Assessment of the Short-Term Impact of Anaerobic Digestate on Soil C Stock and CO2 Emissions in Shallow Water Table Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Emission of CO2 from Soil
2.3. Soil Samplings and Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Soil Water-Filled Pore Space (WFPS) and Temperature
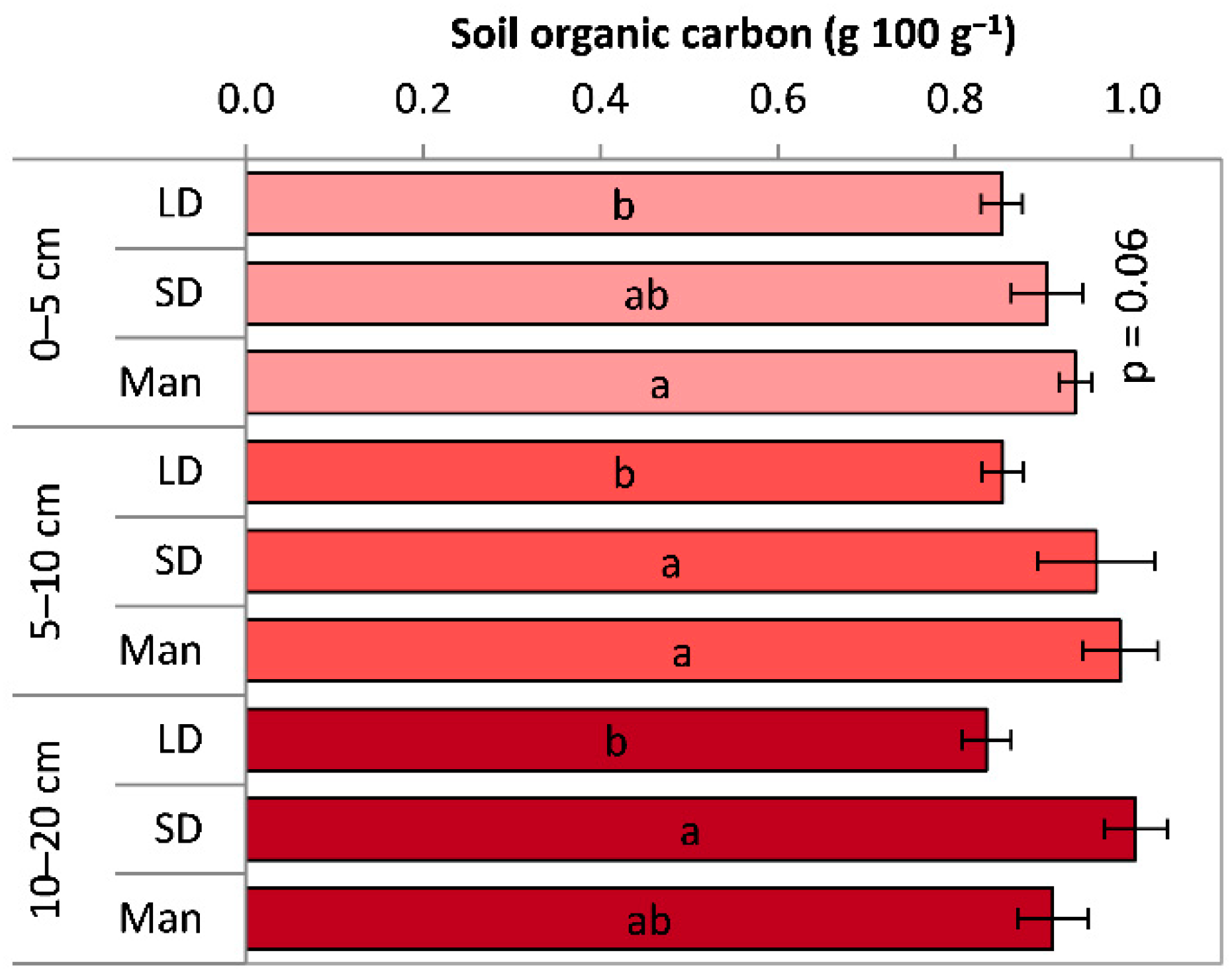
3.2. Soil Organic Carbon
3.2.1. Soil Organic Carbon (SOC) Content
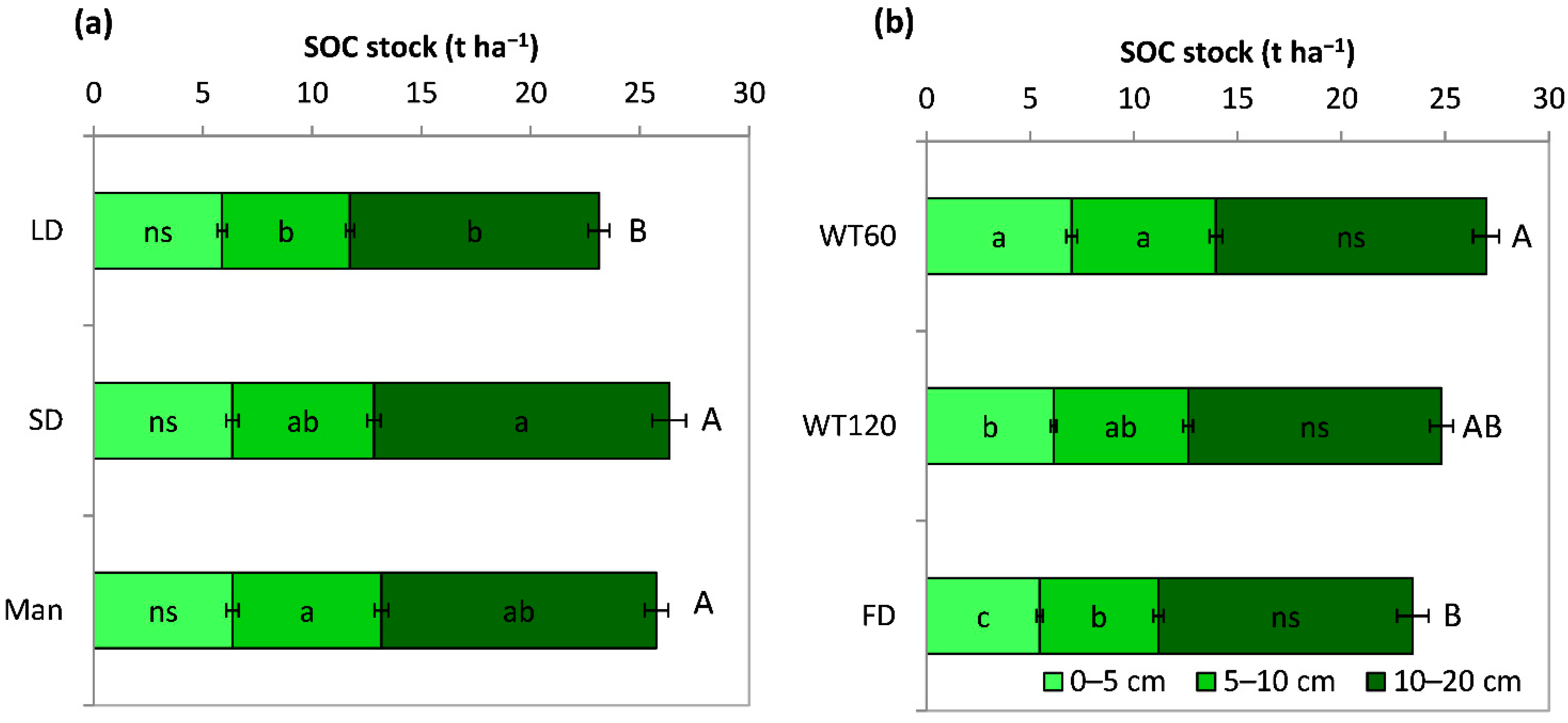
3.2.2. Soil Organic Carbon (SOC) Stock
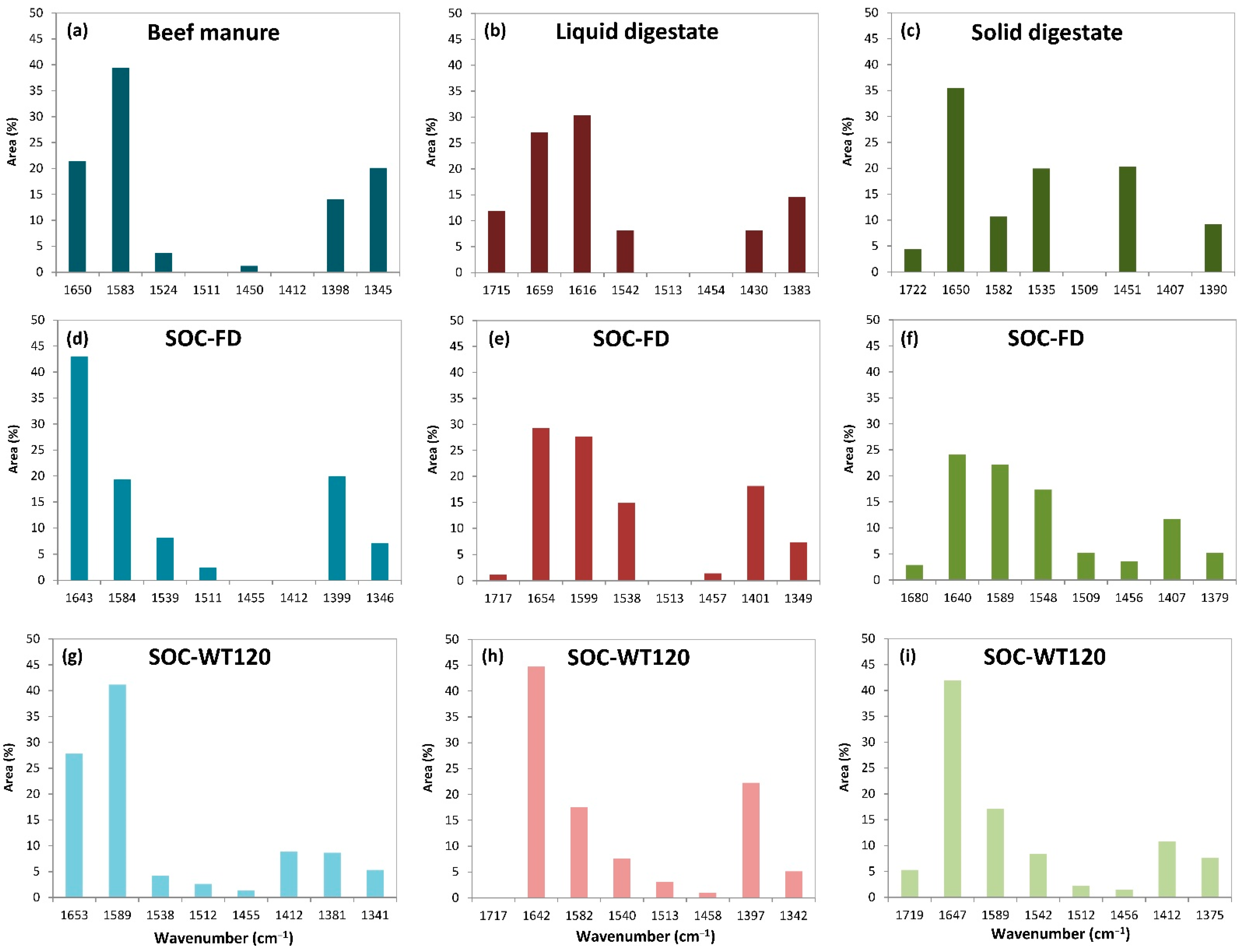
3.2.3. FT-IR Characterization of Soil Organic Carbon (SOC)
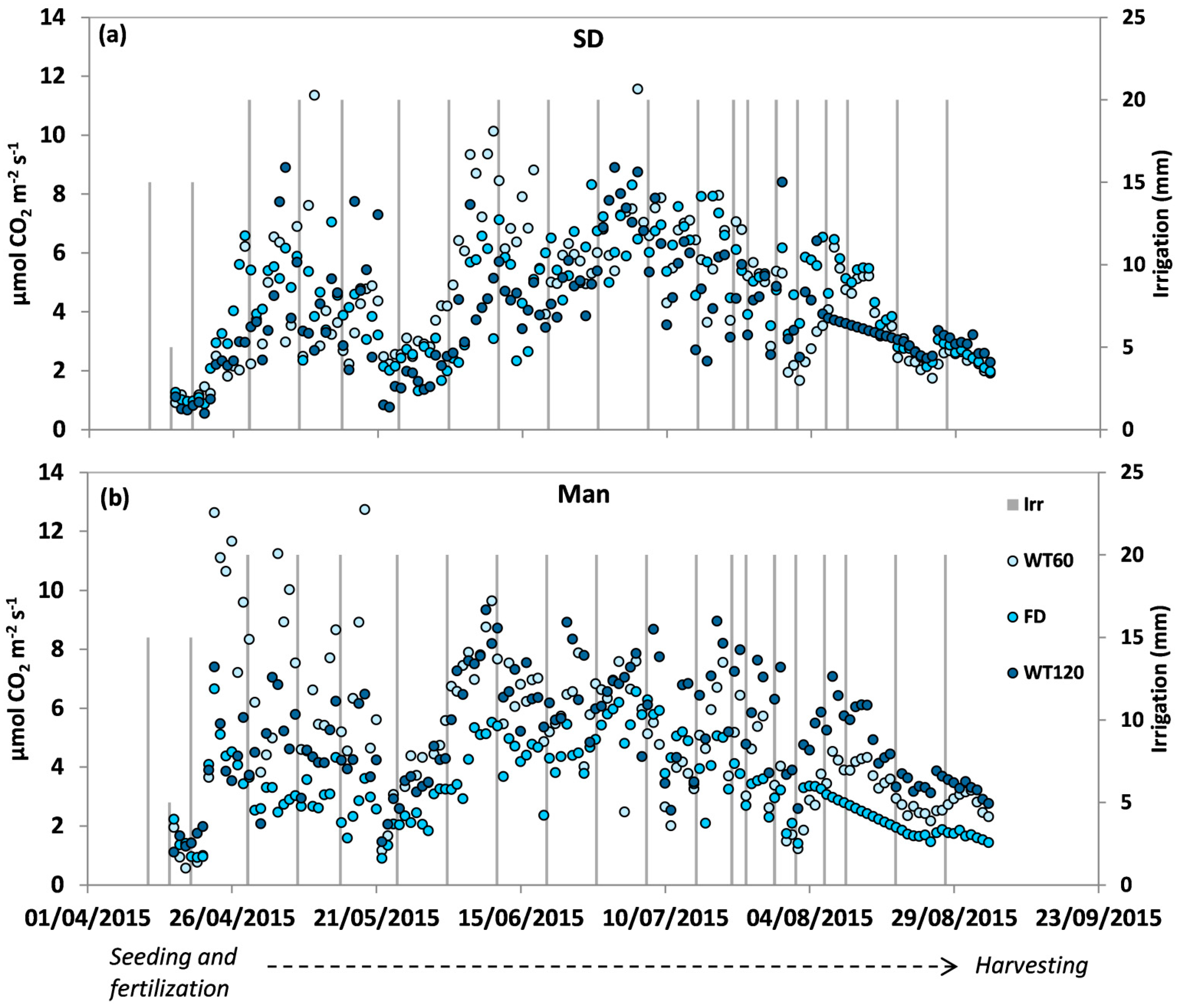
3.3. Soil Carbon Dioxide (CO2) Fluxes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Effect | 0–5 cm | 5–10 cm | 10–20 cm | 0–20 cm |
|---|---|---|---|---|
| Intercept | <0.01 | <0.01 | <0.01 | <0.01 |
| Time | 0.02 | <0.01 | <0.01 | <0.01 |
| Fert | 0.06 | 0.01 | 0.05 | <0.01 |
| WT | 0.15 | 0.78 | 0.16 | 0.15 |
| Time*Fert | 0.81 | 0.53 | 0.95 | 0.68 |
| Time*WT | 0.66 | 0.53 | 0.87 | 0.56 |
| Fert*WT | 0.40 | 0.36 | 0.54 | 0.11 |
| Time*Fert*WT | 0.35 | 0.38 | 0.68 | 0.09 |
| Effect | 0–5 cm | 5–10 cm | 10–20 cm | 0–20 cm |
|---|---|---|---|---|
| Intercept | <0.01 | <0.01 | <0.01 | <0.01 |
| Time | 0.02 | <0.01 | <0.01 | <0.01 |
| Fert | 0.09 | 0.01 | 0.05 | 0.01 |
| WT | <0.01 | <0.01 | 0.53 | 0.01 |
| Time*Fert | 0.76 | 0.46 | 0.93 | 0.31 |
| Time*WT | 0.57 | 0.36 | 0.84 | 0.56 |
| Fert*WT | 0.12 | 0.66 | 0.83 | 0.81 |
| Time*Fert*WT | 0.34 | 0.33 | 0.67 | 0.73 |
References
- EC New. EU Framework to Decarbonise Gas Markets. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6682 (accessed on 20 December 2021).
- EU Amending Directive 2010/31/EU on the Energy Performance of Buildings and Directive 2012/27/EU on Energy Efficiency. Available online: https://eur-lex.europa.eu/eli/dir/2018/844/oj (accessed on 13 February 2022).
- Corden, C.; Bougas, K.; Cunningham, E.; Tyrer, D.; Kreißig, J.; Zetti, E.; Al, E. Digestate and Compost as Fertilisers: Risk Assessment and Risk Management Options. 2019. Available online: https://ec.europa.eu/environment/chemicals/reach/pdf/40039%20Digestate%20and%20Compost%20RMOA%20-%20Final%20report%20i2_20190208.pdf (accessed on 13 February 2022).
- U.N. Transforming Our World: The 2030 Agenda for Sustainable Development; U.N.: New York, NY, USA, 2015. [Google Scholar]
- Arthurson, V. Closing the global energy and nutrient cycles through application of biogas residue to agricultural lLand—Potential benefits and drawback. Energies 2009, 2, 226–242. [Google Scholar] [CrossRef] [Green Version]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-based biogas fermentation residues—Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Grillo, F.; Piccoli, I.; Furlanetto, I.; Ragazzi, F.; Obber, S.; Bonato, T.; Meneghetti, F.; Morari, F. Agro-environmental sustainability of anaerobic digestate fractions in intensive cropping systems: Insights regarding the Nitrogen use efficiency and crop performance. Agronomy 2021, 11, 745. [Google Scholar] [CrossRef]
- Möller, K. Influence of different manuring systems with and without biogas digestion on soil organic matter and nitrogen inputs, flows and budgets in organic cropping systems. Nutr. Cycl. Agroecosyst. 2009, 84, 179–202. [Google Scholar] [CrossRef]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What is the potential for biogas digesters to improve soil carbon sequestration in Sub-Saharan Africa? Comparison with other uses of organic residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Marcato, C.E.; Mohtar, R.; Revel, J.C.; Pouech, P.; Hafidi, M.; Guiresse, M. Impact of anaerobic digestion on organic matter quality in pig slurry. Int. Biodeterior. Biodegrad. 2009, 63, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Molinuevo-Salces, B.; Gómez, X.; Morán, A.; García-González, M.C. Anaerobic co-digestion of livestock and vegetable processing wastes: Fibre degradation and digestate stability. Waste Manag. 2013, 33, 1332–1338. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Gómez, X.; Blanco, D.; Lobato, A.; Calleja, A.; Martínez-Núñez, F.; Martin-Villacorta, J. Digestion of cattle manure under mesophilic and thermophilic conditions: Characterization of organic matter applying thermal analysis and 1H NMR. Biodegradation 2011, 22, 623–635. [Google Scholar] [CrossRef]
- Tambone, F.; Adani, F.; Gigliotti, G.; Volpe, D.; Fabbri, C.; Provenzano, M.R. Organic matter characterization during the anaerobic digestion of different biomasses by means of CPMAS 13C NMR spectroscopy. Biomass Bioenergy 2013, 48, 111–120. [Google Scholar] [CrossRef]
- Tambone, F.; Genevini, P.; D’Imporzano, G.; Adani, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.N.; Tiwari, K.N.; Upadhyay, R.M. Effect of crop residues and biogas slurry incorporation in wheat on yield and soil Fertility. J. Indian Soc. Soil Sci. 2000, 48, 515–520. [Google Scholar]
- Béghin-Tanneau, R.; Guérin, F.; Guiresse, M.; Kleiber, D.; Scheiner, J.D. Carbon sequestration in soil amended with anaerobic digested matter. Soil Tillage Res. 2019, 192, 87–94. [Google Scholar] [CrossRef]
- Askri, A.; Laville, P.; Trémier, A.; Houot, S. Influence of origin and post-treatment on greenhouse gas emissions after anaerobic digestate application to soil. Waste Biomass Valorization 2016, 7, 293–306. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Oenema, O.; Kuikman, P.J.; Bakker, R.R.; Groenigen, J.W. Van Bioenergy by-products as soil amendments? Implications for carbon sequestration and greenhouse gas emissions. GCB Bioenergy 2010, 2, 201–213. [Google Scholar] [CrossRef]
- Schaufler, G.; Kitzler, B.; Schindlbacher, A.; Skiba, U.; Sutton, M.A.; Zechmeister-Boltenstern, S. Greenhouse gas emissions from European soils under different land use: Effects of soil moisture and temperature. Eur. J. Soil Sci. 2010, 61, 683–696. [Google Scholar] [CrossRef]
- Crézé, C.M.; Madramootoo, C.A. Water table management and fertilizer application impacts on CO2, N2O and CH4 fluxes in a corn agro-ecosystem. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Morari, F.; Lugato, E.; Polese, R.; Berti, A.; Giardini, L. Nitrate concentrations in groundwater under contrasting agricultural management practices in the low plains of Italy. Agric. Ecosyst. Environ. 2012, 147, 47–56. [Google Scholar] [CrossRef]
- Cocco, E.; Bertora, C.; Squartini, A.; Delle Vedove, G.; Berti, A.; Grignani, C.; Lazzaro, B.; Morari, F. How shallow water table conditions affect N2O emissions and associated microbial abundances under different nitrogen fertilisations. Agric. Ecosyst. Environ. 2018, 261, 1–11. [Google Scholar] [CrossRef]
- Vedove, G.D.; Alberti, G.; Zuliani, M.; Peressotti, A. Automated Monitoring of Soil Respiration: An Improved Automatic Chamber System. Ital. J. Agron. 2007, 2, 377–382. [Google Scholar] [CrossRef]
- Xu, L.; Furtaw, M.D.; Madsen, R.A.; Garcia, R.L.; Anderson, D.J.; McDermitt, D.K. On maintaining pressure equilibrium between a soil CO2 flux chamber and the ambient air. J. Geophys. Res. Atmos. 2006, 111, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, G.L.; Livingston, G.P. Vents and seals in non-steady-state chambers used for measuring gas exchange between soil and the atmosphere. Eur. J. Soil Sci. 2001, 52, 675–682. [Google Scholar] [CrossRef]
- Delle Vedove, G.; Grignani, C.; Bertora, C. Greenhouse Gases Emissions from Soils. In Handbook of Standardised Ecosystem Protocols; ExpeER: Rio de Janeiro, Brazil, 2013; pp. 65–86. Available online: https://expeeronline.eu/images/ExpeER-Documents/Handbook_of_standardized_ecosystem_protocols.pdf (accessed on 13 February 2022).
- Hutchinson, G.L.; Mosier, A.R. Improved Soil Cover Method for Field Measurement of Nitrous Oxide Fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Livingston, G.P.; Hutchinson, G.L. Enclosure-based measurement of trace gas exchange: Applications and sources of error. In Biogenic Trace Gases: Measuring Emissions from Soil and Water; Blackwell Science: Cambridge, MA, USA, 1995; pp. 14–50. [Google Scholar]
- Grossman, R.B.; Reinsch, T.G. 2.1 Bulk density and linear extensibility. In Methods of Soil Analysis: Part 4 Physical Methods; Dane, J.H., Topp, C.G., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 201–228. [Google Scholar]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardini, L. Soil organic matter properties after 40 years of different use of organic and mineral fertilisers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Rao, C.N.R. Chemical Applications of Infrared Spectroscopy; Academic Press, Inc.: New York, NY, USA; London, UK, 1963. [Google Scholar]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Sinsabaugh, R.L.; Repert, D.A.; Parkhurst, A.D.F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Rep. Ecol. 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, H.L.; Chen, G.; Peng, Y.; Xiao, Y.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS ONE 2014, 9, e88752. [Google Scholar] [CrossRef] [Green Version]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gagnon, B.; Bertrand, N. In situ mineralization of dairy cattle manures as determined using soil-surface carbon dioxide fluxes. Soil Sci. Soc. Am. J. 2006, 70, 744–752. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; Zilio, M.; Adani, F. Measuring the organic amendment properties of the liquid fraction of digestate. Waste Manag. 2019, 88, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, I.; Kaiser, M.; Gunina, A.; Ledesma, P.; Polifka, S.; Wiedner, K.; Mueller, C.W.; Glaser, B.; Ludwig, B. Substitution of mineral fertilizers with biogas digestate plus biochar increases physically stabilized soil carbon but not crop biomass in a field trial. Sci. Total Environ. 2019, 680, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.E.; Davidson, E.A. Interannual variation of soil respiration in two New England forests. Global Biogeochem. Cycles 2001, 15, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Tillage-induced seasonal changes in soil physical properties affecting soil CO2 evolution under intensive cropping. Soil Tillage Res. 1995, 34, 41–60. [Google Scholar] [CrossRef]
- Rochette, P.; Flanagan, L.B.; Gregorich, E.G. Separating soil respiration into plant and soil components using analyses of the natural abundance of carbon-13. Soil Sci. Soc. Am. J. 1999, 63, 1207–1213. [Google Scholar] [CrossRef]
- Maucieri, C.; Barbera, A.C.; Borin, M. Effect of injection depth of digestate liquid fraction on soil carbon dioxide emission and maize biomass production. Ital. J. Agron. 2016, 11, 6–11. [Google Scholar] [CrossRef]

| Fertilizer | Dry Matter (%) | TKN (g kg−1) | C (% Dry Matter) |
|---|---|---|---|
| Beef manure (Man) | 8.0 | 2.1 | 75 |
| Liquid digestate (LD) | 8.2 | 9.6 | 65 |
| Solid digestate (SD) | 22.5 | 8.6 | 85 |
| Period | Fertilizer | WT60 | WT120 | FD | |||
|---|---|---|---|---|---|---|---|
| Seeding–harvest | SD | 4.4 g CO2 m2 d−1 | b | 6.2 g CO2 m2 d−1 | a | 5.5 g CO2 m2 d−1 | a |
| Man | 5.6 g CO2 m2 d−1 | a | 5.9 g CO2 m2 d−1 | a | 4.0 g CO2 m2 d−1 | b | |
| 2 weeks after fertilization | SD | 3.4 g CO2 m2 d−1 | ns | 3.6 g CO2 m2 d−1 | ns | 4.5 g CO2 m2 d−1 | ns |
| Man | 8.2 g CO2 m2 d−1 | a | 4.9 g CO2 m2 d−1 | b | 3.8 g CO2 m2 d−1 | b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccoli, I.; Francioso, O.; Camarotto, C.; Delle Vedove, G.; Lazzaro, B.; Giandon, P.; Morari, F. Assessment of the Short-Term Impact of Anaerobic Digestate on Soil C Stock and CO2 Emissions in Shallow Water Table Conditions. Agronomy 2022, 12, 504. https://doi.org/10.3390/agronomy12020504
Piccoli I, Francioso O, Camarotto C, Delle Vedove G, Lazzaro B, Giandon P, Morari F. Assessment of the Short-Term Impact of Anaerobic Digestate on Soil C Stock and CO2 Emissions in Shallow Water Table Conditions. Agronomy. 2022; 12(2):504. https://doi.org/10.3390/agronomy12020504
Chicago/Turabian StylePiccoli, Ilaria, Ornella Francioso, Carlo Camarotto, Gemini Delle Vedove, Barbara Lazzaro, Paolo Giandon, and Francesco Morari. 2022. "Assessment of the Short-Term Impact of Anaerobic Digestate on Soil C Stock and CO2 Emissions in Shallow Water Table Conditions" Agronomy 12, no. 2: 504. https://doi.org/10.3390/agronomy12020504
APA StylePiccoli, I., Francioso, O., Camarotto, C., Delle Vedove, G., Lazzaro, B., Giandon, P., & Morari, F. (2022). Assessment of the Short-Term Impact of Anaerobic Digestate on Soil C Stock and CO2 Emissions in Shallow Water Table Conditions. Agronomy, 12(2), 504. https://doi.org/10.3390/agronomy12020504






