Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Management Practices, Experimental Arrangements, and Treatments
2.2. Determination of Plant Growth and Physiological Parameters
2.2.1. Soluble Protein
2.2.2. Superoxide Dismutase (SOD), Catalase (CAT), and Peroxidase (POD)
2.2.3. Chlorophyll Content (a, b, and Carotenoids)
2.3. Statistical Analysis
3. Results
3.1. Germination Percentage
3.2. Leaf Parameters
3.3. Stem Weight, Plant Height, and Number of Panicles Plant−1
3.4. Activities of Superoxide Dismutase (SOD), Catalase (CAT), Peroxidase (POD), and Content of Soluble Protein
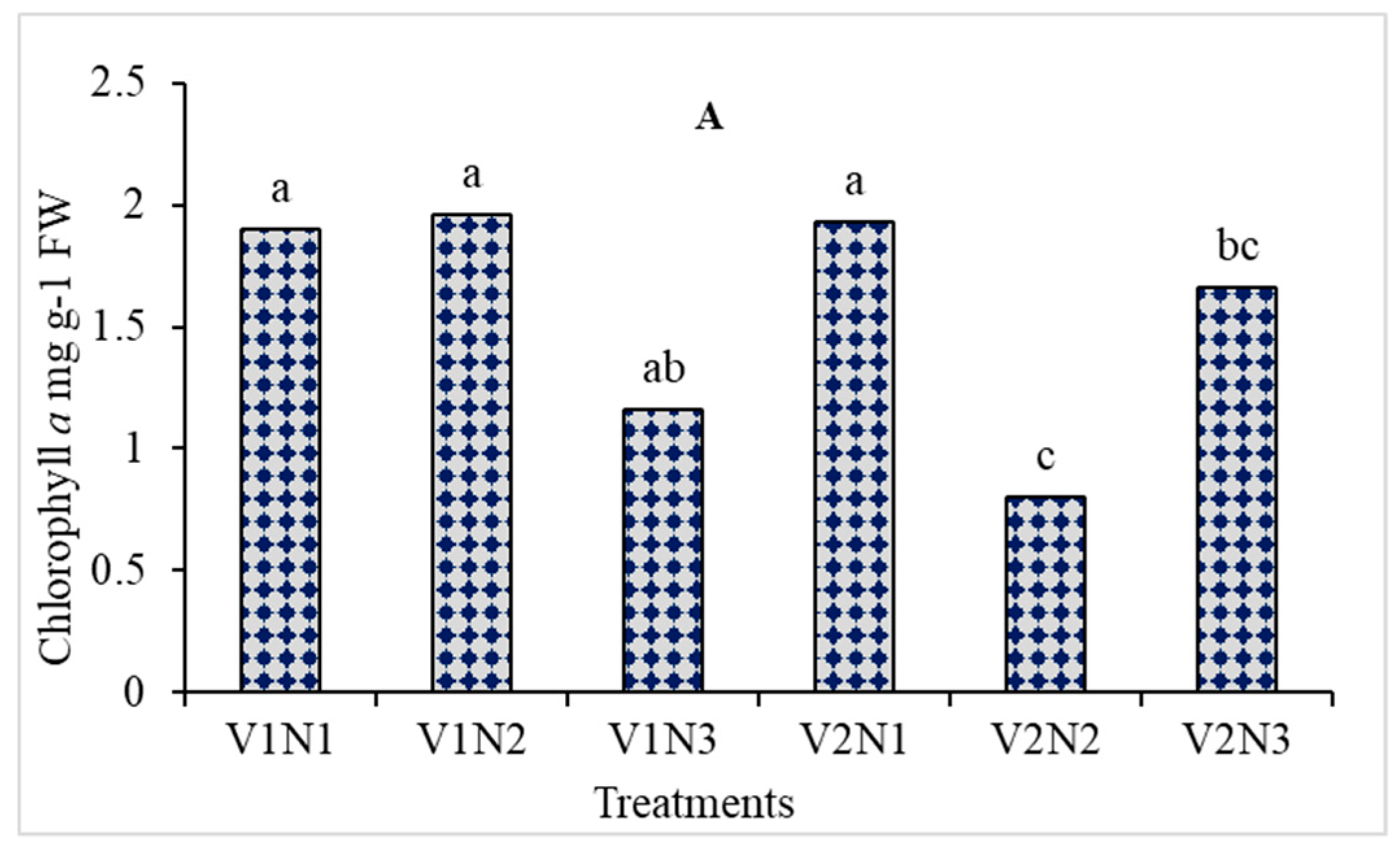
3.5. Chlorophyll a, b, and Carotenoids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Zhou, G.; Zhu, G.; Ahmad, Z.; Song, X.; Jamal, Y.; Ibrahim, M.E.H.; Nimir, N.E.A. Response of boll development to macronutrients application in different cotton genotypes. Agronomy 2019, 9, 322. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.; Khan, M.M.A.; Naeem, M. Effect of nitrogen on growth, nutrient assimilation, essential oil content, yield and quality attributes in Zingiber officinale Rosc. J. Saudi Soc. Agric. Sci. 2016, 15, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Gao, C.; Li, Y.; Li, Y.; Zhu, Y.; Xu, G.; Shen, Q.; Kaldenhoff, R.; Kai, L.; Guo, S. The enhanced drought tolerance of rice plants under ammonium is related to aquaporin (AQP). Plant Sci. 2015, 234, 14–21. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of ammonium and nitrate nutrition on some physiological processes in higher plants-growth, photosynthesis, photorespiration, and water relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E.H.; Zhu, X.; Zhou, G.; Ali, A.A.; Ahmad, I.; Farah, G.A. Nitrogen fertilizer alleviated negative impacts of NaCl on some physiological parameters of wheat. Pak. J. Bot. 2018, 50, 2097–2104. [Google Scholar]
- Prinsi, B.; Espen, L. Mineral nitrogen sources differently affect root glutamine synthetase isoforms and amino acid balance among organs in maize. BMC Plant Biol. 2015, 15, 96. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, Y.; Teraguchi, M.; Kobayashi, Y. Plasma levels of nitrate in congenital heart disease: Comparison with healthy children. Pediatr. Cardiol. 2002, 23, 132–136. [Google Scholar] [CrossRef]
- Yañez-Mansilla, E.; Cartes, P.; Reyes-Díaz, M.; Ribera-Fonseca, A.; Rengel, Z.; Alberdi, M. Leaf nitrogen thresholds ensuring high antioxidant features of Vaccinium corymbosum cultivars. J. Soil Sci. Plant Nutr. 2015, 15, 574–586. [Google Scholar] [CrossRef]
- Kong, L.; Xie, Y.; Hu, L.; Si, J.; Wang, Z. Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Almodares, A.; Jafarinia, M.; Hadi, M. The effects of nitrogen fertilizer on chemical compositions in corn and sweet sorghum. Agri. Enviro. Sci. 2009, 6, 441–446. [Google Scholar]
- Almodares, A.; Taheri, R.; Chung, M.; Fathi, M. The effect of nitrogen and potassium fertilizers on growth parameters and carbohydrate contents of sweet sorghum cultivars. J. Environ. Biol. 2008, 29, 849–852. [Google Scholar] [PubMed]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity, and nitrogen metabolism in ramie under nitrogen fertilizer. J. Agron. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Heitman, A.; Castillo, M.; Smyth, T.; Crozier, C. Stem, Leaf, and Panicle Yield and Nutrient Content of Biomass and Sweet Sorghum. J. Agron. 2018, 110, 1659–1665. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.E.; Woodard, K.R.; Sollenberger, L.E. Optimizing sweet sorghum production for biofuel in the southeastern USA through nitrogen fertilization and top removal. Bioenergy Res. 2012, 5, 86–94. [Google Scholar] [CrossRef]
- Wortmann, C.S.; Liska, A.; Ferguson, R.B.; Lyon, D.J.; Klein, R.; Dweikat, I. Dryland performance of sweet sorghum and grain crops for biofuel in Nebraska. J. Agron. 2010, 102, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Akinseye, F.M.; Ajeigbe, H.A.; Traore, P.C.; Agele, S.O.; Zemadim, B.; Whitbread, A. Improving sorghum productivity under changing climatic conditions: A modelling approach. Field Crops Res. 2020, 246, 107685. [Google Scholar] [CrossRef]
- Assefa, Y.; Staggenborg, S.A.; Prasad, V.P. Grain sorghum water requirement and responses to drought stress: A review. Crop Manag. 2010, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sekoli, M.; Morojele, M. Sorghum productivity trends and growth rate for Lesotho. Glob. J. Agric. Res. 2016, 4, 52–57. [Google Scholar]
- Adams, C.B.; Erickson, J.E.; Singh, M.P. Investigation and synthesis of sweet sorghum crop responses to nitrogen and potassium fertilization. Field Crops Res. 2015, 178, 1–7. [Google Scholar] [CrossRef]
- Ahmad, I.; Zhou, G.; Zhu, G.; Ahmad, Z.; Song, X.; Hao, G.; Jamal, Y.; Ibrahim, M.E.H. Response of leaf characteristics of BT cotton plants to ratio of nitrogen, phosphorus, and potassium. Pak. J. Bot. 2021, 53, 873–881. [Google Scholar] [CrossRef]
- Diallo, B.; Li, M.; Tang, C.; Ameen, A.; Zhang, W.; Xie, G.H. Biomass yield, chemical composition and theoretical ethanol yield for different genotypes of energy sorghum cultivated on marginal land in China. Ind. Crops Prod. 2019, 137, 221–230. [Google Scholar] [CrossRef]
- Giménez Luque, E.; Delgado Fernández, I.C.; Gómez Mercado, F. Effect of salinity and temperature on seed germination in Limonium cossonianum. J. Bot. 2013, 91, 12–16. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Srinivas, V.; Kumar, A.A.; Umakanth, A.V.; Addepally, U.; Rao, P.S. Composting of Sweet Sorghum Bagasse and its Impact on Plant Growth Promotion. Sugar Tech 2020, 22, 143–156. [Google Scholar] [CrossRef]
- Shukla, S.; Felderhoff, T.J.; Saballos, A.; Vermerris, W. The relationship between plant height and sugar accumulation in the stems of sweet sorghum (Sorghum bicolor (L.) Moench). Field Crops Res. 2017, 203, 181–191. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Ali, A.Y.A.; Elsiddig, A.M.I.; Zhou, G.; Nimir, N.E.A.; Ahmad, I.; Suliman, M.S.E.; Elradi, S.B.M.; Salih, E.G.I. Biochar improved sorghum germination and seedling growth under salinity stress. J. Agron. 2020, 112, 911–920. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Pan, K.; Zhu, T.; Li, W.; Zhang, L. Carbon and nitrogen metabolism in leaves and roots of dwarf bamboo (Fargesia denudata Yi) subjected to drought for two consecutive years during sprouting period. J. Plant. Growth Regul. 2014, 33, 243–255. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D. Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 3, pp. 673–686. [Google Scholar]
- Thomas, R.L.; Jen, J.J.; Morr, C.V. Changes in soluble and bound peroxidase—IAA oxidase during tomato fruit development. J. Food Sci. 1982, 47, 158–161. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Freed, R.; Eisensmith, S.; Goetz, S.; Reicosky, D.; Smail, V.; Welberg, P. User’s Guide to MSTAT-C.; Michigan State University: East Lansing, MI, USA, 1991. [Google Scholar]
- Amaducci, S.; Monti, A.; Venturi, G. Non-structural carbohydrates and fibre components in sweet and fibre sorghum as affected by low and normal input techniques. Ind. Crops Prod. 2004, 20, 111–118. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K.K. Effects of atmospheric-pressure N 2, He, air, and O 2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.-J.; Kim, H.-R.; Roy, S.K.; Kim, H.-J.; Boo, H.-O.; Woo, S.-H.; Kim, H.-H. Effects of nitrogen, phosphorus and potassium fertilizers on growth characteristics of two species of bellflower (Platycodon grandiflorum). J. Crop Sci. Biotechnol. 2019, 22, 481–487. [Google Scholar] [CrossRef]
- Ameen, A.; Yang, X.; Chen, F.; Tang, C.; Du, F.; Fahad, S.; Xie, G.H. Biomass yield and nutrient uptake of energy sorghum in response to nitrogen fertilizer rate on marginal land in a semi-arid region. BioEnergy Res. 2017, 10, 363–376. [Google Scholar] [CrossRef]
- Smith, G.; Buxton, D. Temperate zone sweet sorghumethanol production potential. Bioresour. Technol. 1993, 43, 71–75. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, D.; Niu, Z.; Yan, J.; Zhou, X.; Kang, X. Effects of combined organic/inorganic fertilizer application on growth, photosynthetic characteristics, yield and fruit quality of Actinidia chinesis cv ‘Hongyang’. Glob. Ecol. 2020, 22, e00997. [Google Scholar] [CrossRef]
- Cao, T.; Xie, P.; Ni, L.; Zhang, M.; Xu, J. Carbon and nitrogen metabolism of an eutrophication tolerative macrophyte, Potamogeton crispus, under NH4+ stress and low light availability. Environ. Exp. Bot. 2009, 66, 74–78. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, R.; Schachtman, D.P. Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol. 2004, 134, 1135–1145. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.-X.; Zhou, Z.-G.; Guo, W.-Q.; Chen, B.-L.; Oosterhuis, D.M. Effects of N fertilization on root development and activity of water-stressed cotton (Gossypium hirsutum L.) plants. Agric. Water Manag. 2008, 95, 1261–1270. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. cv. Strike) under high NH4NO3 application rates. Sci. Hortic. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Lis Martinez Stark, E.M.; Fernandes, M.S.; de Souza, S.R. Effects of seasonal nitrate flush on nitrogen metabolism and soluble fractions accumulation in two rice varieties. J. Plant Nutr. 2007, 30, 1371–1384. [Google Scholar] [CrossRef]
- Ahmed, S.O.; Abdalla, A.W.H.; Inoue, T.; Ping, A.; Babiker, E.E. Nutritional quality of grains of sorghum cultivar grown under different levels of micronutrients fertilization. Food Chem. 2014, 159, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.M.; Pereira, J.M.M.; Coutinho, J.F.; Björn, L.O.; Torres-Pereira, J.M. Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: A Mediterranean field study. Eur. J. Agron. 2005, 22, 337–347. [Google Scholar] [CrossRef]

| Source | Day after Sowing | |||
|---|---|---|---|---|
| 4 | 10 | 16 | 22 | |
| C (cultivar) | 2187 ** | 2187 ** | 752 ns | 8 ** |
| R (ratio of N) | 501 * | 500 * | 2668 ** | 478 ** |
| Y (year) | 3400 ** | 1045 ** | 7752 ** | 3072 ** |
| C × R | 2185 ** | 1317 ** | 5027 ** | 1272 ** |
| C × Y | 1160 ** | 1452 ** | 352 ns | 75 ns |
| R × Y | 551 * | 730 ** | 277 ns | 267 ns |
| C × R × Y | 479 * | 727 ** | 652 ns | 526 ** |
| Error | 148.70 | 131 | 263 | 99 |
| Year | ||||
| 2017 | 33 a | 34.8 a | 49 b | 67 b |
| 2018 | 22 b | 28.6 b | 66 a | 77 a |
| N levels | ||||
| N1 | 28 ab | 32 ab | 65 a | 74 a |
| N2 | 23 b | 27 b | 48 b | 68 b |
| N3 | 30 a | 35 a | 60 a | 74 a |
| Cultivars | ||||
| CFSH30 | 23 b | 27 b | 55 a | 72 a |
| Siyong3180 | 32 a | 36 a | 60 a | 72 a |
| Years | Cultivars | Rates of N (Kg ha−1) | Day after Sowing | |||
|---|---|---|---|---|---|---|
| 4 | 10 | 16 | 22 | |||
| 2017 | CFSH30 | 0 | 23.3 b | 23.3 b | 41.7 ab | 62.7 ab |
| 150 | 30.0 ab | 30.0 ab | 48.3 ab | 69.7 ab | ||
| 300 | 23.3 b | 26.7 ab | 45.0 ab | 65.7 ab | ||
| Siyong3180 | 0 | 46.7 ab | 48.3 ab | 70.0 a | 78.3 a | |
| 150 | 20.0 b | 22.3 b | 26.7 b | 50.0 b | ||
| 300 | 56.7 a | 58.3 a | 65.0 a | 76.3 ab | ||
| 2018 | CFSH30 | 0 | 18.3 b | 28.3 a | 73.3 a | 80.0 a |
| 150 | 26.0 a | 30.0 a | 70.0 ab | 80.0 a | ||
| 300 | 18.3 b | 25.0 a | 53.3 ab | 75.0 a | ||
| Siyong3180 | 0 | 26.7 a | 30.0 a | 75.0 ab | 78.3 a | |
| 150 | 18.3 b | 28.3 a | 48.3 b | 73.3 a | ||
| 300 | 18.3 b | 30.0 a | 78.3 a | 80.0 a | ||
| Cultivar | N (Kg ha−1) | Leaf Length (cm) | Leaf Width (cm) | Leaf Weight (g) | Specific Leaf Weight (g) |
|---|---|---|---|---|---|
| CFSH30 | 0 | 15.1 b | 4.2 ab | 34.6 b | 1.4 c |
| 150 | 16.2 ab | 4.5 ab | 34.9 b | 1.5 c | |
| 300 | 16.5 ab | 4.9 a | 42.4 ab | 1.6 bc | |
| Siyong3180 | 0 | 16.6 ab | 3.6 b | 46.9 a | 2.1 abc |
| 150 | 18.7 a | 3.8 b | 47.5 a | 2.6 ab | |
| 300 | 17.6 ab | 4.0 ab | 47.9 a | 2.7 a |
| Cultivars | N (kg ha−1) | Stem Weight (g) | Plant Height (cm) | Growth Stages | |
|---|---|---|---|---|---|
| Panicle Initiation | Reproduction | ||||
| Number of Panicle Plant−1 | Number of Panicle Plant−1 | ||||
| CFSH30 | 0 | 73.3 b | 21.0 b | 12.1 a | 53.4 a |
| 150 | 77.1 b | 22.3 ab | 4.7 ab | 34.1 ab | |
| 300 | 104.3 a | 24.1 ab | 12.4 a | 48.8 a | |
| Siyong3180 | 0 | 71.1 b | 26.1 a | 3.4 ab | 19.1 ab |
| 150 | 75.1 b | 25.6 a | 3.5 ab | 1.0 b | |
| 300 | 80 b | 25.8 a | 0.0 b | 0.4 b | |
| Cultivar | N (kg ha−1) | 2017 | 2018 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SOD (µg min−1) | POD (µg min−1) | CAT (µg min−1) | Soluble Protein (mg g−1) | SOD (µg min−1) | POD (µg min−1) | CAT (µg min−1) | Soluble Protein (mg g−1) | ||
| CFSH30 | 0 | 16.2 b | 296.4 b | 307.4 b | 10.8 b | 10.8 b | 382.5 a | 9.3 b | 47.7 ab |
| 150 | 16.2 b | 401.0 ab | 367.9 ab | 33.3 a | 12.2 b | 455.8 a | 60.8 ab | 53.2 ab | |
| 300 | 23.1 ab | 417.0 ab | 482.6 a | 36.0 a | 20.9 a | 448.3 a | 96.8 a | 53.9 a | |
| Siyong3180 | 0 | 18.2 ab | 366.7 ab | 334.6 ab | 10.0 b | 12.1 b | 290.8 a | 9.0 b | 40.5 b |
| 150 | 24.5 a | 373.3 ab | 375.9 ab | 26.8 a | 15.6 ab | 455 a | 40.5 ab | 46.6 ab | |
| 300 | 24.4 a | 435.4 a | 502.3 a | 28.4 a | 20.9 a | 345 a | 82.0 ab | 48.5 ab |
| Source | SOD | POD | CAT | Soluble Protein |
|---|---|---|---|---|
| µg min−1 | µg min−1 | µg min−1 | mg g−1 | |
| C (cultivar) | 200.0 ** | 13,783.7 ns | 284.0 ns | 867.0 ** |
| R (ratio of N) | 597.9 ** | 82,456.0 ** | 145,880.0 ** | 2244.9 ** |
| Y (year) | 633.1 ** | 5883.0 ns | 322,133.0 ** | 15,768.8 ** |
| C × R | 70.3 ** | 2686.0 ns | 871.0 ns | 18.4 ns |
| C × Y | 38.2 * | 49,190.4 ns | 6138.0 ns | 15.0 ns |
| R × Y | 33.0 * | 22,103.0 ns | 22,737.0 ** | 612.8 ** |
| C × R × Y | 11.3 ns | 22,063.0 ns | 34.0 ns | 45.2 ns |
| Error | 8.7 | 15,094.4 | 3867.0 | 28.2 |
| Year | ||||
| 2017 | 20.4 a | 381.5 a | 395.2 a | 24.2 b |
| 2018 | 15.5 b | 396.3 a | 49.8 b | 48.4 a |
| N levels | ||||
| N1 | 14.3 c | 333.9 b | 165.1 c | 27.3 b |
| N2 | 17.2 b | 421.3 a | 211.3 b | 40.0 a |
| N3 | 22.4 a | 411.4 a | 290.9 a | 41.8 a |
| Cultivars | ||||
| CFSH30 | 16.5 b | 400.17 a | 220.9 a | 39.2 a |
| Siyong3180 | 19.3 a | 377.6 a | 224.1 a | 33.5 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Zhu, G.; Zhou, G.; Song, X.; Hussein Ibrahim, M.E.; Ibrahim Salih, E.G. Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum. Agronomy 2022, 12, 501. https://doi.org/10.3390/agronomy12020501
Ahmad I, Zhu G, Zhou G, Song X, Hussein Ibrahim ME, Ibrahim Salih EG. Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum. Agronomy. 2022; 12(2):501. https://doi.org/10.3390/agronomy12020501
Chicago/Turabian StyleAhmad, Irshad, Guanglong Zhu, Guisheng Zhou, Xudong Song, Muhi Eldeen Hussein Ibrahim, and Ebtehal Gabralla Ibrahim Salih. 2022. "Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum" Agronomy 12, no. 2: 501. https://doi.org/10.3390/agronomy12020501
APA StyleAhmad, I., Zhu, G., Zhou, G., Song, X., Hussein Ibrahim, M. E., & Ibrahim Salih, E. G. (2022). Effect of N on Growth, Antioxidant Capacity, and Chlorophyll Content of Sorghum. Agronomy, 12(2), 501. https://doi.org/10.3390/agronomy12020501







