From the Lab to the Field: Combined Application of Plant-Growth-Promoting Bacteria for Mitigation of Salinity Stress in Melon Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Growth Conditions and Plant Material
2.2. Assessment of Bacterial Root Colonization
2.3. Seedling Growth in Germination Paper
2.4. Greenhouse Experiments
2.5. Measurements of Total Antioxidant Enzyme Activity
2.6. Field Experiment
2.7. Fluorescence in Situ Hybridization Experiments
2.8. Statistical Analyses
3. Results
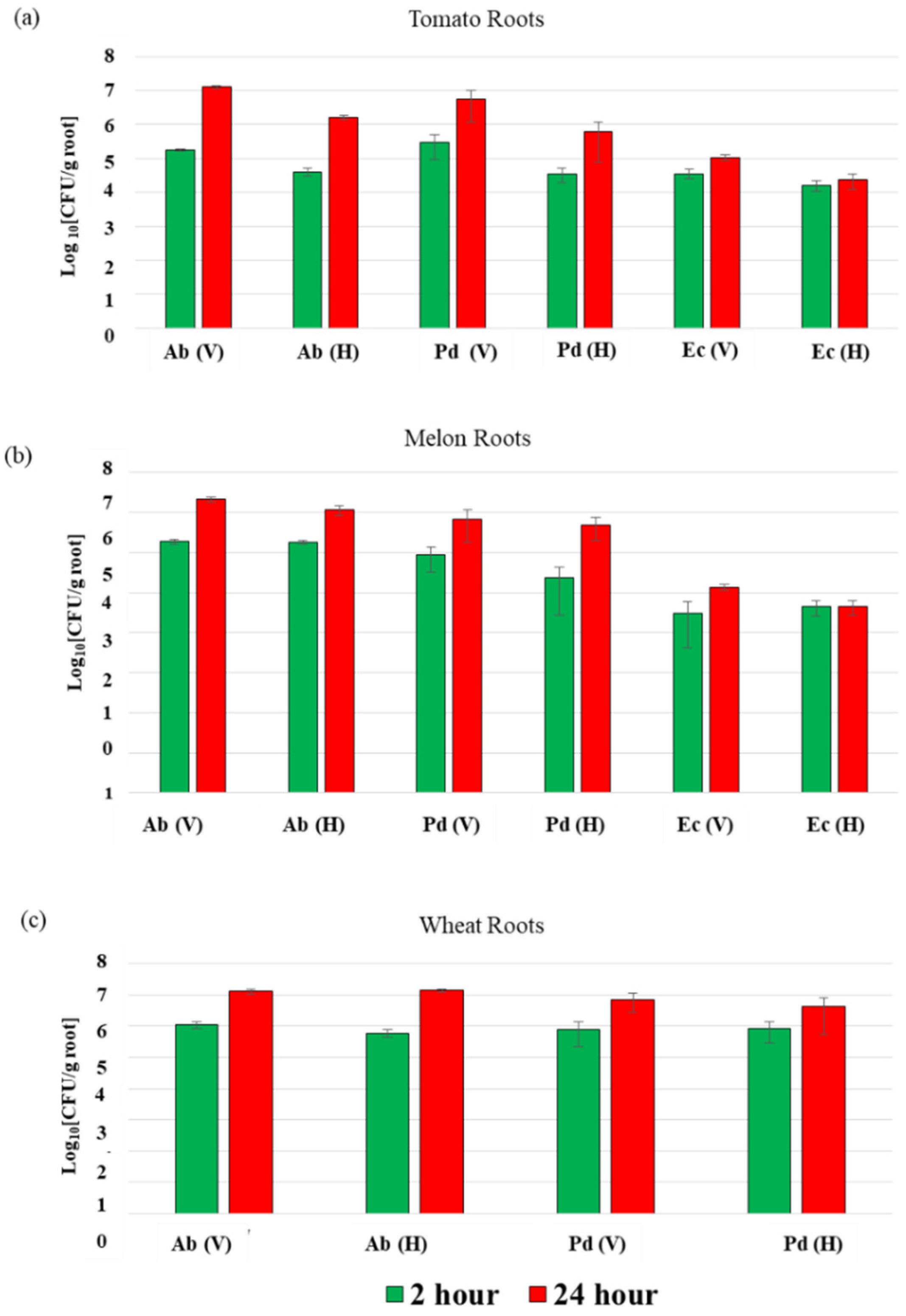
3.1. Azospirillum brasilense Sp7 and Paenibacillus dendritiformis T efficiently Colonize Melon and Tomato Roots
3.2. Azospirillum brasilense Sp7 and P. dendritiformis T Promote Melon Seedling Growth in Germination Paper under Salinity Stress
3.3. Azospirillum brasilense Sp7 and P. dendritiformis T Promote Growth of Melon Plants under Salinity Stress in the Greenhouse
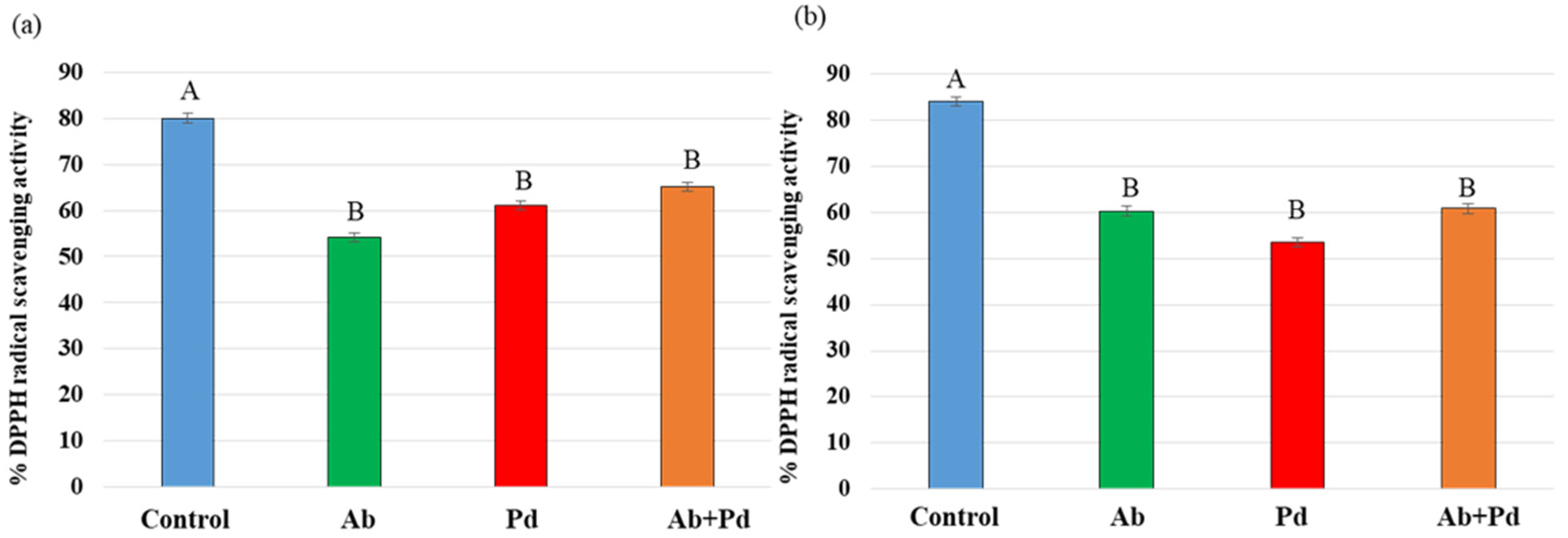
3.4. Effects of A. brasilense Sp7 and P. dendritiformis T Inoculation on Total Antioxidant Activity
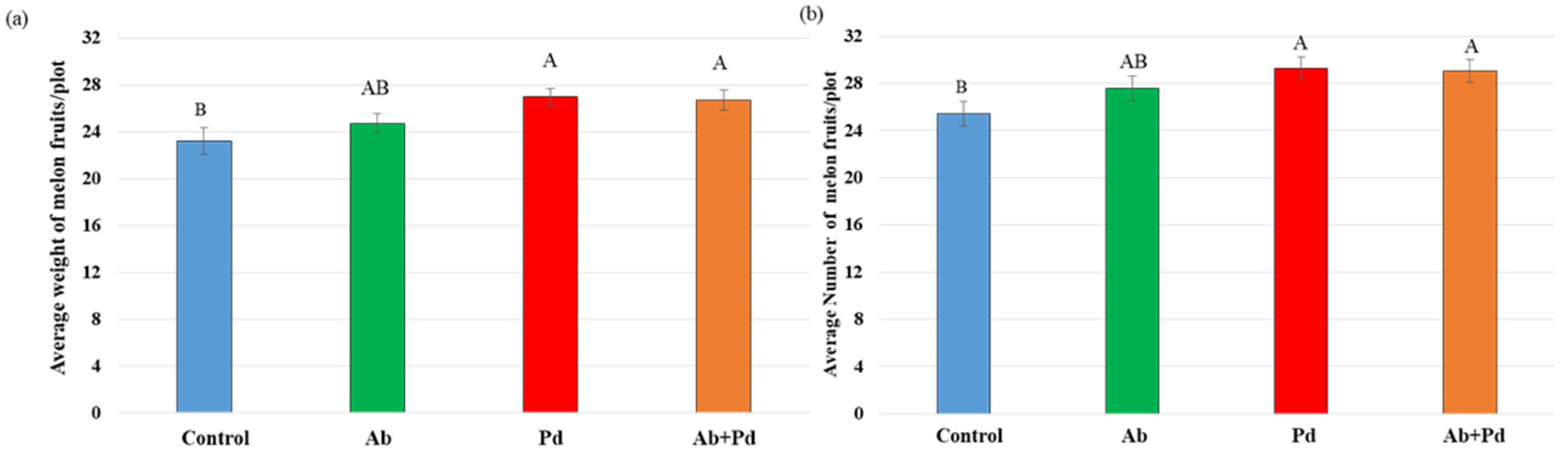
3.5. Combined Inoculation with A. brasilense Sp7 and P. dendritiformis T Increased Melon Yield in the Field under Saline Irrigation
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops-what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. The Effects of moderate and severe salinity on composition and physiology in the biomass crop. Plants 2020, 9, 1266. [Google Scholar] [CrossRef]
- Rengasamy, P. Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 2010, 37, 613–620. [Google Scholar] [CrossRef]
- Gupta, R.K.; Abrol, I.P.; Finkl, C.W.; Kirkham, M.B.; Arbestain, M.C.; Macías, F.; Chesworth, W.; Germida, J.J.; Loeppert, R.H.; Cook, M.G.; et al. Soil Salinity and Salinization. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 699–704. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V.; Maathuis, F.J. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef]
- Pandolfi, C.; Pottosin, I.; Cuin, T.; Mancuso, S.; Shabala, S. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant Cell Physiol. 2010, 51, 422–434. [Google Scholar] [CrossRef]
- Canfora, L.; Bacci, G.; Pinzari, F.; Lo Papa, G.; Dazzi, C.; Benedetti, A. Salinity and bacterial diversity: To what extent does the concentration of salt affect the bacterial community in a saline soil? PLoS ONE 2014, 9, e106662. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Dhuli, P.; Verma, P.; Singh, A.; Choudhary, M.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Culturable microbial diversity in the rhizosphere of different biotypes under variable salinity. Trop. Ecol. 2020, 61, 291–300. [Google Scholar] [CrossRef]
- Matsuguchi, T.; Sakai, M. Influence of soil-salinity on the population and composition of fluorecent pseudomonads in plant rhizosphere. Soil Sci. Plant Nutr. 1995, 41, 497–504. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations; ITPS. Status of the World’s Soil Resources (SWSR)–Main Report; FAO: Rome, Italy, 2015. [Google Scholar]
- Qadir, M.; Ghafoor, A.; Murtaza, G. Amelioration strategies for saline soils: A review. Land Degrad. Dev. 2000, 11, 501–521. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–992. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Lugtenberg, B.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001, 4, 343–350. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Ann. Rev. Microbiol. 2009, 63, 541–556. Available online: https://www.annualreviews.org/doi/full/10.1146/annurev.micro.62.081307.162918 (accessed on 2 January 2022). [CrossRef] [Green Version]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Pal, K.K.; Tilak, K.V.; Saxena, A.K.; Dey, R.; Singh, C.S. Antifungal characteristics of a fluorescent Pseudomonas strain involved in the biological control of Rhizoctonia solani. Microbiol. Res. 2000, 155, 233–242. [Google Scholar] [CrossRef]
- Raza, W.; Yang, W.; Shen, Q.R. Paenibacillus polymyxa: Antibiotics, hydrolytic enzymes and hazard assessment. J. Plant Pathol. 2008, 90, 419–430. [Google Scholar]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Pare, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [Green Version]
- Jjemba, P.K.; Alexander, M. Possible determinants of rhizosphere competence of bacteria. Soil Biol. Biochem. 1999, 31, 623–632. [Google Scholar] [CrossRef]
- Okon, Y.; Labandera-Gonzales, C.; Lage, M.; Lage, P. Agronomic applications of Azospirillum and other PGPR. In Biological Nitrogen Fixation; de Bruijn, F.J., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 925–936. [Google Scholar]
- Burdman, S.; Kigel, J.; Okon, Y. Effects of Azospirillum brasilense on nodulation and growth of common bean (Phaseolus vulgaris L.). Soil Biol. Biochem. 1997, 29, 923–929. [Google Scholar] [CrossRef]
- Volpin, H.; Burdman, S.; CastroSowinski, S.; Kapulnik, Y.; Okon, Y. Inoculation with Azospirillum increased exudation of rhizobial nod-gene inducers by alfalfa roots. Mol. Plant-Microbe Interact. 1996, 9, 388–394. [Google Scholar] [CrossRef]
- Dobbelaere, S.; Croonenborghs, A.; Thys, A.; Ptacek, D.; Vanderleyden, J.; Dutto, P.; Labandera-Gonzalez, C.; Caballero-Mellado, J.; Aguirre, J.F.; Kapulnik, Y.; et al. Responses of agronomically important crops to inoculation with Azospirillum. Aust. J. Plant Physiol. 2001, 28, 871–879. [Google Scholar] [CrossRef]
- Barbosa, J.Z.; Hungria, M.; Sena, J.V.D.; Poggere, G.; dos Reis, A.R.; Correa, R.S. Meta-analysis reveals benefits of co-inoculation of soybean with Azospirillum brasilense and Bradyrhizobium spp. in Brazil. Appl. Soil Ecol. 2021, 163. [Google Scholar] [CrossRef]
- Masciarelli, O.; Llanes, A.; Luna, V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Liu, L.; Jin, X.; Duan, C.; Cui, Y.; Wang, J.; Ma, D.; Zhao, W.; Wang, Y.; Fang, L. Co-inoculation effect of plant-growth-promoting rhizobacteria and rhizobium on EDDS assisted phytoremediation of Cu contaminated soils. Chemosphere 2020, 254, 126724. [Google Scholar] [CrossRef]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb.Cell Fact. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helman, Y.; Burdman, S.; Okon, Y. Plant growth promotion by rhizosphere bacteria through direct reffects. In Beneficial Microorganisms in Multicellular Life Forms; Rosenberg, E., Gophna, U., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 89–103. [Google Scholar] [CrossRef]
- Burdman, S.; Jurkevitch, E.; Okon, Y. Recent Advances in the Use of Plant Growth Promoting Rhizobacteria (PGPR) in Agriculture. In Microbial Interactions in Agriculture and Forestry (Volume II); Subba Rao, N.S., Dommergues, Y.R., Eds.; Science Publishers: Enfield, NH, USA, 2000; pp. 229–250. [Google Scholar]
- Li, J.Y.; Gao, T.T.; Wang, Q. Comparative and functional analyses of two sequenced Paenibacillus polymyxa genomes provides insights into their potential genes related to plant growth-promoting features and biocontrol mechanisms. Front. Genet. 2020, 11, 1374. [Google Scholar] [CrossRef]
- Brito, L.F.; Lopez, M.G.; Straube, L.; Passaglia, L.M.P.; Wendischl, V.F. Inorganic phosphate solubilization by rhizosphere bacterium Paenibacillus sonchi: Gene expression and physiological functions. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J.; Okon, Y. Plant growth-promoting actions of rhizobacteria. Adv. Bot. Res. 2009, 51, 283–320. [Google Scholar] [CrossRef]
- Hadas, R.; Okon, Y. Effect of Azospirillum brasilense inoculation on root morphology and respiration in tomato seedlings. Biol. Fert. Soils 1987, 5, 241–247. [Google Scholar] [CrossRef]
- Mangmang, J.S.; Deaker, R.; Rogers, G. Early seedling growth response of lettuce, tomato and cucumber to Azospirillum brasilense inoculated by soaking and drenching. Hortic. Sci. 2015, 42, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Sirota-Madi, A.; Olender, T.; Helman, Y.; Brainis, I.; Finkelshtein, A.; Roth, D.; Hagai, E.; Leshkowitz, D.; Brodsky, L.; Galatenko, V.; et al. Genome sequence of the pattern-forming social bacterium Paenibacillus dendritiformis C454 chiral morphotype. J. Bacteriol. 2012, 194, 2127–2128. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, D.; Dror, R.; Vered, E.; Mishli, O.; Levy, D.; Helman, Y. Disease protection and growth promotion of potatoes (Solanum tuberosum L.) by Paenibacillus dendritiformis. Plant Pathol. 2014, 64, 545–551. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Ajithkumar, P.; Arun, M.; Sathasivam, R.; Sandhya, S.; Choi, J.; Park, S.U. An endophyte Paenibacillus dendritiformis strain APL3 promotes Amaranthus polygonoides L. sprout growth and their extract inhibits food-borne pathogens. Plant Sci. Today 2021, 8, 941–947. [Google Scholar] [CrossRef]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic resistance in chilli pepper against anthracnose (caused by Colletotrichum truncatum) induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Tarrand, J.J.; Krieg, N.R.; Döbereiner, J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can. J. Microbiol. 1978, 24, 967–980. [Google Scholar] [CrossRef]
- Tcherpakov, M.; Ben-Jacob, E.; Gutnick, D.L. Paenibacillus dendritiformis sp. nov., proposal for a new pattern-forming species and its localization within a phylogenetic cluster. Int. J. Syst. Bacteriol. 1999, 49, 239–246. [Google Scholar] [CrossRef]
- Simon, R.; Priefer, U.; Puhler, A. A broad host range mobilization system for in vivo genetic engineering transposon mutagenesis in Gram negative bacteria. Bio-Technology 1983, 1, 784–791. [Google Scholar] [CrossRef]
- Bai, C.H.; Liang, Y.L.; Hawkesford, M.J. Identification of QTLs associated with seedling root traits and their correlation with plant height in wheat. J. Exp. Bot. 2013, 64, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [Green Version]
- Kumar, Y.; Westram, R.; Behrens, S.; Fuchs, B.; Glockner, F.O.; Amann, R.; Meier, H.; Ludwig, W. Graphical representation of ribosomal RNA probe accessibility data using ARB software package. BMC Bioinform. 2005, 6, 61. [Google Scholar] [CrossRef] [Green Version]
- Michiels, K.W.; Croes, C.L.; Vanderleyden, J. Two different modes of attachment of Azospirillum brasilense Sp7 to wheat roots. J. Gen. Microbiol. 1991, 137, 2241–2246. [Google Scholar] [CrossRef] [Green Version]
- Burdman, S.; Okon, Y.; Jurkevitch, E. Surface characteristics of Azospirillum brasilense in relation to cell aggregation and attachment to plant roots. Crit. Rev. Microbiol. 2000, 26, 91–110. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, D.N.; Dardanelli, M.S.; Ruíz-Saínz, J.E. Attachment of bacteria to the roots of higher plants. FEMS Microbiol. Lett. 2007, 272, 127–136. [Google Scholar] [CrossRef]
- Wheatley, R.M.; Poole, P.S. Mechanisms of bacterial attachment to roots. FEMS Microbiol. Rev. 2018, 42, 448–461. [Google Scholar] [CrossRef]
- Al-Amoudi, S.; Essack, M.; Simões, M.F.; Bougouffa, S.; Soloviev, I.; Archer, J.A.; Lafi, F.F.; Bajic, V.B. Bioprospecting red sea coastal ecosystems for culturable microorganisms and their antimicrobial potential. Mar. Drugs 2016, 14, 165. [Google Scholar] [CrossRef] [Green Version]
- Jangra, M.; Randhawa, H.K.; Kaur, M.; Srivastava, A.; Maurya, N.; Patil, P.P.; Jaswal, P.; Arora, A.; Patil, P.B.; Raje, M.; et al. Purification, Characterization and in vitro evaluation of polymyxin A from Paenibacillus dendritiformis: An underexplored member of the polymyxin family. Front. Microbiol. 2018, 9, 2864. [Google Scholar] [CrossRef]
- Miranda, D.; Fischer, G.; Mewis, I.; Rohn, S.; Ulrichs, C. Salinity effects on proline accumulation and total antioxidant activity in leaves of the cape gooseberry (Physalis peruviana L.). J. Appl. Bot. Food Qual. 2014, 87, 67–73. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranova, E.; van Montagu, M.; Inze, D.; van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A.R.; Dalal, R.C.; Kirchhof, G.; Kopittke, P.M.; Menzies, N.W. The effect of salinity on plant-available water. Plant Soil 2017, 418, 477–491. [Google Scholar] [CrossRef]
- Arif, M.R.; Islam, M.T.; Robin, A.H.K. Salinity stress alters root morphology and root hair traits in Brassica napus. Plants 2019, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J.E.; Rachmilevitch, S. Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 2012, 63, 2717–2727. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gopalakrishnan, V.; Burdman, S.; Jurkevitch, E.; Helman, Y. From the Lab to the Field: Combined Application of Plant-Growth-Promoting Bacteria for Mitigation of Salinity Stress in Melon Plants. Agronomy 2022, 12, 408. https://doi.org/10.3390/agronomy12020408
Gopalakrishnan V, Burdman S, Jurkevitch E, Helman Y. From the Lab to the Field: Combined Application of Plant-Growth-Promoting Bacteria for Mitigation of Salinity Stress in Melon Plants. Agronomy. 2022; 12(2):408. https://doi.org/10.3390/agronomy12020408
Chicago/Turabian StyleGopalakrishnan, Vinoj, Saul Burdman, Edouard Jurkevitch, and Yael Helman. 2022. "From the Lab to the Field: Combined Application of Plant-Growth-Promoting Bacteria for Mitigation of Salinity Stress in Melon Plants" Agronomy 12, no. 2: 408. https://doi.org/10.3390/agronomy12020408
APA StyleGopalakrishnan, V., Burdman, S., Jurkevitch, E., & Helman, Y. (2022). From the Lab to the Field: Combined Application of Plant-Growth-Promoting Bacteria for Mitigation of Salinity Stress in Melon Plants. Agronomy, 12(2), 408. https://doi.org/10.3390/agronomy12020408





