Long-Term Impacts of Different Cropping Patterns on Soil Physico-Chemical Properties and Enzyme Activities in the Low Land Plain of North China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design and Treatment Management
2.3. Soil Sampling and Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil Physical Properties
3.1.1. Soil Aggregate Formation
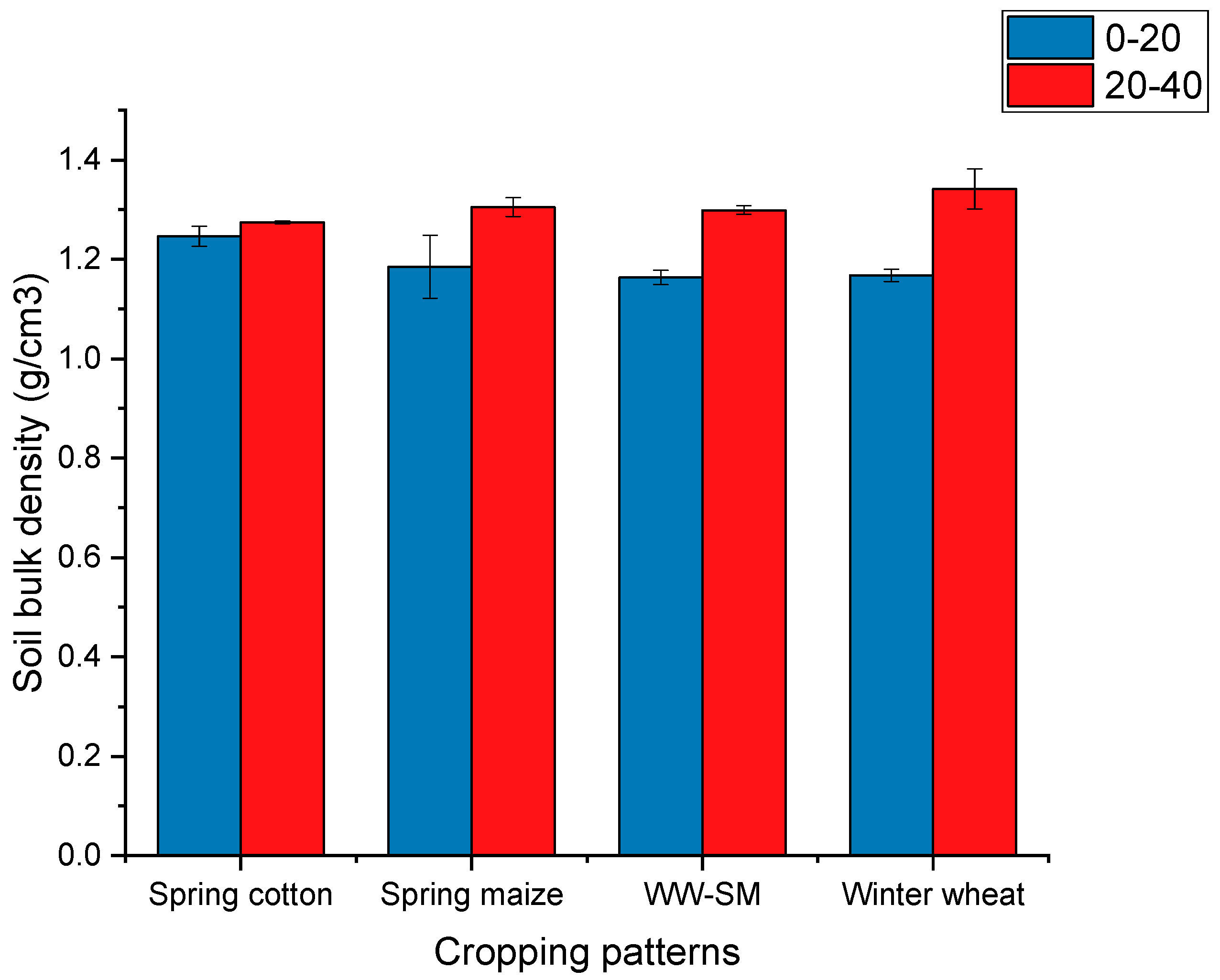
3.1.2. Soil Bulk Density
3.2. Soil Chemical Properties
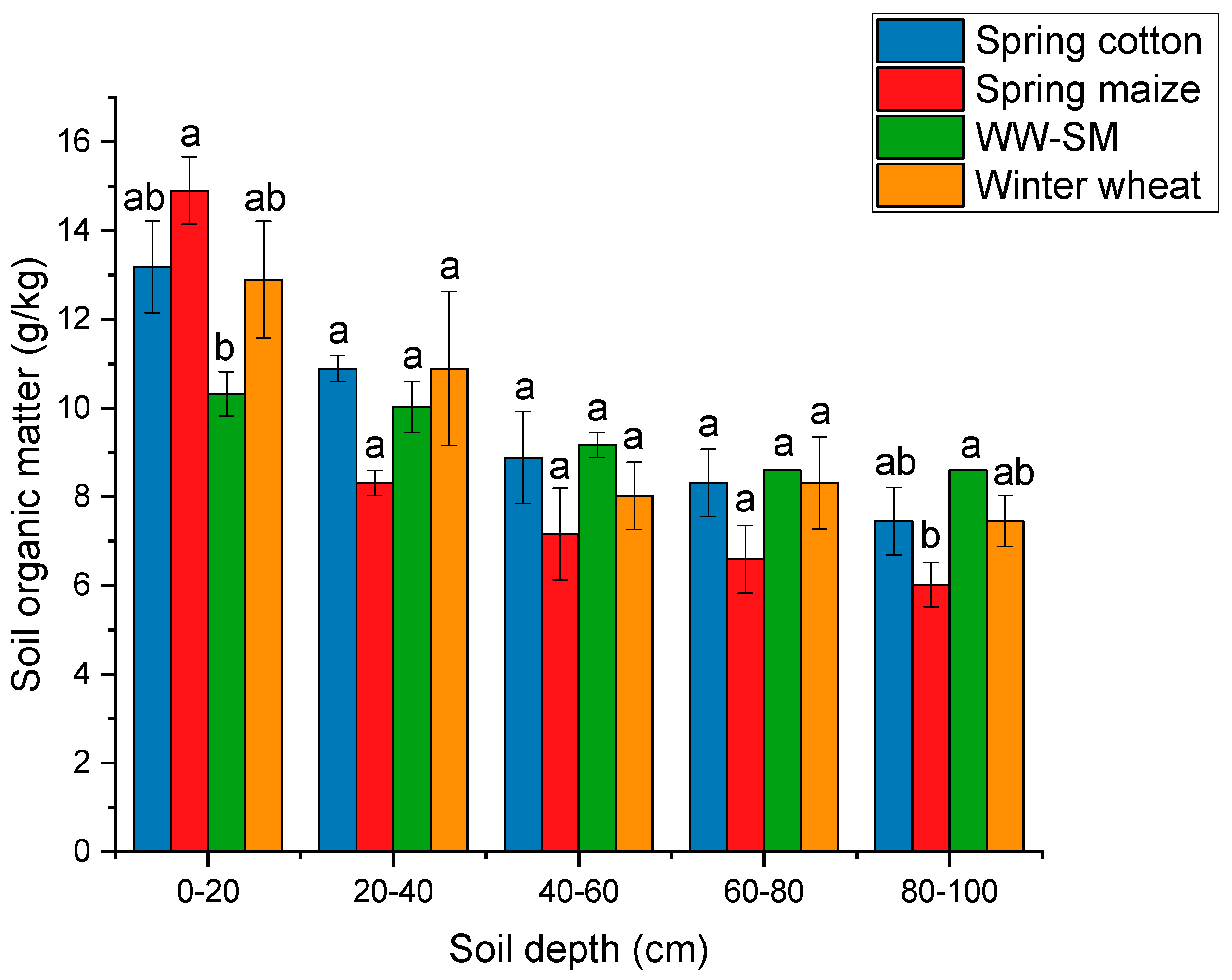
3.2.1. Soil Organic Matter
3.2.2. Soil Available Phosphorous and Available Potassium
3.2.3. Soil pH
3.2.4. Soil Electrical Conductivity
3.2.5. Soil Ions and Total Soluble Salts
3.3. Soil Enzyme Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trivedi, P.; Singh, B.P.; Singh, B.K. Chapter 1. Soil Carbon: Introduction, Importance, Status, Threat, and Mitigation; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Al-Kaisi, M.; Yang, J.; Chen, Y.; Sui, P. Effects of Seven Diversified Crop Rotations on Selected Soil Health Indicators and Wheat Productivity. Agronomy 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Wu, J.; Wang, X.; Ma, L. Economic and Environmental Sustainability of Maize-Wheat Rotation Production When Substituting Mineral Fertilizers with Manure in the North China Plain. J. Clean. Prod. 2020, 271, 122683. [Google Scholar] [CrossRef]
- Li, F.; Li, Z.; Qiao, Y.; Zhu, N.; Du, K.; Leng, P.; Liu, S. Winter Wheat and Summer Maize Roots in Agro-Ecosystems on the North China Plain. The Root Systems in Sustainable Agricultural Intensification, 1st ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 271–288. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Pacenka, S.; Gao, W.; Zhang, M.; Sui, P.; Steenhuis, T.S. Recharge and Groundwater Use in the North China Plain for Six Irrigated Crops for an Eleven Year Period. PLoS ONE 2014, 10, e0115269. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, Y.; Pacenka, S.; Gao, W.; Ma, L.; Wang, G.; Yan, P.; Sui, P.; Steenhuis, T.S. Effect of Diversified Crop Rotations on Groundwater Levels and Crop Water Productivity in the North China Plain. J. Hydrol. 2015, 522, 428–438. [Google Scholar] [CrossRef]
- Gao, B.; Ju, X.; Meng, Q.; Cui, Z.; Christie, P.; Chen, X.; Zhang, F. The Impact of Alternative Cropping Systems on Global Warming Potential, Grain Yield and Groundwater Use. Agric. Ecosyst. Environ. 2015, 203, 46–54. [Google Scholar] [CrossRef]
- Xiao, D.; Shen, Y.; Qi, Y.; Moiwo, J.P.; Min, L.; Zhang, Y.; Guo, Y.; Pei, H. Impact of Alternative Cropping Systems on Groundwater Use and Grain Yields in the North China Plain Region. Agric. Syst. 2017, 153, 109–117. [Google Scholar] [CrossRef]
- Liu, M.; Min, L.; Shen, Y.; Wu, L. Evaluating the Impact of Alternative Cropping Systems on Groundwater Consumption and Nitrate Leaching in the Piedmont Area of the North China Plain. Agronomy 2020, 10, 1635. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Xu, M.; Zhang, H.; Luo, Y. Characteristics of Soil C:N Ratio and Δ13C in Wheat-Maize Cropping System of the North China Plain and Influences of the Yellow River. Sci. Rep. 2017, 7, 16854. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Mao, R.; Bai, P.; Luo, C. Reclaiming the Saline Soils of Nanpi County: Turning Knowledge into Practice. Aciar Monogr. Ser. 2014, 84, 366–370. [Google Scholar]
- Sun, B.; Zhou, S.; Zhao, Q. Evaluation of Spatial and Temporal Changes of Soil Quality Based on Geostatistical Analysis in the Hill Region of Subtropical China. Geoderma 2003, 115, 85–99. [Google Scholar] [CrossRef]
- Gajda, A.M.; Czyz, E.A.; Dexter, A.R. Effects of Long-Term Use of Different Farming Systems on Some Physical, Chemical and Microbiological Parameters of Soil Quality. Int. Agrophys. 2016, 30, 165–172. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Liu, X.; Liu, X.; Ju, Z.; Shao, L. The Long-Term Impact of Irrigation on Selected Soil Properties and Grain Production. J. Soil Water Conserv. 2018, 73, 310–320. [Google Scholar] [CrossRef]
- Mu-Chun, Y.; Ting-Ting, X.; Peng-Hui, S.; Jian-Jun, D. Effects of Different Cropping Patterns of Soybean and Maize Seedlings on Soil Enzyme Activities and MBC and MBN. J. Northeast Agric. Univ. 2012, 19, 42–47. [Google Scholar] [CrossRef]
- Wright, A.L.; Dou, F.; Hons, F.M. Soil Organic C and N Distribution for Wheat Cropping Systems after 20 Years of Conservation Tillage in Central Texas. Agric. Ecosyst. Environ. 2007, 121, 376–382. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M.; Matocha, J.E. Tillage Impacts on Microbial Biomass and Soil Carbon and Nitrogen Dynamics of Corn and Cotton Rotations. Appl. Soil Ecol. 2005, 29, 85–92. [Google Scholar] [CrossRef]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased Contribution of Root Exudates to Soil Carbon Input during Grassland Degradation; Elsevier Ltd: Amsterdam, The Netherlands, 2020; Volume 146. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Cortasa, M.S.; Loges, R. Winter Wheat Roots Grow Twice as Deep as Spring Wheat Roots, Is This Important for N Uptake and N Leaching Losses? Plant Soil 2009, 322, 101–114. [Google Scholar] [CrossRef]
- Zhi, X.; Han, Y.; Li, Y.; Wang, G.; Feng, L.; Yang, B.; Fan, Z.; Lei, Y.; Du, W.; Mao, S. Root Growth and Spatial Distribution Characteristics for Seedlings Raised in Substrate and Transplanted Cotton. PLoS ONE 2017, 12, e0190032. [Google Scholar] [CrossRef] [Green Version]
- Dou, F.; Wright, A.L.; Mylavarapu, R.S.; Jiang, X.; Matocha, J.E. Soil Enzyme Activities and Organic Matter Composition Affected by 26 Years of Continuous Cropping. Pedosphere 2016, 26, 618–625. [Google Scholar] [CrossRef]
- Mcdaniel, M.D.; Grandy, A.S.; Tiemann, L.K.; Weintraub, M.N. Eleven Years of Crop Diversification Alters Decomposition Dynamics of Litter Mixtures Incubated with Soil. Ecosphere 2016, 7, e01426. [Google Scholar] [CrossRef]
- Lal, R. Soils and Food Sufficiency. A Review. Sustain. Agric. 2009, 29, 113–133. [Google Scholar]
- Wright, A.L.; Hons, F.M.; Lemon, R.G.; McFarland, M.L.; Nichols, R.L. Microbial Activity and Soil C Sequestration for Reduced and Conventional Tillage Cotton. Appl. Soil Ecol. 2008, 38, 168–173. [Google Scholar] [CrossRef]
- Feng, Y.; Motta, A.C.; Reeves, D.W.; Burmester, C.H.; Van Santen, E.; Osborne, J.A. Soil Microbial Communities under Conventional-till and No-till Continuous Cotton Systems. Soil Biol. Biochem. 2003, 35, 1693–1703. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Rasaily, R.G.; Wang, Q.; Cai, G.; Su, Y.; Qiao, X.; Liu, L. Soil Properties and Crop Yields after 11 Years of No Tillage Farming in Wheat-Maize Cropping System in North China Plain. Soil Tillage Res. 2011, 113, 48–54. [Google Scholar] [CrossRef]
- Nichols, K. Soil Quality Demonstrations and Procedures; USDA-ARS-Northern Great Plains Research Laboratory: Mandan, ND, USA, 2011; p. 18.
- Kuo, S. Phosphorus. Methods Soil Analysis. Part 3; Chemical Methods-SSSA Book Series No. 5; Wiley: Hoboken, NJ, USA, 1996; Volume 5, pp. 869–919. [Google Scholar] [CrossRef]
- Jadoon, S.; Aljaff, H.K.; Hamawandy, J.K.; Pashdary, S.S.; Zakhoy, A.S. Assessment of the Available Potassium in the Soil of Baharka District, Kurdistan-Iraq. Pharma Chem. 2015, 7, 1–8. [Google Scholar]
- Nelson, D.; Sommers, L. Methods of Soil Analysis. Part 3. Chemical Methods; Chemical Methods Soil Science Society of America Book Series; Bigham, J.M., Ed.; SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Thompson, L.M.; Troeh, F.R. Soils and Soil Fertility. AIBS Bull. 1978, 7, 53. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. Methods Soil Anal. Part 2 Microbiol. Biochem. Prop. 1994, 5, 775–834. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, C.; Wang, J.; Meng, Q.; Yuan, Y.; Ma, X.; Liu, X.; Zhu, Y.; Ding, G.; Zhang, J.; et al. Soil Aggregates Stability and Storage of Soil Organic Carbon Respond to Cropping Systems on Black Soils of Northeast China. Sci. Rep. 2020, 10, 265. [Google Scholar] [CrossRef]
- Baeva, Y.I.; Kurganova, I.N.; Lopes de Gerenyu, V.O.; Pochikalov, A.V.; Kudeyarov, V.N. Changes in Physical Properties and Carbon Stocks of Gray Forest Soils in the Southern Part of Moscow Region during Postagrogenic Evolution. Eurasian Soil Sci. 2017, 50, 327–334. [Google Scholar] [CrossRef]
- Burdukovskii, M.; Kiseleva, I.; Perepelkina, P.; Kosheleva, Y. Impact of Different Fallow Durations on Soil Aggregate Structure and Humus Status Parameters. Soil Water Res. 2020, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Warrington, D.N.; Goldstein, D.; Levy, G.J. Clay Translocation within the Soil Profile as Affected by Intensive Irrigation with Treated Wastewater. Soil Sci. 2007, 172, 692–700. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Zhang, Q. Effects of Soil Aggregate Stability on Soil Organic Carbon and Nitrogen under Land Use Change in an Erodible Region in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartlová, J.; Badalíková, B.; Pospíšilová, L.; Pokorný, E.; Šarapatka, B. Water Stability of Soil Aggregates in Different Systems of Tillage. Soil Water Res. 2015, 10, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Haynes, R.J.; Beare, M.H. Influence of Six Crop Species on Aggregate Stability and Some Labile Organic Matter Fractions. Soil Biol. Biochem. 1997, 29, 1647–1653. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, D.; Chen, S.; Sun, H.; Yang, Y. Performance of Double-Cropped Winter Wheat-Summer Maize under Minimum Irrigation in the North China Plain. Agron. J. 2006, 98, 1620–1626. [Google Scholar] [CrossRef]
- Gold, M.V. Double Cropping and Interplanting: January 1991–February 1994; National Agricultural Library: Baltimore, MD, USA, 1994.
- McKenzie, N.; Jacquier, D.; Isbell, R.; Brown, K. Australian Soils and Landscapes. [Electronic Resource]: An Illustrated Compendium; CSIRO Publishing: Clayton, Australia, 2004. [Google Scholar]
- Huggins, D.R.; Allmaras, R.R.; Clapp, C.E.; Lamb, J.A.; Randall, G.W. Corn-Soybean Sequence and Tillage Effects on Soil Carbon Dynamics and Storage. Soil Sci. Soc. Am. J. 2007, 71, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez-Morales, K.D.; Cadena-Zapata, M.; Zermeño-González, A.; Campos-Magaña, S. Effect of Tillage Systems on Physical Properties of a Clay Loam Soil under Oats. Agriculture 2019, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Kazula, M.J.; Lauer, J.G.; Arriaga, F.J. Crop Rotation Effect on Selected Physical and Chemical Properties of Wisconsin Soils. J. Soil Water Conserv. 2017, 72, 553–563. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Canqui, H.; Lal, R. Mechanisms of Carbon Sequestration in Soil Aggregates. CRC Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Bauer, A. Influence of Soil Organic Matter on Bulk Density and Available Water Capacity of Soils. Int. J. Chem. Stud. 1974, 7, 44–51. [Google Scholar]
- Ouda, S.; Zohry, A.; Noreldin, T. Crop Rotation Maintains Soil Sustainability. In Crop Rotation; Springer: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Wang, H.; Sheng, Y.; Jiang, W.; Pan, F.; Wang, M.; Chen, X.; Shen, X.; Yin, C.; Mao, Z. The Effects of Crop Rotation Combinations on the Soil Quality of Old Apple Orchard. Hortic. Plant J. 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Nyamadzawo, G.; Chikowo, R.; Nyamugafata, P.; Nyamangara, J.; Giller, K.E. Soil Organic Carbon Dynamics of Improved Fallow-Maize Rotation Systems under Conventional and No-Tillage in Central Zimbabwe. Nutr. Cycl. Agroecosyst. 2008, 81, 85–93. [Google Scholar] [CrossRef]
- Mokolobate, M.S.; Haynes, R.J. Increases in PH and Soluble Salts Influence the Effect That Additions of Organic Residues Have on Concentrations of Exchangeable and Soil Solution Aluminium. Eur. J. Soil Sci. 2002, 53, 481–489. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of Soil Organic Matter Dynamics to Physical Protection and Tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Aguilera, J.; Motavalli, P.; Valdivia, C.; Gonzales, M.A. Impacts of cultivation and fallow length on soil carbon and nitrogen availability in the Bolivian andean highland region. Mt. Res. Dev. 2013, 4, 391–403. [Google Scholar] [CrossRef]
- Magdoff, F.; Weil, R. Soil Organic Matter Management Strategies; CRC Press LLCL: Boca Raton, FL, USA, 2004. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Wienhold, B.J.; Black, A.L. Tillage, Nitrogen, and Cropping System Effects on Soil Carbon Sequestration. Soil Sci. Soc. Am. J. 2002, 66, 906–912. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-Term Effects of Organic Amendments on Soil Fertility. Sustain. Agric. 2011, 2, 761–786. [Google Scholar] [CrossRef]
- Hue, N.V.; Silva, J.A. Organic Soil Amendments for Sustainable Agriculture: Organic Sources of Nitrogen, Phosphorus, and Potassium. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture, 1st ed.; University of Hawaii: Honolulu, HI, USA, 2000; pp. 133–144. [Google Scholar]
- Zuber, S.M.; Behnke, G.D.; Nafziger, E.D.; Villamil, M.B. Crop Rotation and Tillage Effects on Soil Physical and Chemical Properties in Illinois. Agron. J. 2015, 107, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Wright, A.L.; Hanlon, E.A.; Sui, D.; Rice, R. Soil PH Effects on Nutrient Availability in the Everglades Agricultural Area 1. EDIS 2009, Volume 2009, 1–5. [Google Scholar] [CrossRef]
- Sainju, U.M.; Allen, B.L.; Caesar-TonThat, T.; Lenssen, A.W. Dryland Soil Chemical Properties and Crop Yields Affected by Long-Term Tillage and Cropping Sequence. Springerplus 2015, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Balemi, T.; Negisho, K. Management of Soil Phosphorus and Plant Adaptation Mechanisms to Phosphorus Stress for Sustainable Crop Production: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef] [Green Version]
- Engels, C. Differences between Maize and Wheat in Growth-Related Nutrient Demand and Uptake of Potassium and Phosphorus at Suboptimal Root Zone Temperatures. Plant Soil 1993, 150, 129–138. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.; Li, X.; Yang, S.; Rengel, Z. Wheat/Maize or Wheat/Soybean Strip Intercropping I. Yield Advantage and Interspecific Interactions on Nutrients. F. Crop. Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]
- Oosterhuis, D.M. Potassium Management of Cotton. In Potassium for Sustainable Crop Production; Pasricha, N.S., Bansal, S.K., Eds.; International Potash Institute: Basel, Switzerland, 2002; pp. 321–346. [Google Scholar]
- Shahzad, A.N.; Rizwan, M.; Asghar, M.G.; Qureshi, M.K.; Bukhari, S.A.H.; Kiran, A.; Wakeel, A. Early Maturing Bt Cotton Requires More Potassium Fertilizer under Water Deficiency to Augment Seed-Cotton Yield but Not Lint Quality. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Abaye, A.O. Potassium Fertilization of Cotton. Virginia Tech. Coop. Ext. 2009, Volume 418-025, 1–4. Available online: https://vtechworks.lib.vt.edu/bitstream/handle/10919/55782/418-025.pdf?sequence=1&isAllowed=y (accessed on 4 January 2022).
- Wakeel, A.; Hafeez-ur-Rehman; Magen, H. Potash Use for Sustainable Crop Production in Pakistan: A Review. Int. J. Agric. Biol. 2017, 19, 381–390. [Google Scholar] [CrossRef]
- Mairura, F.S.; Mugendi, D.N.; Mwanje, J.I.; Ramisch, J.J.; Mbugua, P.K.; Chianu, J.N. Integrating Scientific and Farmers’ Evaluation of Soil Quality Indicators in Central Kenya. Geoderma 2007, 139, 134–143. [Google Scholar] [CrossRef]
- Cui, H.; Luo, Y.; Chen, J.; Jin, M.; Li, Y.; Wang, Z. Straw Return Strategies to Improve Soil Properties and Crop Productivity in a Winter Wheat-Summer Maize Cropping System. Eur. J. Agron. 2022, 133, 126436. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Kahl, K.; Carlson, B.; Huggins, D.R.; Paulitz, T. Soil Acidification Modifies Soil Depth-Microbiome Relationships in a No-till Wheat Cropping System. Soil Biol. Biochem. 2020, 149, 107939. [Google Scholar] [CrossRef]
- Bowman, R.A.; Halvorson, A.D. Soil Chemical Changes after Nine Years of Differential n Fertilization in a No-till Dryland Wheat-Corn-Fallow Rotation. Soil Sci. 1998, 163, 241–247. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the Rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Yadav, J.S.P.; Massoud, F.I.; Abrol, I.P. Salt-Affected Soils and Their Management; Fao Soils Bull. 39; FAO: Rome, Italy, 1988; p. 131. [Google Scholar]
- Othaman, N.N.C.; Isa, M.N.M.; Ismail, R.C.; Ahmad, M.I.; Hui, C.K. Factors That Affect Soil Electrical Conductivity (EC) Based System for Smart Farming Application; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2203. [Google Scholar] [CrossRef]
- Visconti, F.; de Paz, J.M.; Rubio, J.L. What Information Does the Electrical Conductivity of Soil Water Extracts of 1 to 5 Ratio (w/v) Provide for Soil Salinity Assessment of Agricultural Irrigated Lands? Geoderma 2010, 154, 387–397. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Lee, C.H.; Jien, S.H. Reduction of Nutrient Leaching Potential in Coarse-Textured Soil by Using Biochar. Water 2020, 12, 12. [Google Scholar] [CrossRef]
- Meshram, J.H.; Mahajan, S.S.; Nagrale, D.; Gokte-Narkhedkar, N.; Kumbhalkar, H. Understanding Root Biology for Enhancing Cotton Production. In Plant Roots; IntechOpen: London, UK, 2021; p. 13. [Google Scholar]
- Rengasamy, P.; Chittleborough, D.; Helyar, K. Root-Zone Constraints and Plant-Based Solutions for Dryland Salinity. Plant Soil 2020, 257, 249–260. [Google Scholar]
- Rengasamy, P. World Salinization with Emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Raats, P.A.C. Distribution of Salts in the Root Zone. J. Hydrol. 1975, 27, 237–248. [Google Scholar] [CrossRef]
- Williams, A.; Kay, P.; Stirling, G.; Weng, X.; Bell, L. Impacts of Reducing Fallow Periods on Indicators of Soil Function in Subtropical Dryland Farming Systems. Agric. Ecosyst. Environ. 2016, 324, 107727. [Google Scholar] [CrossRef]
- Dick, W.A. Influence of Long-Term Tillage and Crop Rotation Combinations on Soil Enzyme Activities. Soil Sci. Soc. Am. J. 1984, 48, 569–574. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, S.K.; Singh, P.; Tiwari, S.; Nand, M.M.; Chiranjeeb, K.; Majhi, M. Effects of Cropping Systems on Soil Properties and Enzymatic Activities in Calcareous Soil. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1255–1262. [Google Scholar] [CrossRef]
- Junnarkar, N.B.; Sherasiya, M.; Duggirala, S.M.; Duggirala, N.; Nishant, J. Interrelationship between Alkaline Phosphatase Activity and Soil Characteristics. Biosci. Guard. 2011, 1, 473–480. [Google Scholar]

| Soil Depth | Cropping Patterns | >1 mm (%) | 0.25–1 mm (%) | 0.053–0.25 (%) | <0.053 mm (%) | MWD (mm) |
|---|---|---|---|---|---|---|
| 0–20 cm | Spring cotton | 50.62 a (±3.71) | 20.65 a (±9.49) | 15.98 a (±1.93) | 12.75 a (±1.09) | 0.67 a (±0.01) |
| Spring maize | 51.33 a (±3.30) | 39.18 a (±10.7) | 18.68 a (±2.97) | 8.63 a (±2.59) | 0.61 a (±0.09) | |
| WW–SM | 48.80 a (±4.49) | 23.39 a (±6.76) | 15.35 a (±1.58) | 12.46 a (±1.77) | 0.66 a (±0.01) | |
| Winter wheat | 57.95 a (±4.96) | 20.16 a (±9.55) | 13.13 a (±2.87) | 8.76 a (±2.61) | 0.73 a (±0.08) | |
| 20–40 cm | Spring cotton | 43.00 a (±6.94) | 35.62 a (±5.93) | 13.30 a (±4.77) | 8.08 b (±3.71) | 0.68 a (±0.07) |
| Spring maize | 37.53 a (±3.66) | 28.15 a (±3.94) | 20.07 a (±3.76) | 14.25 ab (±4.91) | 0.59 a (±0.10) | |
| WW–SM | 31.60 a (±6.97) | 33.81 a (±3.33) | 22.57 a (±4.86) | 12.02 ab (±4.78) | 0.57 a (±0.03) | |
| Winter wheat | 35.01 a (±3.90) | 25.46 a (±5.99) | 20.27 a (±3.78) | 19.26 a (±3.15) | 0.55 a (±0.08) |
| Cropping patterns | 0–20 cm | 20–40 cm |
|---|---|---|
| Soil Available Phosphorous (mg/kg) | ||
| Spring cotton | 8.01 c (±0.06) | 5.25 c (±0.10) |
| Spring maize | 10.60 a (±0.08) | 6.45 a (±0.22) |
| WW–SM | 10.56 a (±0.07) | 6.04 b (±0.09) |
| Winter wheat | 9.33 b (±0.46) | 4.71 d (±0.04) |
| Soil available potassium (mg/kg) | ||
| Spring cotton | 202.77 a (±2.01) | 176.42 c (±1.31) |
| Spring maize | 195.60 b (±2.35) | 195.61 a (±1.13) |
| WW–SM | 197.16 b (±3.47) | 190.83 b (±0.28) |
| Winter wheat | 181.92 c (±0.43) | 184.10 c (±11.1) |
| Cropping Pattern | pH | ||||
|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 80–100 cm | |
| Spring cotton | 8.41 a (±0.17) | 8.43 a (±0.24) | 8.47 a (±0.25) | 8.49 a (±0.01) | 8.48 a (±0.01) |
| Spring maize | 8.36 a (±0.20) | 8.48 a (±0.12) | 8.53 a (±0.12) | 8.5 a (±0.1) | 8.55 a (±0.01) |
| WW–SM | 8.38 a (±0.11) | 8.29 a (±0.06) | 8.34 a (±0.02) | 8.37 a (±0.01) | 8.43 a (±0.01) |
| Winter wheat | 8.45 a (±0.00) | 8.42 a (±0.09) | 8.46 a (±0.18) | 8.48 a (±0.15) | 8.48 a (±0.14) |
| Correlations | Soil EC | Soil pH | Soil HCO3− | Soil Cl− | Soil Ca2+ | Soil Mg2+ | Soil SO42− | Soil K+ + Na+ | Soil Glucosidase | Soil Urease | Soil Alkaline Phosphatase | Soil Arylsulfatase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil EC | 1 | |||||||||||
| Soil pH | −0.742 | 1 | ||||||||||
| Soil HCO3− | −0.812 | 0.951 * | 1 | |||||||||
| Soil Cl− | 0.953 * | −0.798 | −0.922 | 1 | ||||||||
| Soil Ca2+ | 0.807 | −0.986 * | −0.983 * | 0.881 | 1 | |||||||
| Soil Mg2+ | 0.349 | −0.202 | −0.514 | 0.574 | 0.346 | 1 | ||||||
| Soil SO42− | 0.952 * | −0.821 | −0.955 * | 0.990 * | 0.904 | 0.638 | 1 | |||||
| Soil K+ + Na+ | 0.985 * | −0.648 | −0.773 | 0.954 * | 0.739 | 0.464 | 0.904 | 1 | ||||
| Soil glucosidase | −0.421 | −0.253 | 0.015 | −0.377 | 0.106 | −0.532 | −0.311 | −0.558 | 1 | |||
| Soil urease | 0.001 | −0.629 | −0.576 | 0.224 | 0.584 | 0.249 | 0.343 | −0.073 | 0.67 | 1 | ||
| Soil alkaline phosphatase | 0.958 * | −0.887 | −0.878 | 0.918 | 0.912 | 0.206 | 0.882 | 0.898 | −0.143 | 0.208 | 1 | |
| Soil arylsulfatase | 0.415 | −0.913 | −0.757 | 0.486 | 0.841 | −0.043 | 0.535 | 0.284 | 0.626 | 0.803 | 0.648 | 1 |
| Cropping Pattern | EC (dS/m) | ||||
|---|---|---|---|---|---|
| 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 80–100 cm | |
| Spring cotton | 0.34 a (±0.23) | 0.29 a (±0.13) | 0.28 a (±0.03) | 0.31 a (±0.01) | 0.37 a (±0.01) |
| Spring maize | 0.48 a (±0.11) | 0.31 a (±0.09) | 0.31 a (±0.07) | 0.29 a (±0.01) | 0.31 ab (±0.01) |
| WW–SM | 0.26 a (±0.07) | 0.28 a (±0.07) | 0.29 a (±0.07) | 0.26 a (±0.01) | 0.24 b (±0.01) |
| Winter wheat | 0.22 a (±0.03) | 0.24 a (±0.03) | 0.26 a (±0.06) | 0.28 a (±0.07) | 0.30 ab (±0.09) |
| Cropping Patterns | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | 80–100 cm |
|---|---|---|---|---|---|
| Ca2+ (cmol/kg) | |||||
| Spring cotton | 0.78 ab (±0.36) | 0.46 ab (±0.02) | 0.45 a (±0.25) | 0.28 a (±0.15) | 0.21 a (±0.11) |
| Spring maize | 1.06 a (±0.30) | 0.46 ab (±0.15) | 0.38 a (±0.10) | 0.30 a (±0.05) | 0.25 a (±0.05) |
| WW–SM | 0.88 ab (±0.29) | 0.70 a (±0.20) | 0.43 a (±0.02) | 0.40 a (±0.05 | 0.28 a (±0.05) |
| Winter wheat | 0.43 b (±0.17) | 0.38 b (±0.11) | 0.30 a (±0.13) | 0.28 a (±0.12) | 0.25 a (±0.05) |
| Mg2+ (cmol/kg) | |||||
| Spring cotton | 0.65 a (±0.37) | 0.46 a (±0.02) | 0.31 b (±0.12) | 0.21 b (±0.05) | 0.16 a (±0.02) |
| Spring maize | 0.43 a (±0.20) | 0.20 c (±0) | 0.23 b (±0.05) | 0.18 b (±0.05) | 0.16 a (±0.02) |
| WW–SM | 0.41 a (±0.07) | 0.31 b (±0.02) | 0.53 a (±0.07) | 0.48 a (±0.12) | 0.31 a (±0.12) |
| Winter wheat | 0.31 a (±0.12) | 0.33 b (±0.10) | 0.31 b (±0.14) | 0.31 ab (±0.14) | 0.26 a (±0.10) |
| K++ Na+ (cmol/kg) | |||||
| Spring cotton | 1.25 a (±0.91) | 1.16 a (±0.56) | 1.13 a (±0.20) | 1.21 a (±0.18) | 1.48 ab (±0.18) |
| Spring maize | 1.53 a (±0.17) | 1.35 a (±0.15) | 1.4 a (±0.10) | 1.56 a (±0.11) | 1.73 a (±0.05) |
| WW–SM | 0.91 a (±0.12) | 1.05 a (±0.18) | 1.01 a (±0.12) | 1.10 a (±0.08) | 1.16 b (±0.02) |
| Winter wheat | 0.86 a (±0.34) | 1.00 a (±0.26) | 1.30 a (±0.45) | 1.45 a (±0.58) | 1.45 ab (±0.37) |
| Cl− (cmol/kg) | |||||
| Spring cotton | 0.96 a (±0.80) | 0.66 a (±0.28) | 0.51 a (±0.10) | 0.51 a (±0.02) | 0.43 a (±0.05) |
| Spring maize | 1.18 a (±0.25) | 0.73 a (±0.15) | 0.51 a (±0.20) | 0.51 a (±0.07) | 0.46 a (±0.07) |
| WW–SM | 0.71 a (±0.28) | 0.66 a (±0.23) | 0.56 a (±0.05) | 0.51 a (±0.05) | 0.43 a (±0.05) |
| Winter wheat | 0.43 a (±0.05) | 0.43 a (±0.05) | 0.5 a (±0.10) | 0.46 a (±0.02) | 0.43 a (±0.02) |
| HCO3− (cmol/kg) | |||||
| Spring cotton | 0.63 a (±0.02) | 0.66 b (±0.05) | 0.78 a (±0.02) | 0.8 a (±0) | 0.93 a (±0.23) |
| Spring maize | 0.61 a (±0.07) | 0.68 ab (±0.02) | 0.88 a (±0.27) | 0.93 a (±0.23) | 0.93 a (±0.23) |
| WW–SM | 0.63 a (±0.05) | 0.7 ab (±0) | 0.73 a (±0.02) | 0.76 a (±0.05) | 0.78 a (±0.02) |
| Winter wheat | 0.68 a (±0.10) | 0.76 a (±0.05) | 0.83 a (±0.15) | 0.93 a (±0.32) | 0.96 a (±0.28) |
| SO42− (cmol/kg) | |||||
| Spring cotton | 1.08 a (±0.79) | 0.76 a (±0.35) | 0.60 a (±0.20) | 0.53 a (±0.10) | 0.46 a (±0.11) |
| Spring maize | 1.23 a (±0.34) | 0.60 a (±0.18) | 0.61 a (±0.18) | 0.61 a (±0.10) | 0.56 a (±0.10) |
| WW–SM | 0.86 a (±0.20) | 0.70 a (±0.10) | 0.68 a (±0.10) | 0.63 a (±0.20) | 0.41 a (±0.02) |
| Winter wheat | 0.50 a (±0) | 0.51 a (±0.02) | 0.58 a (±0.14) | 0.58 a (±0.07) | 0.46 a (±0.02) |
| Total soluble salts (cmol/kg) | |||||
| Spring cotton | 1.03 a (±0.69) | 0.8 a (±0.31) | 0.72 a (±0.07) | 0.67 a (±0.04) | 0.72 b (±0.02) |
| Spring maize | 1.27a (±0.24) | 0.80 a (±0.16) | 0.8 a (±0.08) | 0.82 a (±0.07) | 0.85 a (±0.02) |
| WW–SM | 0.88 a (±0.18) | 0.81 a (±0.14) | 0.71 a (±0.08) | 0.71 a (±0.10) | 0.62 b (±0.03) |
| Winter wheat | 0.6 a (±0.07) | 0.63 a (±0.06) | 0.72 a (±0.11) | 0.77 a (±0.16) | 0.72 b (±0.10) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gikonyo, F.N.; Dong, X.; Mosongo, P.S.; Guo, K.; Liu, X. Long-Term Impacts of Different Cropping Patterns on Soil Physico-Chemical Properties and Enzyme Activities in the Low Land Plain of North China. Agronomy 2022, 12, 471. https://doi.org/10.3390/agronomy12020471
Gikonyo FN, Dong X, Mosongo PS, Guo K, Liu X. Long-Term Impacts of Different Cropping Patterns on Soil Physico-Chemical Properties and Enzyme Activities in the Low Land Plain of North China. Agronomy. 2022; 12(2):471. https://doi.org/10.3390/agronomy12020471
Chicago/Turabian StyleGikonyo, Florence Nyambura, Xinliang Dong, Peter Semba Mosongo, Kai Guo, and Xiaojing Liu. 2022. "Long-Term Impacts of Different Cropping Patterns on Soil Physico-Chemical Properties and Enzyme Activities in the Low Land Plain of North China" Agronomy 12, no. 2: 471. https://doi.org/10.3390/agronomy12020471
APA StyleGikonyo, F. N., Dong, X., Mosongo, P. S., Guo, K., & Liu, X. (2022). Long-Term Impacts of Different Cropping Patterns on Soil Physico-Chemical Properties and Enzyme Activities in the Low Land Plain of North China. Agronomy, 12(2), 471. https://doi.org/10.3390/agronomy12020471






