Relationship between Grain Yield and Quality Traits under Optimum and Low-Nitrogen Stress Environments in Tropical Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germplasm, Experimental Site and Trial Management
2.2. Statistical Analysis
3. Results
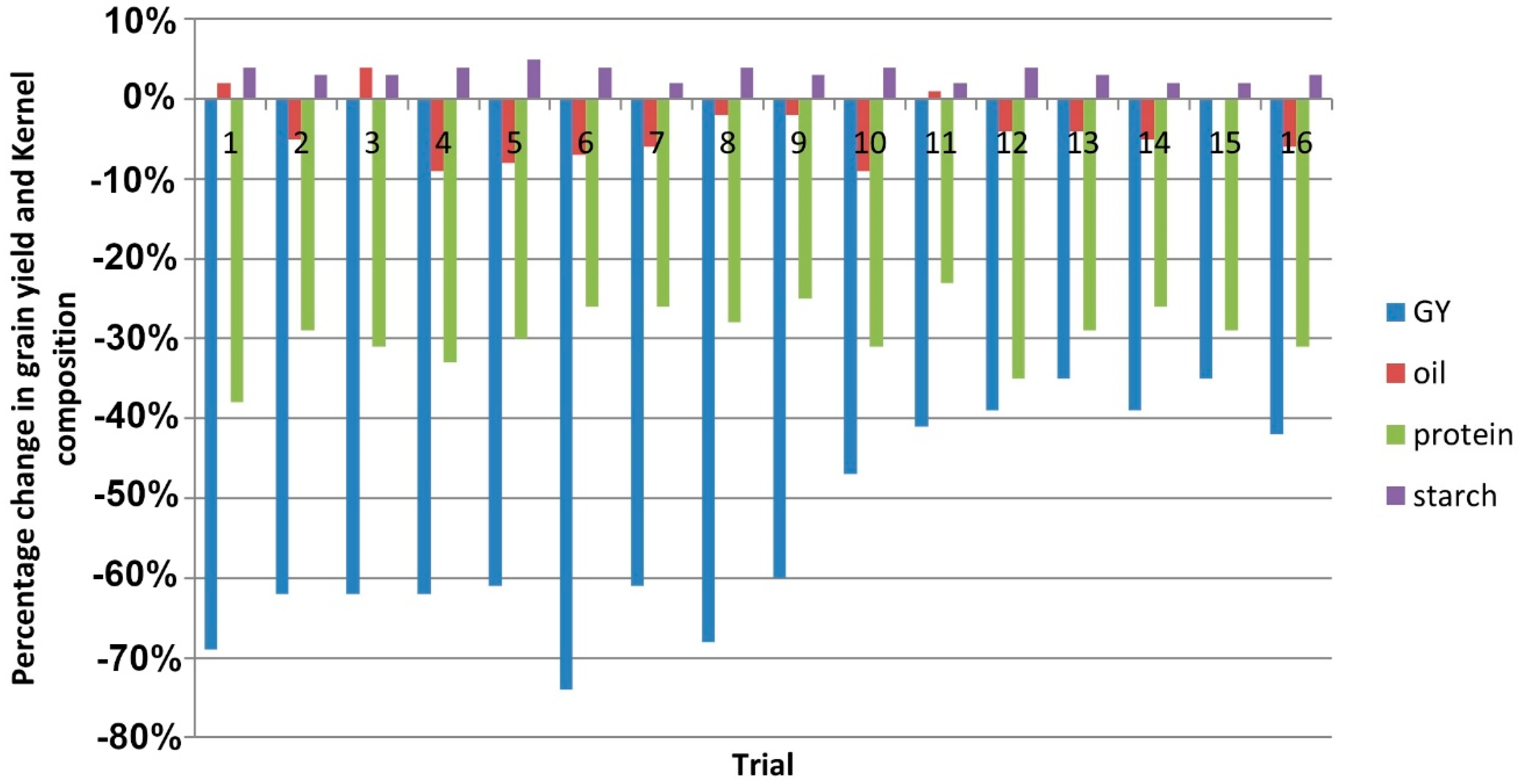
3.1. Grain Yield and Kernel Weight
3.2. Grain Quality Traits
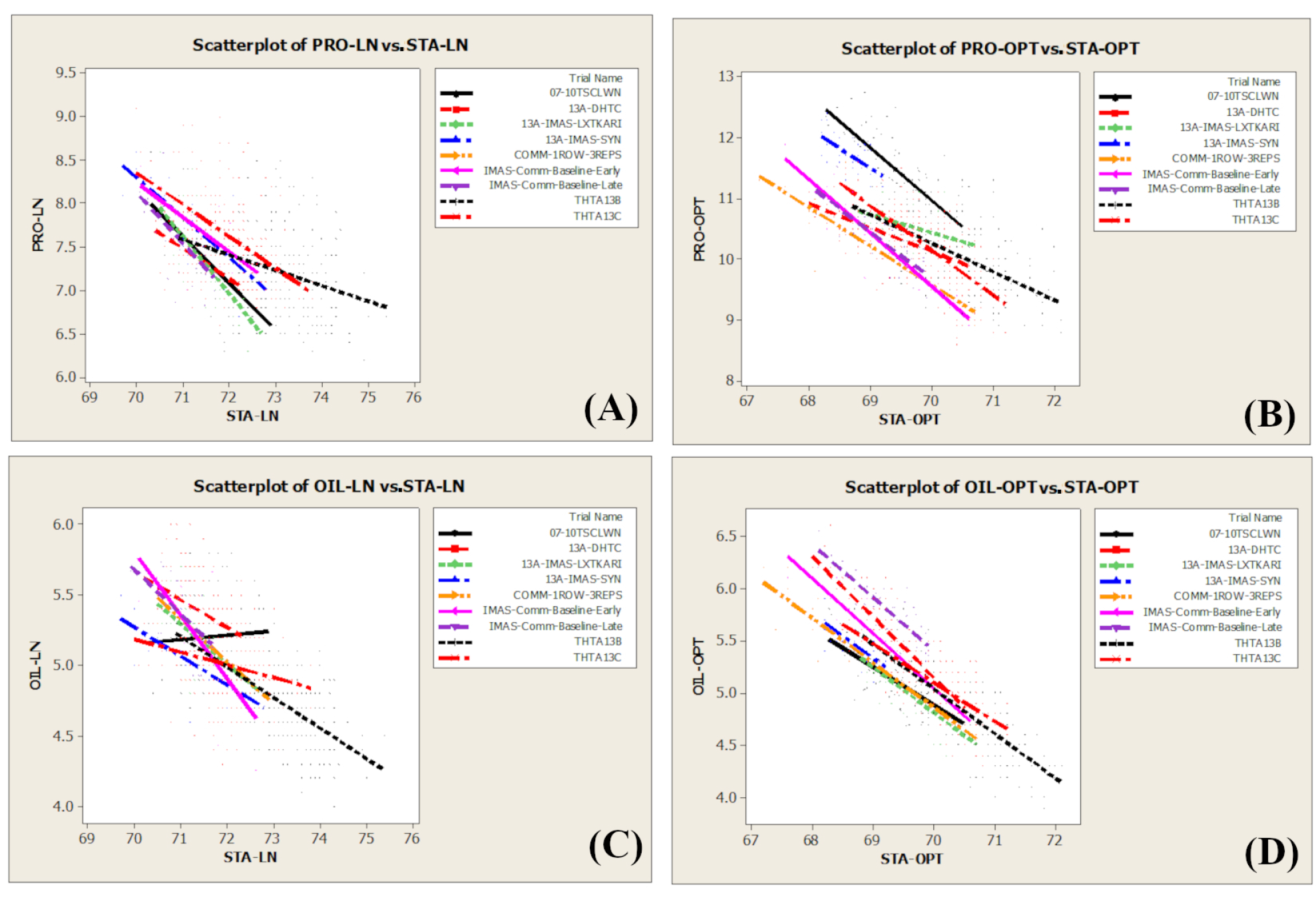
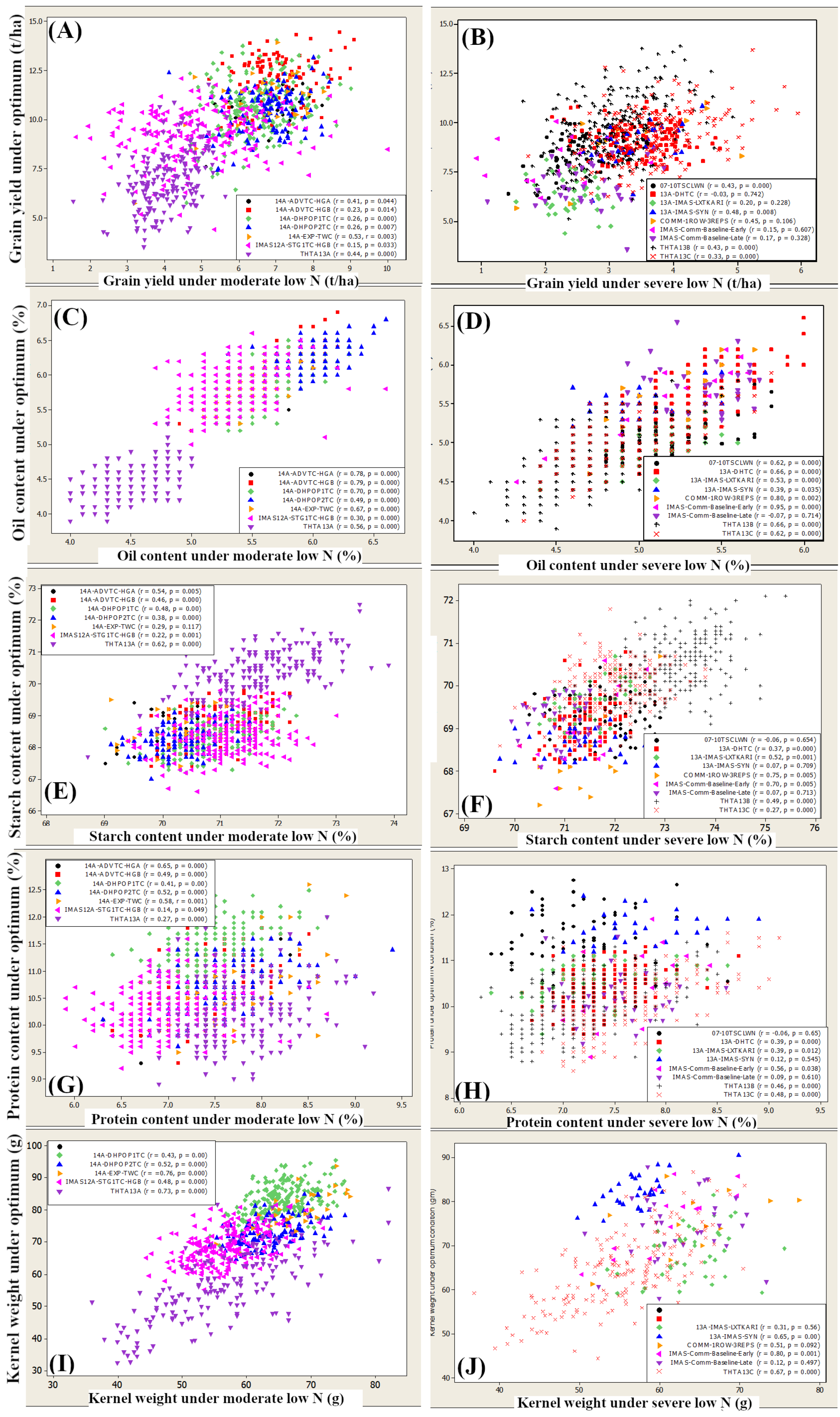
3.3. Phenotypic Correlation among Traits and between N Environments
3.4. Relationship between Traits under Low N and Optimum
4. Discussion
4.1. Mean Grain Yield and Grain Composition
4.2. Relationship between Grain Yield and Grain Quality Traits
4.3. Relation between Optimum and Low-Nitrogen Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasanna, B.M.; Cairns, J.E.; Zaidi, P.H.; Beyene, Y.; Makumbi, D.; Gowda, M.; Magorokosho, C.; Zaman-Allah, M.; Olsen, M.; Das, A.; et al. Beat the stress: Breeding for climate resilience in maize for the tropical rainfed environments. Theor. Appl. Genet. 2021, 134, 1729–1752. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAOSTAT), Rome. 2020. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 31 August 2021).
- Cairns, J.E.; Prasanna, B.M. Developing and deploying climate-resilient maize varieties in the developing world. Curr. Opin. Plant Biol. 2018, 45, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Atlin, G.N.; Olsen, M.; Burgueño, J.; Tarekegne, A.; Babu, R.; Ndou, E.N.; Mashingaidze, K.; Moremoholo, L.; Ligeyo, D. Identification of donors for low-nitrogen stress with maize lethal necrosis (MLN) tolerance for maize breeding in sub-Saharan Africa. Euphytica 2019, 215, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.L.; Kelly, V.A.; Kopicki, R.J.; Byerlee, D. Fertilizer Use in African agriculture: Lessons Learned and Good Practice Guidelines; The World Bank: Washington, DC, USA, 2007. [Google Scholar]
- Tsai, C.Y.; Dweikat, I.; Huber, D.M.; Warren, H.L. Interrelationship of nitrogen nutrition with maize (Zea mays) grain yield, nitrogen use efficiency and grain quality. J. Sci. Food Agric. 1992, 58, 1–8. [Google Scholar] [CrossRef]
- Presterl, T.; Seitz, G.; Schmidt, W.; Geiger, H.H. Improving nitrogen-use efficiency in European maize—Comparison between line per se and testcross performance under high and low soil nitrogen. Maydica 2002, 47, 83–91. [Google Scholar]
- Worku, M.; Bänziger, M.; Erley, G.S.A.; Friesen, D.; Diallo, A.O.; Horst, W.J. Nitrogen uptake and utilization in contrasting nitrogen efficient tropical maize hybrids. Crop Sci. 2007, 47, 519. [Google Scholar] [CrossRef] [Green Version]
- Worku, M.; Banziger, M.; Friesen, D.; Erley, G.S.A.; Diallo, A.O.; Vivek, B.; Horst, W.J. Protein quantity and quality, and grain yield performance of quality protein maize and normal endosperm maize under different levels of nitrogen. In Crop Research, Technology Dissemination and Adoption to Increase Food Supply, Reducing Hunger and Poverty in Africa; African Crop Science Society: El-Minia, Egypt, 2007; pp. 1905–1909. [Google Scholar]
- Worku, M.; Banziger, M.; Friesen, D.; Schulte, G.; Horst, W.J.; Vivek, B.S. Relative importance of general combining ability and specific combining ability among tropical maize (Zea mays L.) inbreds under contrasting nitrogen environments. Maydica 2008, 53, 279–288. [Google Scholar]
- Worku, M.; Banziger, M.; Schulte auf’m Erley, G.; Friesen, D.; Diallo, A.O.; Horst, W.J. Nitrogen efficiency as related to dry matter partitioning and root system size in tropical mid-altitude maize hybrids under different levels of nitrogen stress. Field Crops Res. 2012, 130, 57–67. [Google Scholar] [CrossRef]
- Duvick, D.N. What Is Yield? In Developing Drought-and Low N-Tolerant Maize; Edmeades, G.O., Banziger, M., Mickelson, H.R., Pena-Valdivia, C.B., Eds.; CIMMYT: Mexico City, Mexico, 1996; pp. 332–335. [Google Scholar]
- Liu, Z.H.; Xie, H.L.; Tian, G.W.; Chen, S.J.; Wang, C.L.; Hu, Y.M.; Tang, J.H. QTL mapping of nutrient components in maize kernels under low nitrogen conditions. Plant Breed. 2008, 127, 279–285. [Google Scholar] [CrossRef]
- Borrás, L.; Zinselmeier, C.; Senior, M.L.; Westgate, M.E.; Muszynski, M.G. Characterization of grain-filling patterns in diverse maize germplasm. Crop Sci. 2009, 49, 999. [Google Scholar] [CrossRef]
- Borrás, L.; Otegui, M.E. Seed dry weight response to source-sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Crop Sci. 2001, 41, 1816–1822. [Google Scholar] [CrossRef]
- Duarte, A.P.; Mason, S.C.; Jackson, D.S.; De Kiehl, J.C. Grain quality of Brazilian maize genotypes as influenced by nitrogen level. Crop Sci. 2005, 45, 1958–1964. [Google Scholar] [CrossRef] [Green Version]
- Ertiro, B.T.; Olsen, M.; Das, B.; Gowda, M.; Labuschagne, M. Efficiency of indirect selection for grain yield in maize (Zea mays L.) under low nitrogen conditions through secondary traits under low nitrogen and grain yield under optimum conditions. Euphytica 2020, 216, 134. [Google Scholar] [CrossRef]
- Borrás, L.; Curá, J.A.; Otegui, M.E. Maize kernel composition and post-flowering source-sink ratio. Crop Sci. 2002, 42, 781–790. [Google Scholar]
- Mayer, L.I.; Cirilo, A.G.; Maddonni, G.A. Kernel hardness-related traits in response to heat stress during the grain-filling period of maize crops. Crop Sci. 2019, 59, 318–332. [Google Scholar] [CrossRef]
- CIMMYT. Kiboko Crops Research Station: A Brief and Visitors’ Guide. (Field Visitors Manual); CIMMYT: Nairobi, Kenya, 2013; Available online: https://repository.cimmyt.org/handle/10883/3396?locale-attribute=en (accessed on 17 November 2021).
- Patterson, H.D.; Williams, E.R. A new class of resolvable incomplete block designs. Biometrika 1976, 63, 83–89. [Google Scholar] [CrossRef]
- SAS Institute Inc. JMP® 13 Documentation Library; SAS Institute Inc.: Cary, NC, USA, 2017. [Google Scholar]
- Minitab (Version 16). 2013. Available online: http://www.minitab.com/en-US/products/minitab/default.aspx (accessed on 15 November 2021).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Miti, F.; Tongoona, P.; Derera, J. S1 selection of local maize landraces for low soil nitrogen tolerance in Zambia. Afr. J. Plant Sci. 2010, 4, 67–81. [Google Scholar]
- Bänziger, M.; Betran, F.J.; Lafitte, H.R. Efficiency of high-N selection environments for improving maize for low-N target environments. Crop Sci. 1997, 37, 1103–1109. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Labuschagne, M.; Olsen, M.; Das, B.; Prasanna, B.M.; Gowda, M. Genetic dissection of nitrogen use efficiency in tropical maize through genome-wide association and genomic prediction. Front. Plant Sci. 2020, 11, 474. [Google Scholar] [CrossRef]
- Ertiro, B.T.; Olsen, M.; Das, B.; Gowda, M.; Labuschagne, M. Genetic dissection of grain yield and agronomic traits in maize under optimum and low-nitrogen stressed Environments. Int. J. Mol. Sci. 2020, 21, 543. [Google Scholar] [CrossRef] [Green Version]
- Eckhoff, S.A.; Paulsen, M.R. Maize. In Cereal Grain Quality; Henry, R.J., Kettlewell, P.S., Eds.; Chapman & Hall: London, UK, 1996; pp. 77–112. [Google Scholar]
- Duvic, D.N.; Cassman, K.G. Post—Green revolution trends in yield potential of temperate maize in the North-Central United States. Crop Sci. 1999, 39, 1622–1630. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Malhi, S. Effects of fertilization and other agronomic measures on nutritional quality of crops: A review. J. Sci. Food Agric. 2008, 88, 7–23. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Huber, D.M.; Warren, H.L. A proposed role of zein and glutelin as N sinks in maize. Plant Physiol. 1980, 66, 330–333. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Napier, J.A.; Tatham, A.S. Seed storage proteins: Structures and biosynthesis. Plant Cell 1995, 7, 945–956. [Google Scholar]
- Uribelarrea, M.; Below, F.E.; Moose, S.P. Grain composition and productivity of maize hybrids derived from the Illinois protein strains in response to variable nitrogen supply. Crop Sci. 2004, 44, 1593. [Google Scholar] [CrossRef]
- Triboi, E.; Triboi-Blondel, A.-M. Productivity and grain or seed composition: A new approach to an old problem. Eur. J. Agron. 2002, 16, 163–186. [Google Scholar] [CrossRef]
- Smith, A.M.; Martin, C. Starch biosynthesis and the potential for its manipulation. In Biosynthesis and Manipulation of Plant Products; Grierson, D., Ed.; Blackie Academic and Professional Publishers: Glasgow, UK, 1993; pp. 1–54. [Google Scholar]
- Wang, T.L.; Bogracheva, T.Y.; Hedley, C.L. Starch: As simple as A, B, C? J. Exp. Bot. 1998, 49, 481–502. [Google Scholar] [CrossRef] [Green Version]
| Trial Code | Trial Name | Experimental Design | Rep | Year | No. of Genotypes | Type of Germplasm | MIN T (°C) | MAX T (°C) | RH (%) | RF (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OP | LN | OP | LN | OP | LN | OP | LN | |||||||
| 1 | 07-10TSCLWN | Alpha lattice | 2 | 2011 | 126 | Experimental hybrids | 30.9 | 30.9 | 16.4 | 16.4 | 79.8 | 79.8 | 0.1 | 0.1 |
| 2 | 13A-DHTC | Alpha lattice | 2 | 2013 | 120 | DH testcrosses | 29.0 | 29.4 | 15.1 | 16.4 | 87.5 | 90.0 | 5.0 | 311.7 |
| 3 | 13A-IMAS-LXTKARI | Alpha lattice | 2 | 2013 | 40 | Experimental hybrids | 28.6 | 30.6 | 15.3 | 17.0 | 88.6 | 86.7 | 39.8 | 188.5 |
| 4 | 13A-IMAS-SYN | Alpha lattice | 2 | 2013 | 30 | Synthetics | 31.1 | 30.6 | 18.1 | 17.0 | 87.8 | 86.7 | 241.5 | 188.5 |
| 5 | COMM-1ROW-3REPS | RCBD | 3 | 2012 | 12 | Commercial varieties | 29.6 | 31.6 | 15.1 | 17.8 | 88.5 | 85.2 | 76.5 | 331.2 |
| 6 | IMAS-Baseline-Early | RCBD | 3 | 2011 | 14 | Commercial varieties | 30.5 | 30.9 | 16.0 | 16.4 | 77.8 | 79.8 | 7.0 | 7.0 |
| 7 | IMAS-Baseline-Late | Alpha lattice | 3 | 2011 | 35 | Commercial varieties | 30.5 | 31.6 | 16.0 | 17.8 | 77.8 | 85.2 | 7.0 | 331.2 |
| 8 | THTA13B | Alpha lattice | 2 | 2013 | 250 | Experimental hybrids | 29.0 | 29.5 | 16.0 | 16.6 | 89.1 | 89.9 | 252.9 | 323.0 |
| 9 | THTA13C | Alpha lattice | 2 | 2013 | 180 | Experimental hybrids | 29.0 | 29.5 | 16.0 | 16.6 | 89.1 | 89.9 | 252.9 | 323.0 |
| 10 | IMAS12A-STG1TC-HGB | Alpha lattice | 2 | 2012 | 210 | Experimental hybrids | 29.6 | 29.6 | 15.1 | 15.1 | 88.5 | 88.5 | 76.5 | 76.5 |
| 11 | THTA13A | Alpha lattice | 2 | 2013 | 168 | Experimental hybrids | 29.2 | 29.5 | 16.2 | 16.6 | 90.2 | 89.9 | 252.9 | 323.0 |
| 12 | 14A-DHPOP1TC | Alpha lattice | 2 | 2014 | 215 | DH testcrosses | 29.3 | 29.3 | 15.4 | 15.4 | 84.1 | 84.1 | 18.3 | 18.3 |
| 13 | 14A-DHPOP2TC | Alpha lattice | 2 | 2014 | 110 | DH testcrosses | 29.3 | 29.3 | 15.4 | 15.4 | 84.1 | 84.1 | 18.3 | 18.3 |
| 14 | 14A-EXP-TWC | Alpha lattice | 2 | 2014 | 30 | Experimental hybrids | 29.3 | 29.3 | 15.4 | 15.4 | 84.1 | 84.1 | 18.3 | 18.3 |
| 15 | 14A-ADVTC-HGA | RCBD | 2 | 2014 | 25 | Experimental hybrids | 29.3 | 29.3 | 15.4 | 15.4 | 84.1 | 84.1 | 18.3 | 18.3 |
| 16 | 14A-ADVTC-HGB | Alpha lattice | 2 | 2014 | 114 | Experimental hybrids | 29.3 | 29.3 | 15.4 | 15.4 | 84.1 | 84.1 | 18.3 | 18.3 |
| Trial Code | Grain Yield (t/ha) | % Yield Reduction | Oil Content (%) | Protein Content (%) | Starch Content (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OPT | h2 | LN | h2 | OPT | h2 | LN | h2 | OPT | h2 | LN | h2 | OPT | h2 | LN | h2 | ||
| 1 | 8.35 ** | 0.52 | 2.56 ** | 0.71 | 69 | 5.12 ** | 0.85 | 5.21 ** | 0.79 | 11.53 ** | 0.53 | 7.15 ** | 0.70 | 69.35 ** | 0.67 | 71.80 ** | 0.60 |
| 2 | 9.09 ** | 0.47 | 3.48 ** | 0.33 | 62 | 5.66 ** | 0.92 | 5.37 ** | 0.84 | 10.47 ** | 0.56 | 7.38 ** | 0.68 | 69.12 ** | 0.83 | 71.29 ** | 0.56 |
| 3 | 6.30 ** | 0.63 | 2.40 ** | 0.65 | 62 | 4.97 ** | 0.88 | 5.15 ** | 0.76 | 10.55 ** | 0.68 | 7.29 ** | 0.85 | 69.64 ** | 0.66 | 71.52 ** | 0.57 |
| 4 | 8.90 ** | 0.80 | 3.38 * | 0.47 | 62 | 5.49 ** | 0.70 | 5.00 ** | 0.65 | 11.74 ** | 0.77 | 7.69 ** | 0.77 | 68.63 * | 0.52 | 71.30 * | 0.55 |
| 5 | 8.69 ** | 0.91 | 3.42 * | 0.58 | 61 | 5.62 ** | 0.94 | 5.20 ** | 0.86 | 10.71 ** | 0.91 | 7.54 NS | 0.37 | 68.22 ** | 0.85 | 71.41 * | 0.50 |
| 6 | 8.08 ** | 0.79 | 2.11 ** | 0.88 | 74 | 5.62 ** | 0.97 | 5.22 ** | 0.95 | 10.48 ** | 0.95 | 7.71 * | 0.63 | 68.91 ** | 0.92 | 71.35 ** | 0.78 |
| 7 | 6.87 ** | 0.79 | 2.70 ** | 0.55 | 61 | 5.76 ** | 0.68 | 5.41 ** | 0.88 | 10.22 * | 0.44 | 7.61 ** | 0.71 | 69.29 * | 0.56 | 70.94 ** | 0.69 |
| 8 | 9.65 ** | 0.82 | 3.09 ** | 0.53 | 68 | 4.85 ** | 0.90 | 4.74 ** | 0.83 | 10.06 ** | 0.86 | 7.21 ** | 0.80 | 70.43 ** | 0.74 | 73.13 ** | 0.71 |
| 9 | 9.36 ** | 0.80 | 3.74 ** | 0.48 | 60 | 5.09 ** | 0.85 | 4.98 ** | 0.85 | 10.14 ** | 0.88 | 7.62 ** | 0.69 | 70.02 ** | 0.67 | 72.00 ** | 0.65 |
| 10 | 9.25 ** | 0.66 | 4.93 ** | 0.37 | 47 | 5.84 ** | 0.73 | 5.30 ** | 0.53 | 10.29 ** | 0.71 | 7.05 ** | 0.59 | 68.36 ** | 0.63 | 71.11 ** | 0.40 |
| 11 | 6.92 ** | 0.86 | 4.08 ** | 0.62 | 41 | 4.53 ** | 0.79 | 4.57 ** | 0.82 | 10.21 ** | 0.78 | 7.81 ** | 0.78 | 70.44 ** | 0.77 | 71.69 ** | 0.77 |
| 12 | 10.60 ** | 0.55 | 6.49 ** | 0.35 | 39 | 5.89 ** | 0.88 | 5.66 ** | 0.76 | 11.46 ** | 0.74 | 7.49 ** | 0.51 | 68.27 ** | 0.63 | 70.85 ** | 0.44 |
| 13 | 10.48 ** | 0.63 | 6.82 * | 0.33 | 35 | 6.24 ** | 0.79 | 6.02 ** | 0.61 | 10.77 ** | 0.72 | 7.69 ** | 0.59 | 68.28 ** | 0.52 | 70.34 NS | 0.30 |
| 14 | 10.92 ** | 0.78 | 6.63 ** | 0.82 | 39 | 5.79 ** | 0.73 | 5.49 ** | 0.80 | 10.73 ** | 0.95 | 7.92 NS | 0.37 | 68.59 ** | 0.75 | 70.21 * | 0.57 |
| 15 | 10.42 ** | 0.88 | 6.79 NS | 0.44 | 35 | 5.65 ** | 0.78 | 5.63 ** | 0.73 | 10.39 ** | 0.86 | 7.32 ** | 0.69 | 68.51 NS | 0.47 | 69.98 * | 0.54 |
| 16 | 12.10 ** | 0.69 | 7.07 * | 0.51 | 42 | 5.95 ** | 0.84 | 5.58 ** | 0.82 | 10.70 ** | 0.84 | 7.41 ** | 0.71 | 68.62 ** | 0.71 | 70.93 ** | 0.58 |
| Mean | 9.10 | 0.68 | 4.29 | 0.54 | 54 | 5.51 | 0.78 | 5.28 | 0.78 | 10.71 | 0.72 | 7.49 | 0.66 | 69.03 | 0.64 | 71.24 | 0.59 |
| Min | 6.30 | 0.00 | 2.11 | 0.33 | 35 | 4.53 | 0.00 | 4.57 | 0.53 | 10.06 | 0.01 | 7.05 | 0.37 | 68.22 | 0.04 | 69.98 | 0.30 |
| Max | 12.10 | 0.91 | 7.07 | 0.88 | 74 | 6.24 | 0.97 | 6.02 | 0.95 | 11.74 | 0.95 | 7.92 | 0.85 | 70.44 | 0.92 | 73.13 | 0.78 |
| No | Trial Name | Optimum N | Low N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OIL | PRO | STA | KNWT | OIL | PRO | STA | KNWT | ||
| 1 | 07-10TSCLWN | −0.12 | 0.04 | 0.13 | NA | −0.34 ** | −0.13 | −0.08 | NA |
| 2 | 13A-DHTC | 0.14 | −0.10 | −0.14 | NA | 0.09 | 0.02 | −0.19 * | NA |
| 3 | 13A-IMAS-LXTKARI | 0.24 | −0.32 | −0.23 | 0.42 ** | 0.29 * | −0.32 * | 0.05 | 0.22 |
| 4 | 13A-IMAS-SYN | −0.29 | −0.03 | 0.17 | 0.35 | −0.14 | −0.05 | 0.02 | 0.29 |
| 5 | COMM-1ROW-3REPS | 0.05 | −0.47 | 0.22 | 0.38 | 0.15 | NA | −0.18 | 0.71 ** |
| 6 | IMAS-Comm-Baseline-Early | 0.01 | −0.37 | 0.19 | 0.61 * | 0.28 | 0.03 | −0.23 | −0.14 |
| 7 | IMAS-Comm-Baseline-Late | −0.16 | −0.34 * | 0.21 | 0.41 ** | 0.09 | −0.39 * | 0.28 | 0.03 |
| 8 | THTA13B | 0.45 ** | −0.30 ** | −0.26 ** | NA | 0.03 | −0.15 * | −0.34 ** | 0.29 ** |
| 9 | THTA13C | 0.20 ** | −0.31 ** | −0.06 | 0.60 ** | 0.15 * | −0.02 | −0.35 ** | 0.32 ** |
| 10 | 14A-DHPOP1TC | 0.15 * | −0.29 ** | 0.03 | 0.30 ** | 0.06 | 0.18 ** | −0.12 | 0.25 ** |
| 11 | 14A-DHPOP2TC | 0.12 | −0.15 | −0.03 | 0.25 ** | −0.00 | 0.24 ** | −0.26 ** | 0.45 ** |
| 12 | 14A-EXP-TWC | 0.29 | 0.12 | −0.40 * | 0.62 ** | 0.10 | −0.28 | 0.29 | 0.40 * |
| 13 | IMAS12A-STG1TC-HGB | −0.10 | −0.34 ** | 0.13 | 0.43 ** | 0.08 | 0.03 | −0.19 ** | 0.37 ** |
| 14 | THTA13A | 0.03 | −0.57 ** | −0.37 ** | 0.67 ** | −0.15 | −0.12 | −0.38 ** | 0.46 ** |
| 15 | 14A-ADVTC-HGA | 0.02 | −0.05 | −0.04 | NA | 0.12 | −0.20 | 0.20 | NA |
| 16 | 14A-ADVTC-HGB | 0.09 | −0.20 * | 0.12 | NA | −0.26 ** | −0.02 | 0.11 | NA |
| Mean | 0.07 | −0.23 | −0.02 | 0.46 | 0.03 | −0.08 | −0.09 | 0.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ertiro, B.T.; Das, B.; Kosgei, T.; Tesfaye, A.T.; Labuschagne, M.T.; Worku, M.; Olsen, M.S.; Chaikam, V.; Gowda, M. Relationship between Grain Yield and Quality Traits under Optimum and Low-Nitrogen Stress Environments in Tropical Maize. Agronomy 2022, 12, 438. https://doi.org/10.3390/agronomy12020438
Ertiro BT, Das B, Kosgei T, Tesfaye AT, Labuschagne MT, Worku M, Olsen MS, Chaikam V, Gowda M. Relationship between Grain Yield and Quality Traits under Optimum and Low-Nitrogen Stress Environments in Tropical Maize. Agronomy. 2022; 12(2):438. https://doi.org/10.3390/agronomy12020438
Chicago/Turabian StyleErtiro, Berhanu Tadesse, Biswanath Das, Titus Kosgei, Amsal Tarekegne Tesfaye, Maryke T. Labuschagne, Mosisa Worku, Michael S. Olsen, Vijay Chaikam, and Manje Gowda. 2022. "Relationship between Grain Yield and Quality Traits under Optimum and Low-Nitrogen Stress Environments in Tropical Maize" Agronomy 12, no. 2: 438. https://doi.org/10.3390/agronomy12020438
APA StyleErtiro, B. T., Das, B., Kosgei, T., Tesfaye, A. T., Labuschagne, M. T., Worku, M., Olsen, M. S., Chaikam, V., & Gowda, M. (2022). Relationship between Grain Yield and Quality Traits under Optimum and Low-Nitrogen Stress Environments in Tropical Maize. Agronomy, 12(2), 438. https://doi.org/10.3390/agronomy12020438







