Effect of Spectral Sensitivity and Light Intensity Response on the Phototactic Behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a Pest of Sugarcane in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects Source
2.2. Soil Preparation as Feeding Source
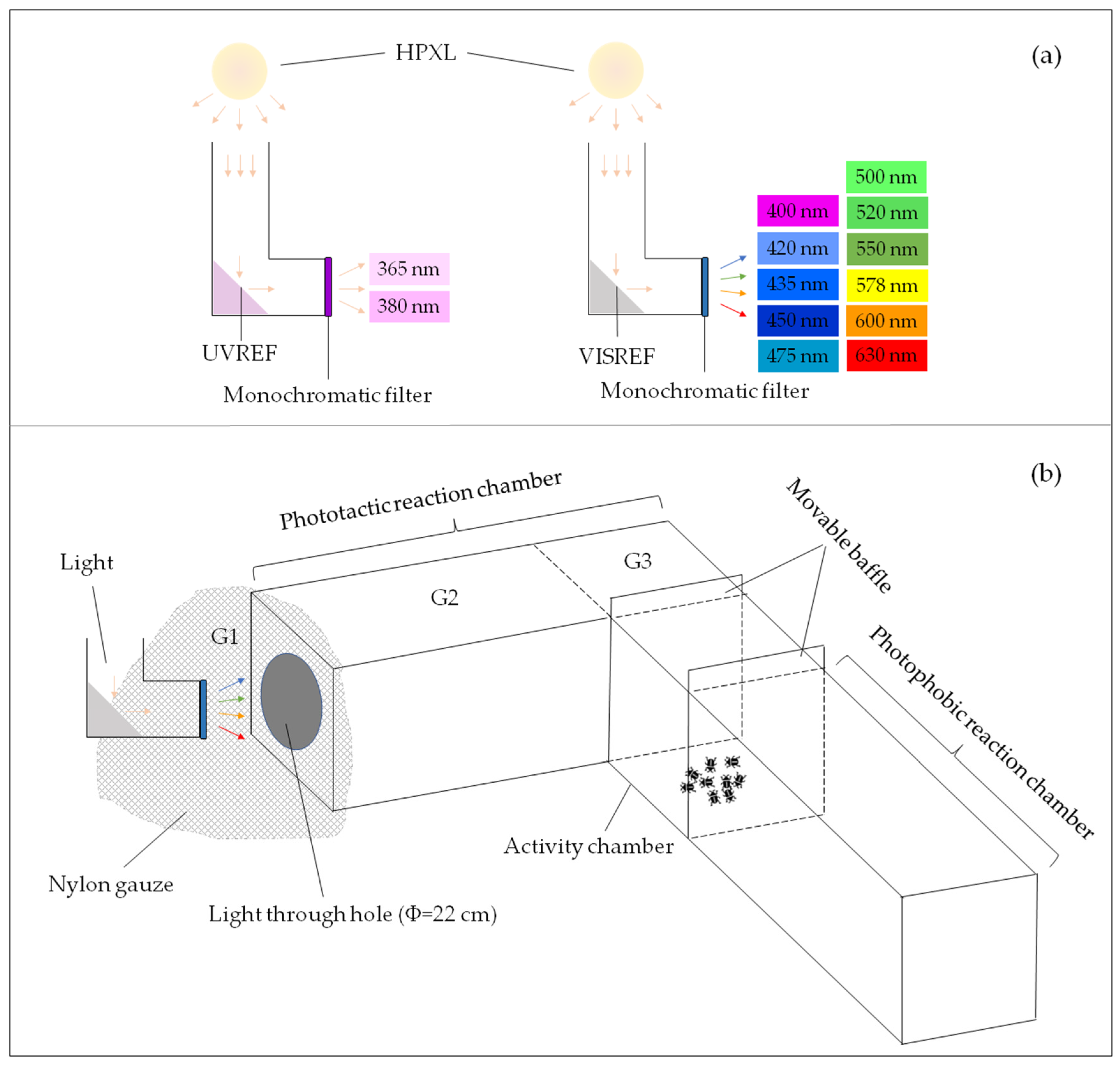
2.3. Optical Path System
2.4. Phototactic Behavior Reaction Device
2.5. Effect of Spectrum on Phototactic Behavior of Males and Females
2.6. Effect of Light Intensity on Phototactic Behavior of Males and Females
2.7. Statistical Analyses
3. Results
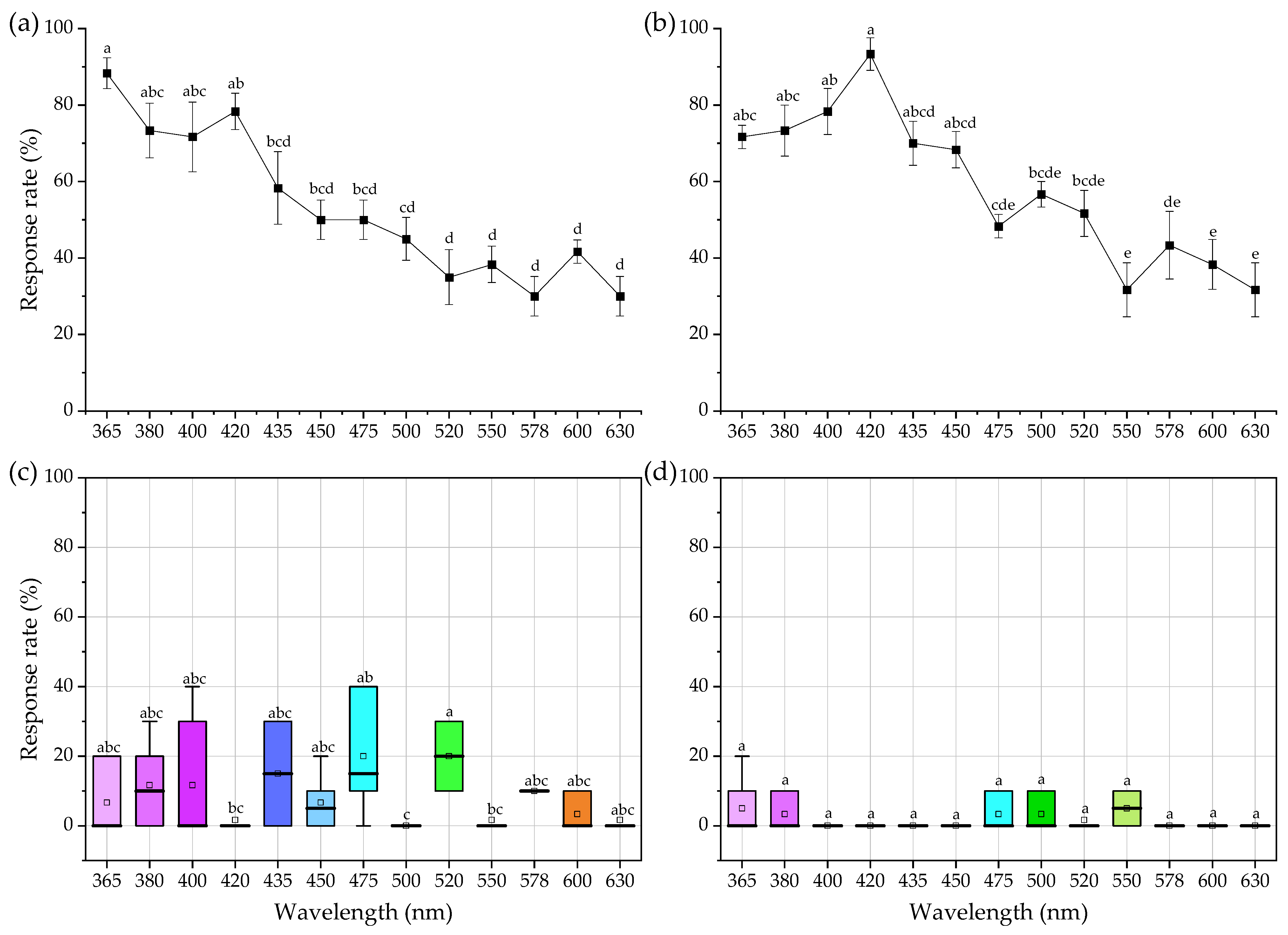
3.1. Spectral Sensitivity of Females and Males
3.2. Phototactic Response Intensity of Females and Males
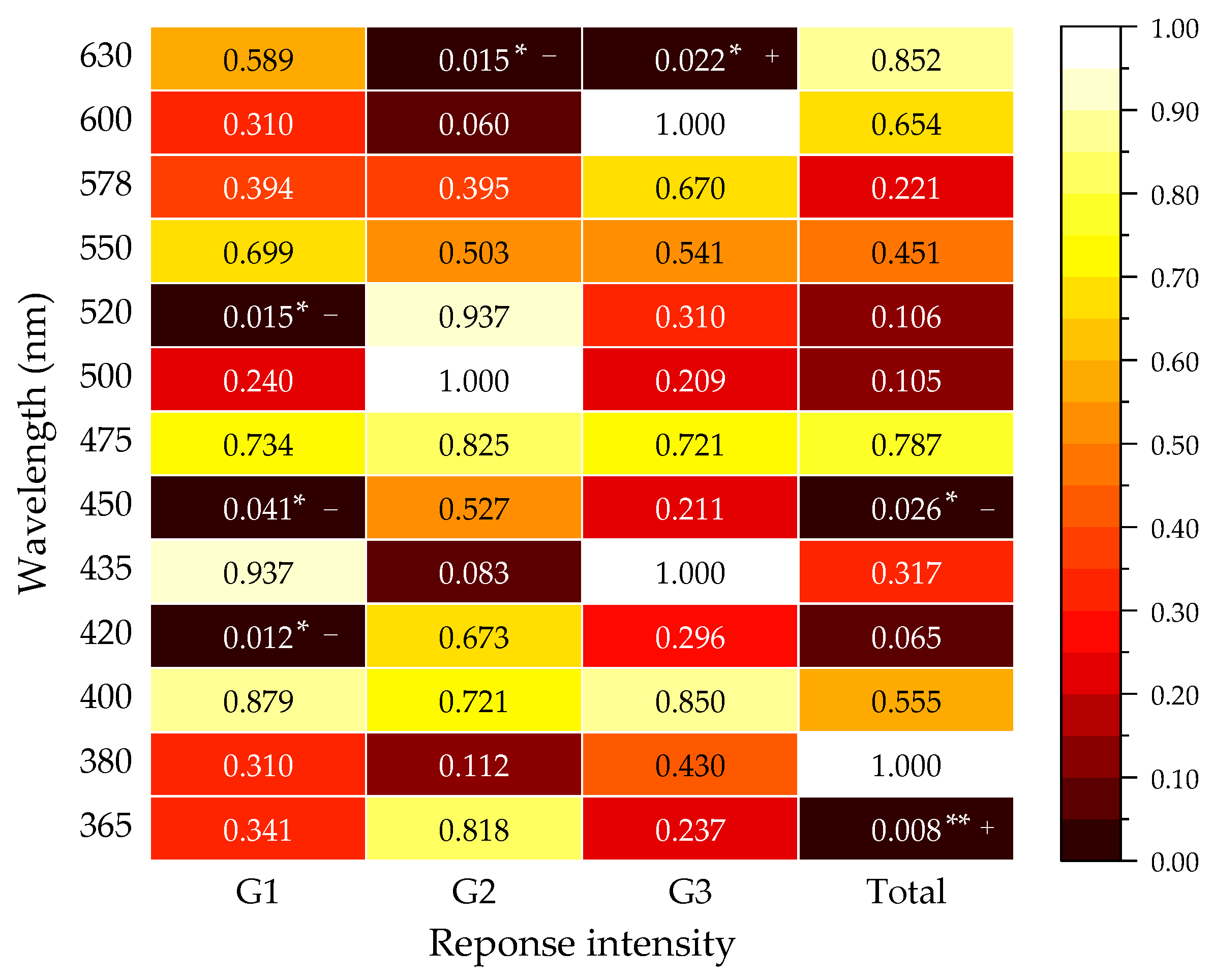
3.3. Comparison of Spectral Sensitivity between Males and Females at the Same Spectrum
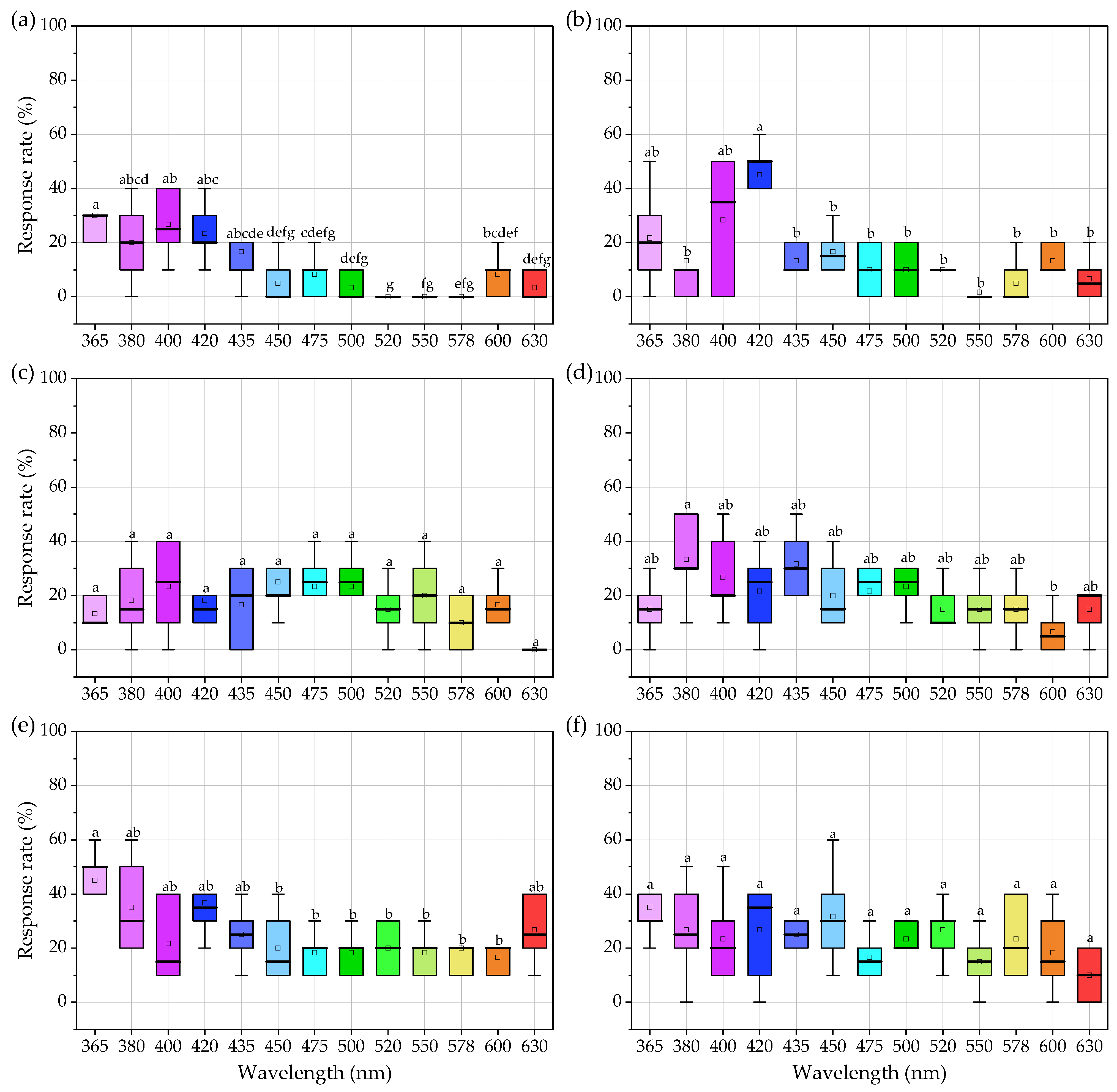
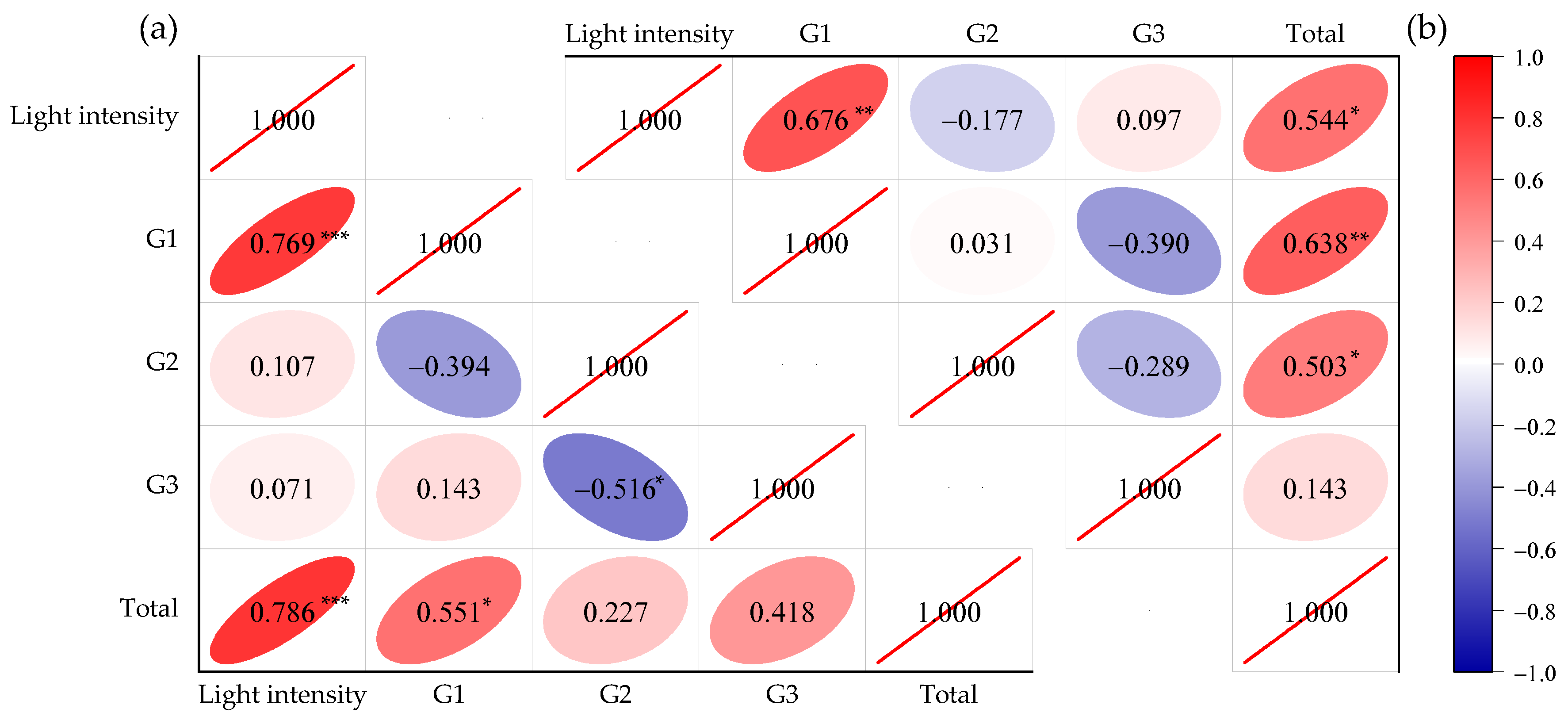
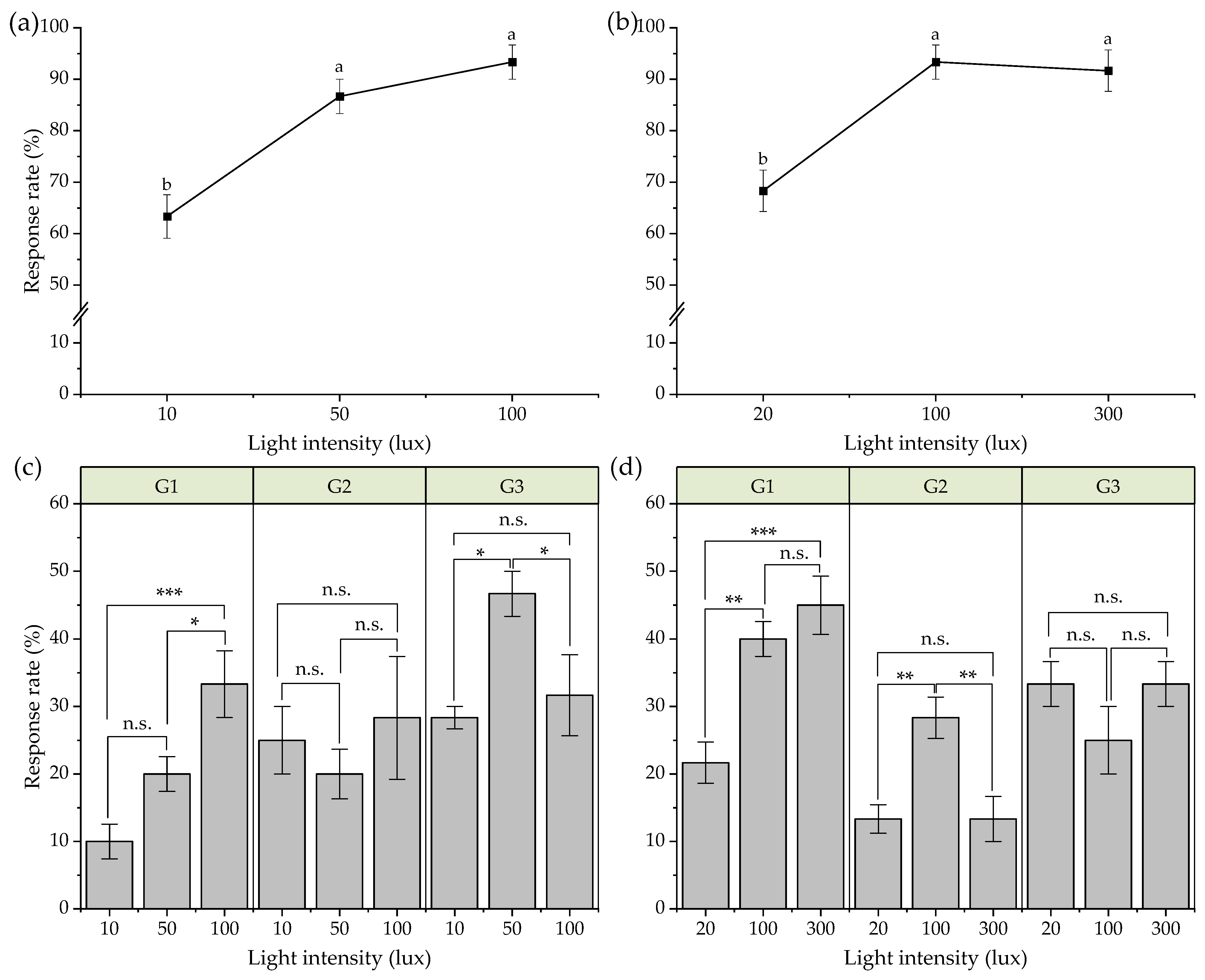
3.4. Effects of Light Intensity on Phototactic Behavior of Females
3.5. Effects of Light Intensity on Phototactic Behavior of Males
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.R.; Yang, L.T. Sugarcane agriculture and sugar industry in China. Sugar Tech 2015, 17, 1–8. [Google Scholar] [CrossRef]
- Li, B.Y. The present situation, problems and countermeasures of sugarcane cultivation in Guangxi. Chin. J. Trop. Agric. 2018, 38, 119–127. [Google Scholar]
- Li, Y.R. Innovation and prospect of sugarcane in Guangxi. J. Guangxi Agric. 2019, 34, 1–7. [Google Scholar]
- Goebel, F.R.; Sallam, N. New pest threats for sugarcane in the new bioeconomy and how to manage them. Curr. Opin. Env. Sust. 2011, 3, 81–89. [Google Scholar] [CrossRef]
- Rossato, J.A.S.; Costa, G.H.G.; Madaleno, C.L.; Mutton, M.J.R.; Higley, L.G.; Fernandes, O.A. Characterization and impact of the sugarcane borer on sugarcane yield and quality. Agron. J. 2013, 105, 643–648. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, B.H. Management of Sugarcane Diseases and Pests; Guangxi Science and Technology Press: Nanning, China, 2014; pp. 2–164. [Google Scholar]
- Huang, C.H.; Shang, X.K.; Wei, J.L.; Pan, X.H. Investigation on the species of sugarcane borers in sugarcane planting areas of Guangxi. Plant Prot. 2021, 47, 186–190. [Google Scholar]
- Gong, H.L.; An, Y.X. Underground Insect Pests of Sugar Crops in China; Jinan University Press: Guangzhou, China, 2010; pp. 7–65. [Google Scholar]
- Long, X.Z.; Yu, Y.H.; Peng, M.G.; Zeng, X.R.; Wei, D.W.; Wang, Z.Y.; Zeng, T.; Jiang, X.D. Occurrence and damage of cockchafer in sugarcane planting areas of Guangxi and its comprehensive prevention strategy. J. South. Agric. 2015, 46, 1038–1041. [Google Scholar]
- Yu, Y.H.; Zeng, T.; Wei, D.W.; Zeng, X.R.; Li, L.F.; Wang, Z.Y. Damage status of Dorysthenes granulosus (Thomson) and its control strategies in Guangxi sugarcane region. Guangxi Agric. Sci. 2006, 37, 545–547. [Google Scholar]
- Deng, T.J.; Zhang, Y.J.; Lu, Y.Y.; Zhang, W.J.; Liu, L.H. Sugarcane damages caused by termite and dominant termite populations in Guangxi. J. South. Agric. 2017, 48, 1009–1013. [Google Scholar]
- Shang, X.K.; Huang, C.H.; Ouyang, J.; Wei, X.; Pan, Z.L.; Pan, X.H.; Wei, J.L.; Wang, B.H. Damage and control of Exolontha castanea Chang in Laibin sugarcane area of Guangxi. Plant Prot. 2016, 42, 193–196. [Google Scholar]
- Shang, X.K.; Huang, C.H.; Wang, B.H. Research progress on chemical control of sugarcane beetles in China. J. South. Agric. 2011, 42, 1229–1232. [Google Scholar]
- Xie, J.J.; Yang, J.X.; Yang, C.Q.; Zhou, R.Q.; Wu, G.; Chen, S.; Luo, Q.W. Effects of 4% imidacloprid-bisultap granule on sugarcane pests and the effects on sugarcane yield. Chin. Plant Prot. 2021, 41, 83–85. [Google Scholar]
- Gong, H.L.; Sun, D.L.; Chen, L.J.; Zhao, H.H.; An, Y.X. Monitoring and management of insecticide resistance in Alissonotum impressicolle Arrow. Sugarcane Canesugar 2016, 4, 23–31. [Google Scholar]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Baker, G.H.; Tann, C.R.; Fitt, G.P. A tale of two trapping methods: Helicoverpa spp. (Lepidoptera, Noctuidae) in pheromone and light traps in Australian cotton production systems. Bull. Entomol. Res. 2011, 101, 9–23. [Google Scholar] [CrossRef]
- Sang, W.; Huang, Q.Y.; Wang, X.P.; Guo, S.H.; Lei, C.L. Progress in research on insect phototaxis and future prospects for pest light-trap technology in China. Chin. J. Appl. Entomol. 2019, 56, 907–916. [Google Scholar]
- Pan, H.; Liang, G.; Lu, Y. Response of different insect groups to various wavelengths of light under field conditions. Insects 2021, 12, 427. [Google Scholar] [CrossRef]
- Wei, J.; Kong, D.S.; Sun, M.H.; Hui, X.H.; Zhao, Y.L. The influence of peanut grubs occurrence degree by frequency trembler grid lamps in peanut field. Chin. Plant Prot. 2008, 28, 17–19. [Google Scholar]
- Chong, J.H.; Hinson, K.R. A comparison of trap types for assessing diversity of Scarabaeoidea on South Carolina golf courses. J. Econ. Entomol. 2015, 108, 2383–2396. [Google Scholar] [CrossRef][Green Version]
- Shimoda, M.; Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Erler, F.; Bayram, Y. Efficacy of mass trapping of tomato moth, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae), using a new-designed light trap in reducing leaf and fruit damages in greenhouse-grown tomatoes. J. Plant Dis. Protect. 2021, 128, 1177–1185. [Google Scholar] [CrossRef]
- Specht, A.; Sosa-Gómez, D.R.; Rios, D.A.M.; Claudino, V.C.M.; Paula-Moraes, S.V.; Malaquias, J.V.; Silva, F.A.M.; Roque-Specht, V.F. Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil: The big outbreak monitored by light traps. Neotrop. Entomol. 2021, 50, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.R.; Xiong, G.R.; Yang, B.P.; Zhan, R.L.; Cai, W.W.; Yang, N.B.; Li, G.P.; Zhang, S.Z. Population dynamics of phototactic insect in the sugarcane producing regions of Hainan province. Chin. J. Trop. Crops. 2013, 34, 2430–2435. [Google Scholar]
- Shang, X.K.; Huang, C.H.; Pan, X.H.; Wei, J.L.; Yang, Z.W.; Chen, J.X. Species composition and occurrence dynamics of scarabs under light traps in Beihai sugarcane area of Guangxi. J. Plant Prot. 2017, 44, 693–694. [Google Scholar]
- Shang, X.K.; Wei, J.L.; Liu, W.; Pan, X.H.; Huang, C.H.; Nikpay, A.; Goebel, F.R. Investigating population dynamics and sex structure of Exolontha castanea Chang (Coleoptera: Melolonthidae) using light traps in sugarcane fields in China. Sugar Tech 2022, 1–8. [Google Scholar] [CrossRef]
- Qu, Q.; Qu, M.J.; Chen, J.F.; Zhao, Z.Q.; Niu, H.L.; Zhou, Q.; Yu, S.L. The influence of spectral and sexual differences on phototaxis action of several kinds of beetles. Chin. Bull. Entomol. 2010, 47, 512–516. [Google Scholar]
- Lü, F.; Hai, X.X.; Fan, F.; Zhou, X.; Liu, S. The phototactic behavior of oriental brown chafer Serica orientalis to different monochromatic lights and light intensities. J. Plant Prot. 2016, 43, 656–661. [Google Scholar]
- Xu, L. Research on the Phototaxis of Harmonia axyridis, Ostrinia furnacalis and Bemisia tabaci. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2016. [Google Scholar]
- Cheng, W.J.; Zheng, X.L.; Wang, P.; Zhou, L.L.; Si, S.Y.; Wang, X.P. Male-biased capture in light traps in Spodoptera exigua (Lepidoptera: Noctuidae): Results from the studies of reproductive activities. J. Insect Behav. 2016, 29, 368–378. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Lau, T.F.S. Sexual dimorphism in the compound eye of the moth Operophtera brumata (Lepidoptera, Geometridae). Invertebr. Biol. 2008, 127, 201–216. [Google Scholar] [CrossRef]
- Cheng, W.J.; Zheng, X.L.; Wang, P.; Lei, C.L.; Wang, X.P. Sexual difference of insect phototactic behavior and related affecting factors. Chin. J. Appl. Ecol. 2011, 22, 3351–3357. [Google Scholar]
- Wen, C.; Ji, Y.C.; Zhang, G.Y.; Tan, S.B.; Wen, J.B. Phototactic behaviour of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) adults. Biocontrol Sci. Technol. 2018, 28, 544–561. [Google Scholar] [CrossRef]
- Wei, G.S.; Zhang, Q.W.; Zhou, M.Z.; Wu, W.G. Studies on the phototaxis of Helicoverpa armigera Hubner. Acta Biophys. Sin. 2000, 16, 89–94. [Google Scholar]
- Miljeteig, C.; Olsen, A.J.; Båtnes, A.S.; Jenssen, B.M. Sex and life stage dependent phototactic response of the marine copepod Calanus finmarchicus (Copepoda: Calanoida). J. Exp. Mar. Biol. Ecol. 2014, 451, 16–24. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, H.H.; Liu, S.; Zhai, Y.F.; Wei, G.S.; Yu, Y. A comparative study on the phototaxis behaviors of Drosophila suzukii and D. melanogaster. J. Plant Prot. 2019, 46, 499–500. [Google Scholar]
- Kim, K.N.; Huang, Q.Y.; Lei, C.L. Advances in insect phototaxis and application to pest management: A review. Pest Manag. Sci. 2019, 75, 3135–3143. [Google Scholar] [CrossRef]
- Kecskeméti, S.; Geösel, A.; Fail, J.; Egri, Á. In search of the spectral composition of an effective light trap for the mushroom pest Lycoriella ingenua (Diptera: Sciaridae). Sci. Rep. 2021, 11, 12770. [Google Scholar] [CrossRef]
- Fakhari, H.; Karimzadeh, J.; Moharramipour, S.; Ahadiyat, A.; Doranian, D. Phototactic behavior of Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) under visible wavelengths. J. Asia-Pac. Entomol. 2020, 23, 1181–1187. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Guo, Z.; Zhang, J.T.; Chen, Y.S.; Zhou, J.Y.; Pan, Y.L.; Liu, X.P. Phototactic behavioral response of the ectoparasitoid beetle Dastarcus helophoroides (Coleoptera: Bothrideridae): Evidence for attraction by near-infrared light. J. Econ. Entomol. 2021, 114, 1549–1556. [Google Scholar] [CrossRef]
- Yang, X.Y. Study on Microstructure of the Compound Eye and Phototactic Behavior of Athetis lepigone. Master’s Thesis, Agricultural University of Hebei, Baoding, China, 2015. [Google Scholar]
- Johansen, N.S.; Vänninen, I.; Pinto, D.M.; Nissinen, A.I.; Shipp, L. In the light of new greenhouse technologies: 2. Direct effects of artificial lighting on arthropods and integrated pest management in greenhouse crops. Ann. Appl. Biol. 2011, 159, 1–27. [Google Scholar] [CrossRef]
- Jing, X.F.; Lei, C.L. Advances in research on phototaxis of insects and the mechanism. Entomol. Knowl. 2004, 41, 198–202. [Google Scholar]
- Warrant, E.; Nilsson, D.E. Invertebrate Vision; Cambridge University Press: Cambridge, UK, 2006; pp. 1–42. [Google Scholar]
- Jiang, Y.L.; Guo, Y.Y.; Wu, Y.Q.; Li, T.; Duan, Y.; Miao, J.; Gong, Z.J.; Huang, Z.J. Spectral sensitivity of the compound eyes of Anomala corpulenta Motschulsky (Coleoptera: Scarabaeoidea). J. Integr. Agr. 2015, 14, 706–713. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.L.; Pan, H.S.; Liang, G.M.; Yang, Y.Z.; Lu, Y.H. Field evaluation of light-emitting diodes with different wavelengths as traps of Anomala corpulenta and Holotrichia parallela. Xinjiang Agric. Sci. 2020, 57, 2028–2033. [Google Scholar]
- Chen, N.S. The nature of phototactic behavior and the principle of navigation of insects in Noctuidae. Entomol. Knowl. 1979, 24, 193–200. [Google Scholar]
- Liu, L.C. Preliminary study on phototactic behavior of insects. J. Nanjing Agric. Univ. 1982, 2, 52–59. [Google Scholar]
- Sang, W.; Zhu, Z.Z.; Lei, C.L. Review of phototaxis in insects and an introduction to the light stress hypothesis. Chin. J. Appl. Entomol. 2016, 53, 915–920. [Google Scholar]
- Baliota, G.V.; Athanassiou, C.G.; Cohnstaedt, L.W. Response of phosphine-resistant and esusceptible Lasioderma serricorne adults to different light spectra. J. Stored Prod. Res. 2021, 92, 101808. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wu, Y.Q.; Li, T.; Gong, Z.J.; Duan, Y.; Miao, J.; Guo, Y.Y. Behavioural responses of Anomala corpulenta Motschulsky (Coleoptera: Scarabaeoidea) to different spectral light. Acta Entomol. Sin. 2015, 58, 1146–1150. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, X.-K.; Pan, X.-H.; Liu, W.; Wei, J.-L.; Huang, C.-H.; Goebel, F.-R.; Nikpay, A. Effect of Spectral Sensitivity and Light Intensity Response on the Phototactic Behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a Pest of Sugarcane in China. Agronomy 2022, 12, 481. https://doi.org/10.3390/agronomy12020481
Shang X-K, Pan X-H, Liu W, Wei J-L, Huang C-H, Goebel F-R, Nikpay A. Effect of Spectral Sensitivity and Light Intensity Response on the Phototactic Behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a Pest of Sugarcane in China. Agronomy. 2022; 12(2):481. https://doi.org/10.3390/agronomy12020481
Chicago/Turabian StyleShang, Xian-Kun, Xue-Hong Pan, Wei Liu, Ji-Li Wei, Cheng-Hua Huang, François-Régis Goebel, and Amin Nikpay. 2022. "Effect of Spectral Sensitivity and Light Intensity Response on the Phototactic Behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a Pest of Sugarcane in China" Agronomy 12, no. 2: 481. https://doi.org/10.3390/agronomy12020481
APA StyleShang, X.-K., Pan, X.-H., Liu, W., Wei, J.-L., Huang, C.-H., Goebel, F.-R., & Nikpay, A. (2022). Effect of Spectral Sensitivity and Light Intensity Response on the Phototactic Behavior of Exolontha castanea Chang (Coleoptera: Melolonthidae), a Pest of Sugarcane in China. Agronomy, 12(2), 481. https://doi.org/10.3390/agronomy12020481





