellier, France, 28 June–1 July 2010; pp. 1–11. [Google Scholar]
- Thorburn, C. Empire strikes back: The making and unmaking of Indonesia's national Integrated Pest Management program. Agroecol. Sustain. Food Syst. 2014, 38, 3–24. [Google Scholar] [CrossRef]
- Campera, M.; Budiadi, B.; Adinda, E.; Ahmad, N.; Balestri, M.; Hedger, K.; Imron, M.A.; Manson, S.; Nijman, V.; Nekaris, K.A.I. Fostering a wildlife-friendly program for sustainable coffee farming: The case of small-holder farmers in Indonesia. Land 2021, 10, 121. [Google Scholar] [CrossRef]
- Ibrahim, H.W.; Zailani, S. A review on the competitiveness of global supply chain in a coffee industry in Indonesia. Int. Bus. Manag. 2010, 4, 105–115. [Google Scholar]
- DaMatta, F.M.; Rahn, E.; Läderach, P.; Ghini, R.; Ramalho, J.C. Why could the coffee crop endure climate change and global warming to a greater extent than previously estimated? Clim. Change 2019, 152, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, M.T.K.; Armesto, J.J.; Primack, R.B. Community studies in pollination ecology in the high temperate Andes of central Chile II. Effect of temperature on visitation rates and pollination possibilities. Plant. Syst. Evol. 1985, 149, 187–203. [Google Scholar] [CrossRef]
- Nekaris, K.A.I.; Poindexter, S.; Reinhardt, K.D.; Sigaud, M.; Cabana, F.; Wirdateti, W.; Nijman, V. Coexistence between Javan slow lorises (Nycticebus javanicus) and humans in a dynamic agroforestry landscape in West Java, Indonesia. Int. J. Primatol. 2017, 38, 303–320. [Google Scholar] [CrossRef] [Green Version]
- Campera, M.; Balestri, M.; Manson, S.; Hedger, K.; Ahmad, N.; Nijman, V.; Budiadi, B.; Imron, M.A.; Nekaris, K.A.I. Shade trees and agrochemical use affect butterfly assemblages in coffee home gardens. Agric. Ecosyst. Environ. 2021, 319, 107547. [Google Scholar] [CrossRef]
- Ricketts, T.H. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv. Biol. 2004, 18, 1262–1271. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A powerful new tool for measuring fractional green canopy cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef] [Green Version]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Hartig, J. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Available online: https://cran.r-project.org/web/packages/DHARMa (accessed on 28 January 2022).
- Engel, E.C.; Irwin, R.E. Linking pollinator visitation rate and pollen receipt. Am. J. Bot. 2013, 90, 1612–1618. [Google Scholar] [CrossRef]
- Andersson, K.S.; Ekroos, J.; Stiernman, M.; Rundlöf, M.; Smith, H.G. Effects of farming intensity, crop rotation and landscape heterogeneity on field bean pollination. Agric. Ecosyst. Environ. 2014, 184, 145–148. [Google Scholar] [CrossRef]
- Kremen, C.; Miles, A. Ecosystem services in biologically diversified versus conventional farming systems: Benefits, externalities, and trade-offs. Ecol. Soc. 2012, 17, 40. [Google Scholar] [CrossRef]
- Putra, D.; Susanti, T.; Risnita, R.; Kurniawan, B.; Ampa, B. Indirect effect of pesticides utilization towards diversity of pollinator insects in chili plantation. IOP Conf. Ser. Mater. 2021, 1098, 052054. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Luo, S.; Hodgson, J.A.; Bianchi, F.J.J.A.; He, H.; van der Werd, W.; Zou, Y. Can landscape level semi-natural habitat compensate for pollinator biodiversity loss due to farmland consolidation? Agric. Ecosyst. Environ. 2021, 319, 107519. [Google Scholar] [CrossRef]
- Tommasi, N.; Biella, P.; Guzzetti, L.; Lasway, J.V.; Njovu, H.K.; Tapparo, A.; Agostinetto, G.; Peters, M.K.; Steffan-Dewenter, I.; Labra, M. Impact of land use intensification and local features on plants and pollinators in Sub-Saharan smallholder farms. Agric. Ecosyst. Environ. 2021, 319, 107560. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Carvalheiro, L.G.; Vaissière, B.E.; Gemmil-herren, B.; Hipólito, J.; Freitas, B.M.; Ngo, H.T.; Azzu, N.; Sáez, A.; Åström, J.; et al. Mutually beneficial pollinator diversity and crop yield outcomes in small and large farms. Science 2016, 351, 388–391. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M.; Steffan-Dewenter, I.; Tscharntke, T. Fruit set of highland coffee increases with the diversity of pollinating bees. Proc. R. Soc. B 2003, 270, 955–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushmann, S.L.; Drummond, F.A. Analysis of pollination services provided by wild and managed bees (Apoidea) in wild blueberry (Vaccinium angustifolium Aiton) production in Maine, USA, with a literature review. Agronomy 2020, 10, 1413. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A global synthesis reveals biodiversity-mediated benefits for crop production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [Green Version]
- Rader, R.; Reilly, J.; Bartomeus, I.; Winfree, R. Native bees buffer the negative impact of climate warming on honey bee pollination of watermelon crops. Glob. Chang. Biol. 2013, 19, 3103–3110. [Google Scholar] [CrossRef]
- Primack, R.B.; Inouye, D.W. Factors affecting pollinator visitation rates: A biogeographic comparison. Curr. Sci. 1993, 65, 257–262. [Google Scholar]
- Perfecto, I.; Mas, A.; Dietsch, T.; Vandermeer, J. Conservation of biodiversity in coffee agroecosystems: A tri-taxa comparison in southern Mexico. Biodivers. Conserv. 2013, 12, 1239–1252. [Google Scholar] [CrossRef]
- Campera, M.; Hedger, K.; Birot, H.; Manson, S.; Balestri, M.; Budiadi, B.; Imron, M.A.; Nijman, V.; Nekaris, K.A.I. Does the presence of shade trees and distance to the forest affect detection rates of terrestrial vertebrates in coffee home gardens? Sustainability 2021, 13, 8540. [Google Scholar] [CrossRef]
- Hennig, E.I. Plant Diversity Effects on Plant-Pollinator Interactions in Urban and Agricultural Settings. Ph.D. Thesis, University Duisburg-Essen, Duisburg, Germany, 2011. [Google Scholar]
- Prado, S.G.; Collazo, J.A.; Marand, M.H.; Irwin, R.E. The influence of floral resources and microclimate on pollinator visitation in an agro-ecosystem. Agric. Ecosyst. Environ. 2021, 307, 107196. [Google Scholar] [CrossRef]
- Hardman, C.J.; Norris, K.; Nevard, T.D.; Hughes, B.; Potts, S.G. Delivery of floral resources and pollination services on farmland under three different wildlife-friendly schemes. Agric. Ecosyst. Environ. 2016, 220, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M. Nearby rainforest promotes coffee pollination by increasing spatio-temporal stability in bee species richness. For. Ecol. Manag. 2009, 258, 1838–1845. [Google Scholar] [CrossRef]
- Pywell, R.F.; Heard, M.S.; Woodcock, B.A.; Hinsley, S.; Ridding, L.; Nowakowski, M.; Bullock, J.M. Wildlife-friendly farming increases crop yield: Evidence for ecological intensification. Proc. R. Soc. B 2015, 282, 20151740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardaker, A.; Pagella, T.; Rayment, M. Ecosystem service and dis-service impacts of increasing tree cover on agricultural land by land-sparing and land-sharing in the Welsh uplands. Ecosyst. Serv. 2021, 48, 101253. [Google Scholar] [CrossRef]
- Grass, I.; Kubitza, C.; Krishna, V.V.; Corre, M.D.; Mußhoff, O.; Pütz, P.; Drescher, J.; Rembold, K.; Ariyanti, E.S.; Barnes, A.D.; et al. Trade-offs between multifunctionality and profit in tropical smallholder landscapes. Nat. Comm. 2020, 11, 1186. [Google Scholar] [CrossRef] [Green Version]
- Wanger, T.C.; Dennig, F.; Toledo-Hernández, M.; Tscharntke, T.; Lambin, E.F. Cocoa pollination, biodiversity-friendly production, and the global market. arXiv 2021, arXiv:2112.02877. [Google Scholar]

| Order | Family | N Species | Pollinator Visitation Time (s) |
|---|---|---|---|
| Lepidoptera | Nymphalidae | 5 | 1636 |
| Papilionidae | 5 | 560 | |
| Pieridae | 1 | 65 | |
| Lycaenidae | 1 | 3 | |
| Hymenoptera | Apidae | 8 | 1336 |
| Vespidae | 2 | 211 | |
| Diptera | Syrphidae | 5 | 1194 |
| Drosophilidae | 2 | 11 | |
| Total | 29 | 5016 |
| Response | Predictor | Estimate | St. Error | Z-Value | p-Value |
|---|---|---|---|---|---|
| Number of visitor species a | Intercept | 0.366 | 1.225 | 0.299 | 0.765 |
| Chemicals: mixed c | 0.129 | 0.192 | 0.672 | 0.501 | |
| Chemicals: organic c | 0.418 | 0.194 | 2.152 * | 0.031 | |
| Humidity (%) | 0.004 | 0.006 | 0.567 | 0.571 | |
| Shade cover (%) | 0.001 | 0.005 | 0.112 | 0.911 | |
| Shade tree richness | 0.029 | 0.046 | 0.619 | 0.536 | |
| Temperature (°C) | 0.011 | 0.039 | 0.293 | 0.769 | |
| Pollinator visitation time b | Intercept | −8.905 | 1.754 | −5.077 * | <0.001 |
| Chemicals: mixed c | 0.580 | 0.266 | 2.183 * | 0.029 | |
| Chemicals: organic c | 0.845 | 0.308 | 2.745 * | 0.006 | |
| Humidity (%) | 0.015 | 0.008 | 1.900 | 0.057 | |
| Shade cover (%) | 0.003 | 0.007 | 0.486 | 0.627 | |
| Shade tree richness | −0.002 | 0.070 | −0.034 | 0.973 | |
| Temperature (°C) | 0.152 | 0.056 | 2.709 * | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manson, S.; Nekaris, K.A.I.; Hedger, K.; Balestri, M.; Ahmad, N.; Adinda, E.; Budiadi, B.; Imron, M.A.; Nijman, V.; Campera, M. Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens. Agronomy 2022, 12, 509. https://doi.org/10.3390/agronomy12020509
Manson S, Nekaris KAI, Hedger K, Balestri M, Ahmad N, Adinda E, Budiadi B, Imron MA, Nijman V, Campera M. Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens. Agronomy. 2022; 12(2):509. https://doi.org/10.3390/agronomy12020509
Chicago/Turabian StyleManson, Sophie, K. A. I. Nekaris, Katherine Hedger, Michela Balestri, Nabil Ahmad, Esther Adinda, Budiadi Budiadi, Muhammad Ali Imron, Vincent Nijman, and Marco Campera. 2022. "Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens" Agronomy 12, no. 2: 509. https://doi.org/10.3390/agronomy12020509
APA StyleManson, S., Nekaris, K. A. I., Hedger, K., Balestri, M., Ahmad, N., Adinda, E., Budiadi, B., Imron, M. A., Nijman, V., & Campera, M. (2022). Flower Visitation Time and Number of Visitor Species Are Reduced by the Use of Agrochemicals in Coffee Home Gardens. Agronomy, 12(2), 509. https://doi.org/10.3390/agronomy12020509






