Poultry Litter Biochar as a Gentle Soil Amendment in Multi-Contaminated Soil: Quality Evaluation on Nutrient Preservation and Contaminant Immobilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Soil and Analyses
2.2. Studied Poultry Litter Biochars (PLBs) and Analyses
2.3. Incubation Experiment
2.4. Statistical Analysis
3. Results
3.1. Heavy Metal Concentration of the Initial Soil and PLBs
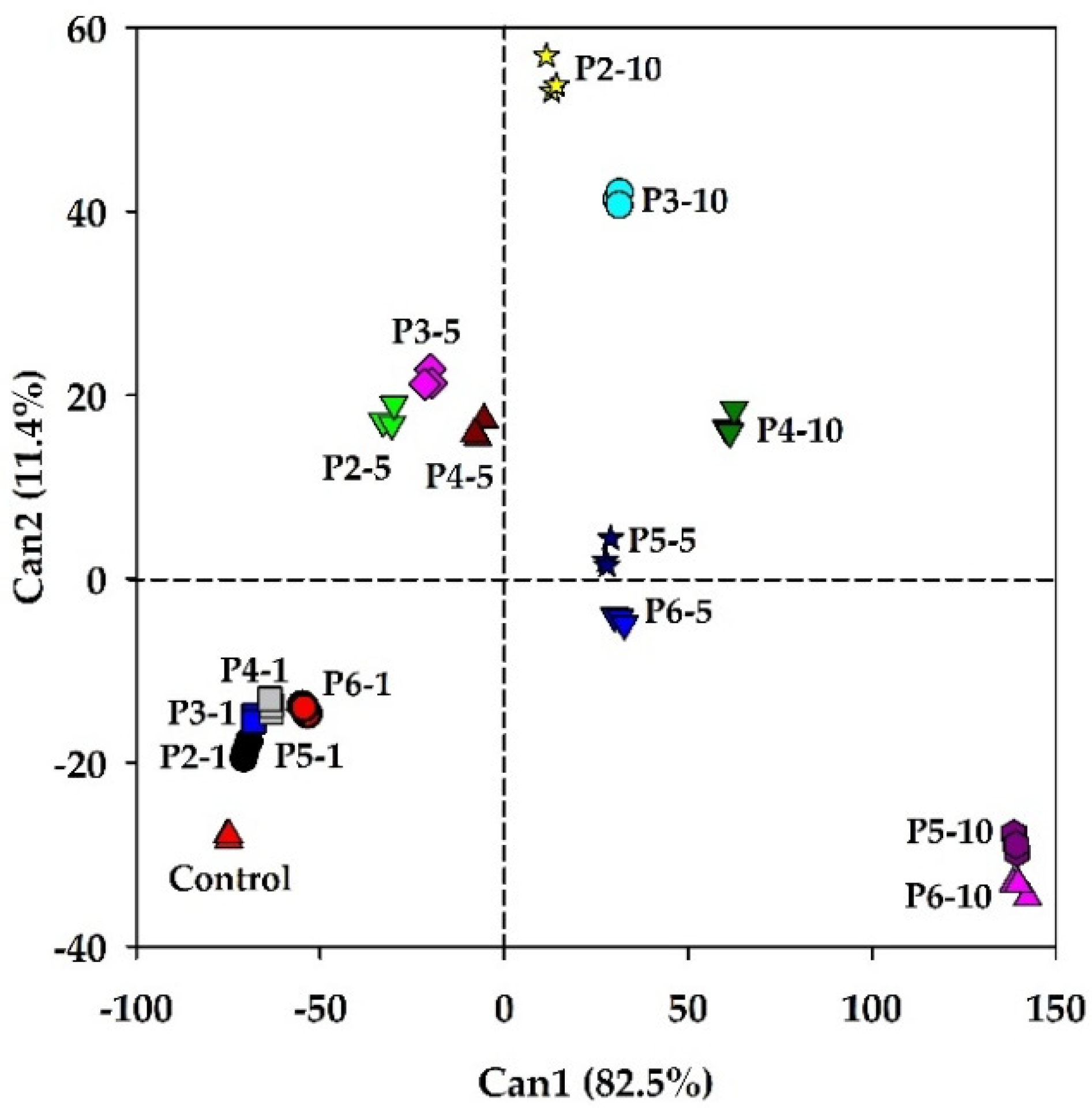
3.2. Results of the two-way ANOVA and CDA
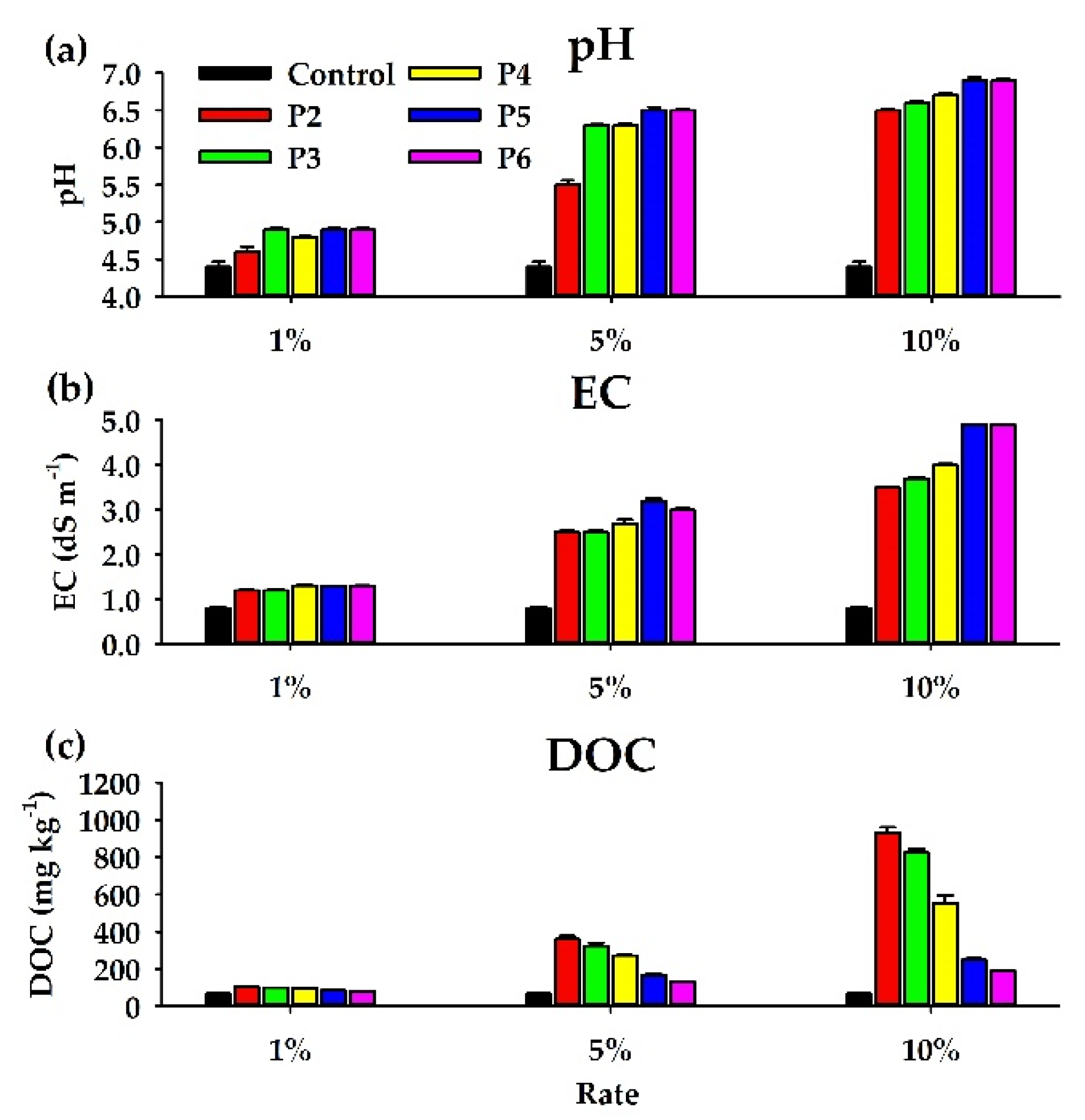
3.3. pH, EC, and DOC
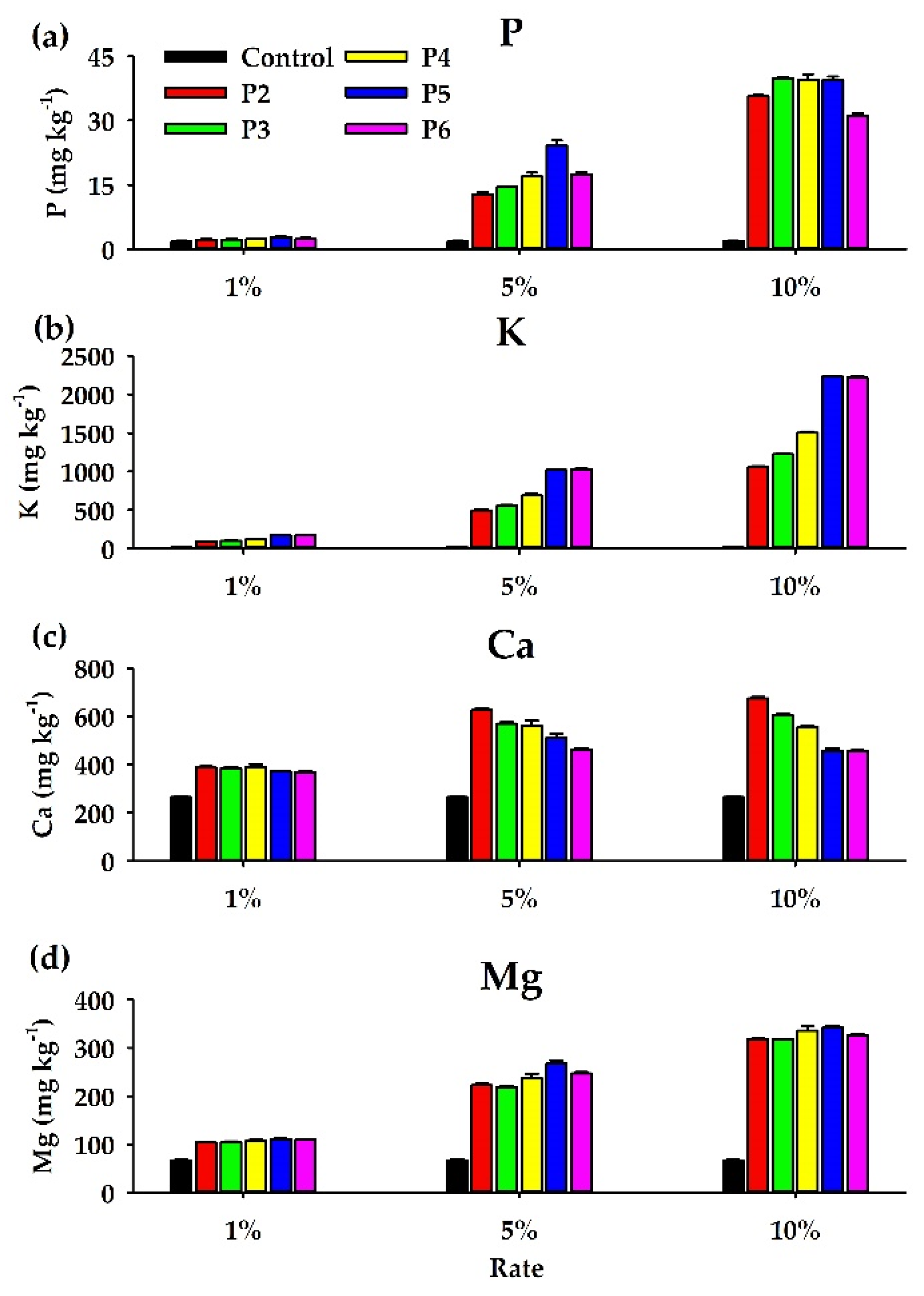
3.4. Available Nutrients (P, K, Ca, and Mg)
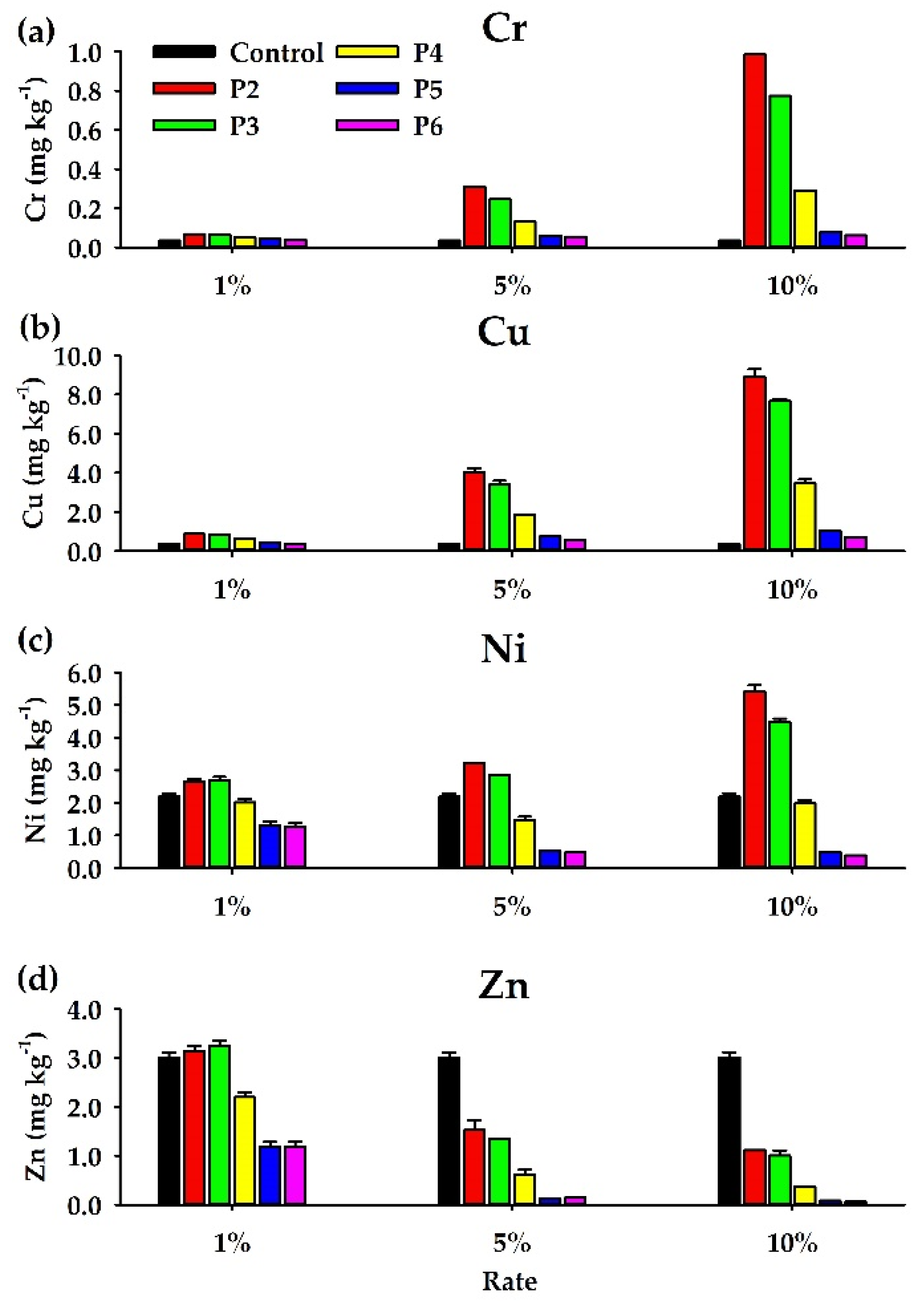
3.5. Cr, Cu, Ni, and Zn Concentrations
4. Discussion
4.1. Effects of the PLB Treatments on pH, EC, and DOC
4.2. Effects of the PLB Treatments on the Available Nutrients (P, K, Ca, and Mg)
4.3. Effects of the PLB Treatments on the Cr, Cu, Ni, and Zn Solubility in Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Marschner, B.; Itanna, F.; Gebrekidan, H. Effects of biochar and alkaline amendments on cadmium immobilization, selected nutrient and cadmium concentrations of lettuce (Lactuca sativa) in two contrasting soils. SpringerPlus 2016, 5, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonangelo, J.A.; Zhang, H. Heavy metal phytoavailability in a contaminated soil of northeastern Oklahoma as affected by biochar amendment. Environ. Sci. Pollut. Res. 2019, 26, 33582–33593. [Google Scholar] [CrossRef]
- Virkutyte, J.; Sillanpää, M.; Latostenmaa, P. Electrokinetic soil remediation—critical review. Sci. Total Environ. 2002, 289, 97–121. [Google Scholar] [CrossRef]
- Yeung, A.T.; Hsu, C.N. Electrokinetic remediation of cadmium contaminated clay. J. Environ. Eng. 2005, 131, 298–304. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Metal-contaminated soils: Remediation practices and treatment technologies. J. Hazard. Toxic Radioact. Waste 2008, 12, 188–209. [Google Scholar] [CrossRef]
- Peng, J.F.; Song, Y.E.; Yuan, P.; Cui, X.Y.; Qiu, G.L. The remediation of heavy metals contaminated sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Surfactant-enhanced remediation of contaminated soil: A review. Eng. Geol. 2001, 60, 371–380. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Bio. Technol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of its by-products. As. J. Energy Env. 2005, 6, 214–231. Available online: https://www.thaiscience.info/Journals/Article/AJEE/10262422.pdf (accessed on 24 November 2021).
- Ahumada, I.; Sepúlveda, K.; Fernández, P.; Ascar, L.; Pedraza, C.; Richter, P.; Brown, S. Effect of biosolid application to Mollisol Chilean soils on the bioavailability of heavy metals (Cu, Cr, Ni, and Zn) as assessed by bioassays with sunflower (Helianthus annuus) and DGT measurements. J. Soils Sedim. 2014, 14, 886–896. [Google Scholar] [CrossRef]
- Sidhu, V.; Sarkar, D.; Datta, R. Effects of biosolids and compost amendment on chemistry of soils contaminated with copper from mining activities. Environ. Monit. Assess. 2016, 188, 176. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.Y.; Chiou, C.T.; Xing, B.S. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.S.; Oh, S.E.; Ahmad, A.; Hyun, S.; Kim, K.R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth. Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Klasson, T.; Wartelle, L.H. Contaminant immobilization and nutrient release by biochar soil amendment: Roles of natural organic matter. Chemosphere 2010, 80, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.N.; Chen, H.S.; Cai, P.; Lianga, W.; Huang, Q.Y. Immobilization and phytotoxicity of Cd in contaminated soil amended with chicken manure compost. J. Hazard. Mater. 2009, 163, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Lim, J.E.; El-Azeem, S.A.M.A.; Choi, B.; Oh, S.E.; Moon, D.H.; Ok, Y.S. Heavy metal immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ. Sci. Pollut. Res. 2013, 20, 1719–1726. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gomez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.N.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Cao, X.D.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macedo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.P.; Lin, M. Equilibrium and kinetics of metal ion adsorption onto a commercial H-type granular activated carbon: Experimental and modeling studies. Water Res. 2001, 35, 2385–2394. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Jiang, J.; Xu, R.K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xu, R.K.; Li, Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012, 229, 145–150. [Google Scholar] [CrossRef]
- Uchimiya, M.; Lima, I.M.; Thomas Klasson, K.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Comis, D. Better ballfields and rain gardens start below the surface. Agric. Res. Mag. 2010, 58. Available online: https://agresearchmag.ars.usda.gov/2010/nov/gardens. (accessed on 2 November 2021).
- Guo, M.; Qiu, G.; Song, W. Poultry litter-based activated carbon for removing heavy metal ions in water. Waste Manag. 2010, 30, 308–315. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenerg. 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M. Effect of low-temperature biochar derived from pig manure and poultry litter on mobile and organic matter-bound forms of Cu, Cd, Pb and Zn in sandy soil. Soil Use Manag. 2016, 32, 357–367. [Google Scholar] [CrossRef]
- Choppala, G.; Bolan, N.; Kunhikrishnan, A.; Skinner, W.; Seshadri, B. Concomitant reduction and immobilization of chromium in relation to its bioavailability in soil. Environ. Sci. Pollut. Res. 2015, 22, 8969–8978. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xu, X.; Wang, L.; Chen, Q.; Fang, J.; Shen, X.; Lou, L.; Tian, G. The speciation, leachability and bioaccessibility of Cu and Zn in animal manure-derived biochar: Effect of feedstock and pyrolysis temperature. Front. Environ. Sci. Eng. 2017, 11, 5. [Google Scholar] [CrossRef]
- Sehrish, A.K.; Aziz, R.; Hussain, M.M.; Rafiq, M.T.; Rizwan, M.; Muhammad, N.; Rafiq, M.K.; Sehar, A.; ud Din, J.; Al-Wabel, M.I.; et al. Effect of poultry litter biochar on chromium (Cr) bioavailability and accumulation in spinach (Spinacia oleracea) grown in Cr-polluted soil. Arab. J. Geosci. 2019, 12, 57. [Google Scholar] [CrossRef]
- Banik, C.; Lawrinenko, M.; Bakshi, S.; Laird, D.A. Impact of pyrolysis temperature and feedstock on surface charge and functional group chemistry of biochars. J. Environ. Qual. 2018, 47, 452. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Guo, X.; Zhao, Z.; He, Q.; Wang, S.; Zhu, Y.; Yan, Y.; Liu, X.; Sun, K.; Zhao, Y.; et al. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ. Pollut. 2016, 218, 513–522. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Usman, A.R.; El-Naggar, A.H.; Aly, A.A.; Ibrahim, H.M.; Elmaghraby, S.; Al-Omran, A. Conocarpus biochar as a soil amendment for reducing heavy metal availability and uptake by maize plants. Saudi J. Biol. Sci. 2015, 22, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Yang, X.; Shen, J.; Robinson, B.; Huang, H.; Liu, D.; Bolan, N.; Pei, J.; Wang, H. Effect of bamboo and rice straw biochars on the bioavailability of Cd, Cu, Pb and Zn to Sedum plumbizincicola. Agric. Ecosyst. Environ. 2014, 191, 124–132. [Google Scholar] [CrossRef]
- Puga, A.; Abreu, C.; Melo, L.; Beesley, L. Biochar application to a contaminated soil reduces the availability and plant uptake of zinc, lead and cadmium. J. Environ. Manag. 2015, 159, 86–93. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chang, Y.F. Quality evaluation of poultry litter biochar produced at different pyrolysis temperatures as a sustainable management approach and its effects on soil carbon mineralization. Agronomy 2021, 11, 1692. [Google Scholar] [CrossRef]
- Environmental Protection Administration (EPA). Total Concentration of Metals in Soils; Method Code No: NIEA S321.63B; Environmental Protection Administration of Taiwan: Taipei, Taiwan, 2003; 11p. [Google Scholar]
- Baker, D.E.; Amacher, M.C. Nickel, Copper, Zinc and Cadmium. In Methods of Soil Analysis, Part 2, Chemical and Microbiological Properties, Agronomy Monograph 9, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy Society of America and Soil Science Society of America: Madison, WI, USA, 1982; pp. 323–336. [Google Scholar]
- Novozamsky, I.; Lexmond, T.H.M.; Houba, V.J.G. A single extraction procedure of soil for evaluation of uptake of some heavy metals by plant. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Degryse, F.; Broos, K.; Smolders, E.; Merckx, R. Soil solution concentration of Cd and Zn can be predicted with a CaCl2 soil extract. Eur. J. Soil Sci. 2003, 54, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Case, V.W.; Westerman, R. Sampling, Handling, and Analyzing Plant Tissue Samples. In Methods of Soil Analysis, Part 5-Mineralogical Methods; Soil Science Society of America: Madison, WI, USA, 2018; pp. 389–427. [Google Scholar]
- Kalderis, D.; Kotti, M.S.; Méndez, A.; Gascó, G. Characterization of hydrochars produced by hydrothermal carbonization of rice husk. Solid Earth 2014, 5, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Mimmo, T.; Panzacchi, P.; Baratieri, M.; Davies, C.A.; Tonon, G. ScienceDirect effect of pyrolysis temperature on miscanthus (Miscanthus 3 giganteus) biochar physical, chemical and functional properties. Biomass Bioenergy 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Dandamudi, K.P.R.; Luboowa, K.M.; Laideson, M.; Murdock, T.; Seger, M.; McGowen, J.; Lammers, P.J.; Deng, S. Hydrothermal liquefaction of Cyanidioschyzon merolae and Salicornia bigelovii Torr.: The interaction effect on product distribution and chemistry. Fuel 2020, 277, 118146. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. High temperature-produced biochar can be efficient in nitrate loss prevention and carbon sequestration. Geoderma 2019, 338, 48–55. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V.; Radovich, T.J.K.; Ahmad, A.A. Biochars improve nutrient phyto-availability of Hawai’i’s highly weathered soils. Agronomy 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Chintala, R.; Mollinedo, J.; Schumacher, T.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2013, 60, 393–404. [Google Scholar] [CrossRef]
- Li, Y.; Zhiu, C.; Qiu, Y.; Tigabu, M.; Ma, X. Effects of biochar and litter on carbon and nitrogen mineralization and soil microbial community structure in a China fir plantation. J. For. Res. 2019, 30, 1913–1923. [Google Scholar] [CrossRef]
- Jiang, X.; Denef, K.; Stewart, C.E.; Cotrufo, M.F. Controls and dynamics of biochar decomposition and soil microbial abundance, composition, and carbon use efficiency during long-term biochar-amended soil incubations. Biol. Fertil. Soils 2016, 52, 1–14. [Google Scholar] [CrossRef]
- Shen, Y.H. Sorption of natural dissolved organic matter on soil. Chemosphere 1999, 38, 1505–1515. [Google Scholar] [CrossRef]
- Whittinghill, K.A.; Hobbie, S.E. Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochemistry 2012, 111, 569–581. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Spokas, K.A.; Novak, J.M.; Lentz, R.D.; Cantrell, K.B. Biochar elemental composition and factors influencing nutrient retention. In Biochar for Environmental Management: Science and Technology, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015; pp. 137–162. [Google Scholar]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Angst, T.E.; Sohi, S.P. Establishing release dynamics for plant nutrients from biochar. GCB Bioenergy 2013, 5, 221–226. [Google Scholar] [CrossRef]
- Murphy, P.N.C.; Stevens, R.J. Lime and gypsum as source measures to decrease phosphorus loss from soils to water. Water Air Soil Pollut. 2010, 212, 101–111. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.Q.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Contrasting effects of biochar on phosphorus dynamics and bioavailability in different soil types. Sci. Total Environ. 2018, 627, 963–974. [Google Scholar] [CrossRef]
- Parfitt, R.L. Anion adsorption by soils and soil materials. Adv. Agron. 1979, 30, 1–50. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Davey, L.J. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science and Technology, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2015; pp. 421–455. [Google Scholar]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of biochar in promoting circular economy in the agriculture sector. Part 1: A review of the biochar roles in soil N, P and K cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application Research of Biochar for the Remediation of Soil Heavy Metals Contamination: A Review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Ge, L.; Khan, M.H.; Chan, W.P.; Bilal, M.; Lisak, G.; Nafee, M. Effects of different biochars on physicochemical properties and immobilization of potentially toxic elements in soil-A geostatistical approach. Chemosphere 2021, 277, 130350. [Google Scholar] [CrossRef]
- Arabi, Z.; Rinklebe, J.; El-Naggar, A.; Hou, D.; Sarmah, A.K.; Moreno-Jiménez, E. (Im)mobilization of arsenic, chromium, and nickel in soils via biochar: A meta-analysis. Environ. Pollut. 2021, 286, 117199. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Liu, K.; Ly, J.; He, W.; Zhang, H.; Cao, Y.; Dai, Y. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol. Environ. Saf. 2015, 113, 207–213. [Google Scholar] [CrossRef]
- Rehman, M.Z.; Rizwan, M.; Ali, S.; Fatima, N.; Yousaf, B.; Naeem, A.; Sabir, M.; Ahmad, H.R.; Ok, Y.S. Contrasting effects of biochar compost and farm manure on alleviation of nickel toxicity in maiz (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol. Environ. Saf. 2016, 133, 218–255. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
- Gell, K.; van Groenigen, J.W.; Cayuela, M.L. Residues of bioenergy production chains as soil amendments: Immediate and temporal phytotoxicity. J. Hazard. Mater. 2011, 186, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Yu, J.; Li, Z.; Wu, L.; Christie, P. Effects of biochar on the migration and transformation of metal species in a highly acid soil contaminated with multiple metals and leached with solutions of different pH. Chemosphere 2021, 278, 130344. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Beesley, L.; Marmiroli, M. The immobilisation and retention of soluble arsenic, cadmium and zinc by biochar. Environ. Pollut. 2011, 159, 474–480. [Google Scholar] [CrossRef]
- Oste, L.A.; Temminghoff, J.M.E.; Van Riemsdijk, W.H. Solid-solution Partitioning of Organic Matter in Soils as Influenced by an Increase in pH or Ca Concentration. Environ. Sci. Technol. 2002, 36, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Impellitteri, C.A.; Lu, Y.F.; Saxe, J.K.; Allen, H.E.; Peijnenburg, W.J. Correlation of the partitioning of dissolved organic matter fractions with the desorption of Cd, Cu, Ni, Pb and Zn from 18 Dutch soils. Environ. Int. 2002, 28, 401–410. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Qiao, Y.; Crowley, D.; Wang, K.; Zhang, H.; Li, H. Effects of biochar and Arbuscular mycorrhizae on bioavailability of potentially toxic elements in an aged contaminated soil. Environ. Pollut. 2015, 206, 636–643. [Google Scholar] [CrossRef]
- Boostani, H.R.; Najafi-Ghiri, M.; Hardie, A.G. Nickel immobilization in a contaminated calcareous soil with application of organic amendments and their derived biochars. Commun. Soil Sci. Plant Anal. 2020, 51, 503–514. [Google Scholar] [CrossRef]
- Rafique, M.I.; Usman, A.R.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. In situ immobilization of Cr and its availability to maize plants in tannery waste–contaminated soil: Effects of biochar feedstock and pyrolysis temperature. J. Soils Sedim. 2020, 20, 330–339. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choppala, G.; Lee, S.J.; Bolan, N.; Chung, J.W.; Edraki, M. Comparative sorption of Pb and Cd by biochars and its implication for metal immobilization in soils. Water Air Soil Pollut. 2013, 224, 1711. [Google Scholar] [CrossRef]
| Soil/Biochars | Cd | Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| mg kg−1 | |||||||
| Soil | 0.1 N HCl | ND | 29.7 | 329 | 101 | 15.6 | 216 |
| Aqua Regia | ND | 1017 | 488 | 901 | 43.9 | 824 | |
| Raw | 0.1N HCl | ND 2 | 53.5 | 13.0 | ND | ND | 168 |
| P2 1 | 0.1N HCl | ND 2 | 51.3 | 10.7 | 0.64 | 0.07 | 144 |
| 0.01M CaCl2 | 0.01 | 43.1 | 18.2 | 1.04 | 0.02 | 35.2 | |
| Total | 0.08 | 271 | 57.3 | 3.18 | ND | 304 | |
| P3 | 0.1 N HCl | ND | 42.8 | 8.14 | 0.48 | 0.07 | 140 |
| 0.01 M CaCl2 | 0.01 | 35.4 | 14.6 | 0.83 | 0.03 | 31.1 | |
| Total | 0.05 | 351 | 73.4 | 4.85 | ND | 409 | |
| P4 | 0.1 N HCl | ND | 24.5 | 4.21 | 0.17 | 0.01 | 105 |
| 0.01 M CaCl2 | 0.004 | 17.3 | 7.99 | 0.35 | 0.01 | 13.8 | |
| Total | 0.06 | 442 | 92.8 | 4.84 | ND | 523 | |
| P5 | 0.1 N HCl | ND | 4.95 | 0.33 | ND | ND | 8.02 |
| 0.01 M CaCl2 | ND | 2.02 | 0.79 | ND | ND | 0.46 | |
| Total | 0.11 | 672 | 143 | 6.83 | ND | 718 | |
| P6 | 0.1 N HCl | ND | 3.4 | ND | ND | ND | 14.9 |
| 0.01 M CaCl2 | ND | 0.43 | 0.04 | ND | ND | ND | |
| Total | 0.14 | 597 | 129 | 7.17 | ND | 752 | |
| Sources | df 1 | pH | EC | DOC | P | K | Ca | Mg | Cr | Cu | Ni | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | 2 | *** 2 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Treatment | 4 | *** | *** | *** | ns | *** | *** | ns | *** | *** | *** | *** |
| Rate × Treatment | 8 | ** | ** | *** | ns | *** | *** | ns | *** | *** | *** | *** |
| Sources | Df 1 | pH | EC | DOC | P | K | Ca | Mg | Cr | Cu | Ni | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | dS m–1 | mg kg−1 | ||||||||||
| Control | 3 | 4.45d 2 | 0.81d | 68.0d 1 | 1.80c | 19.1d | 264c | 66.3d | 0.030d | 0.37d | 2.19b | 3.01a |
| 1% | 15 | 4.82c | 1.28c | 95.0c | 2.47c | 133c | 380b | 107c | 0.055c | 0.64c | 1.99c | 2.19b |
| 5% | 15 | 6.11b | 2.67b | 252b | 15.6b | 699b | 535a | 228b | 0.160b | 2.12b | 1.71d | 0.75c |
| 10% | 15 | 6.73a | 4.17a | 550a | 37.1a | 1644a | 549a | 328a | 0.439a | 4.36a | 2.55a | 0.52d |
| Treatment 3 | ||||||||||||
| Control | 3 | 4.45c | 0.81d | 68.0f | 1.80b 2 | 19.1d | 264d | 66.3b | 0.030e | 0.37f | 2.19c | 3.01a |
| P2 | 9 | 5.54b | 2.40c | 466a | 16.9a | 549c | 562a | 215a | 0.454a | 4.60a | 3.76a | 1.92b |
| P3 | 9 | 5.93a | 2.47c | 417b | 18.9a | 626c | 519b | 213a | 0.362b | 3.97b | 3.34b | 1.86b |
| P4 | 9 | 5.94a | 2.67bc | 308c | 19.6a | 770b | 502b | 227a | 0.160c | 1.99c | 1.83d | 1.06c |
| P5 | 9 | 5.89a | 2.92ab | 168d | 19.6a | 1046a | 428c | 222a | 0.061d | 0.75d | 0.78e | 0.46d |
| P6 | 9 | 6.13a | 3.07a | 135e | 17.0a | 1136a | 429c | 228a | 0.051d | 0.57e | 0.72e | 0.46d |
| Parameter | PC1 | PC2 |
|---|---|---|
| Total variance | 64.2% | 30.1% |
| Cumulative CO2-C | 0.816 *,1 | 0.573 * |
| pH | 0.859 * | −0.466 (p = 0.0008) |
| Electrical conductivity | 0.829 * | −0.530 (p = 0.0001) |
| Dissolve organic carbon | 0.892 * | 0.427 (p = 0.0025) |
| P | 0.927 * | −0.283 |
| K | 0.723 * | −0.628 * |
| Ca | 0.870 * | 0.193 |
| Mg | 0.924 * | −0.371 (p = 0.0094) |
| Cr | 0.790 * | 0.586 * |
| Cu | 0.810 * | 0.582 * |
| Ni | 0.400 (p =0.0049) | 0.907 * |
| Zn | −0.619 * | 0.668 * |
| Parameters 1 | pH | EC | DOC | P | K | Ca | Mg | Cr | Cu | Ni | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.00 | 0.95 2 | 0.56 | 0.91 | 0.89 | 0.69 | 0.97 | 0.40 (0.005) | 0.42 (0.003) | −0.08 | −0.85 |
| EC | 1.00 | 0.51 (0.0002) 3 | 0.93 | 0.98 | 0.59 | 0.97 | 0.34 | 0.36 | –0.13 | –0.80 | |
| DOC | 1.00 | 0.73 | 0.38 (0.007) | 0.81 | 0.66 | 0.97 | 0.97 | 0.74 | –0.27 | ||
| P | 1.00 | 0.89 | 0.67 | 0.97 | 0.57 | 0.58 | 0.13 | –0.71 | |||
| K | 1.00 | 0.42 (0.003) | 0.91 | 0.22 | 0.22 | –0.25 | –0.78 | ||||
| Ca | 1.00 | 0.74 | 0.75 | 0.81 | 0.50 (0.0003) | –0.48 (0.001) | |||||
| Mg | 1.00 | 0.50 (0.0003) | 0.53 (0.0001) | 0.04 | –0.80 | ||||||
| Cr | 1.00 | 0.99 | 0.84 | –0.12 | |||||||
| Cu | 1.00 | 0.85 | –0.13 | ||||||||
| Ni | 1.00 | 0.41 (0.004) | |||||||||
| Zn | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Chang, Y.-F. Poultry Litter Biochar as a Gentle Soil Amendment in Multi-Contaminated Soil: Quality Evaluation on Nutrient Preservation and Contaminant Immobilization. Agronomy 2022, 12, 405. https://doi.org/10.3390/agronomy12020405
Tsai C-C, Chang Y-F. Poultry Litter Biochar as a Gentle Soil Amendment in Multi-Contaminated Soil: Quality Evaluation on Nutrient Preservation and Contaminant Immobilization. Agronomy. 2022; 12(2):405. https://doi.org/10.3390/agronomy12020405
Chicago/Turabian StyleTsai, Chen-Chi, and Yu-Fang Chang. 2022. "Poultry Litter Biochar as a Gentle Soil Amendment in Multi-Contaminated Soil: Quality Evaluation on Nutrient Preservation and Contaminant Immobilization" Agronomy 12, no. 2: 405. https://doi.org/10.3390/agronomy12020405
APA StyleTsai, C.-C., & Chang, Y.-F. (2022). Poultry Litter Biochar as a Gentle Soil Amendment in Multi-Contaminated Soil: Quality Evaluation on Nutrient Preservation and Contaminant Immobilization. Agronomy, 12(2), 405. https://doi.org/10.3390/agronomy12020405







