Impact of Rotational Sequence Selection on Weed Seedbank Composition in Australian Broadacre Crops
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Establishment of Field Trials and Crop Harvest
2.3. Soil Collection and Weed Seedbank Assessment
2.4. Statistical Analysis
3. Results
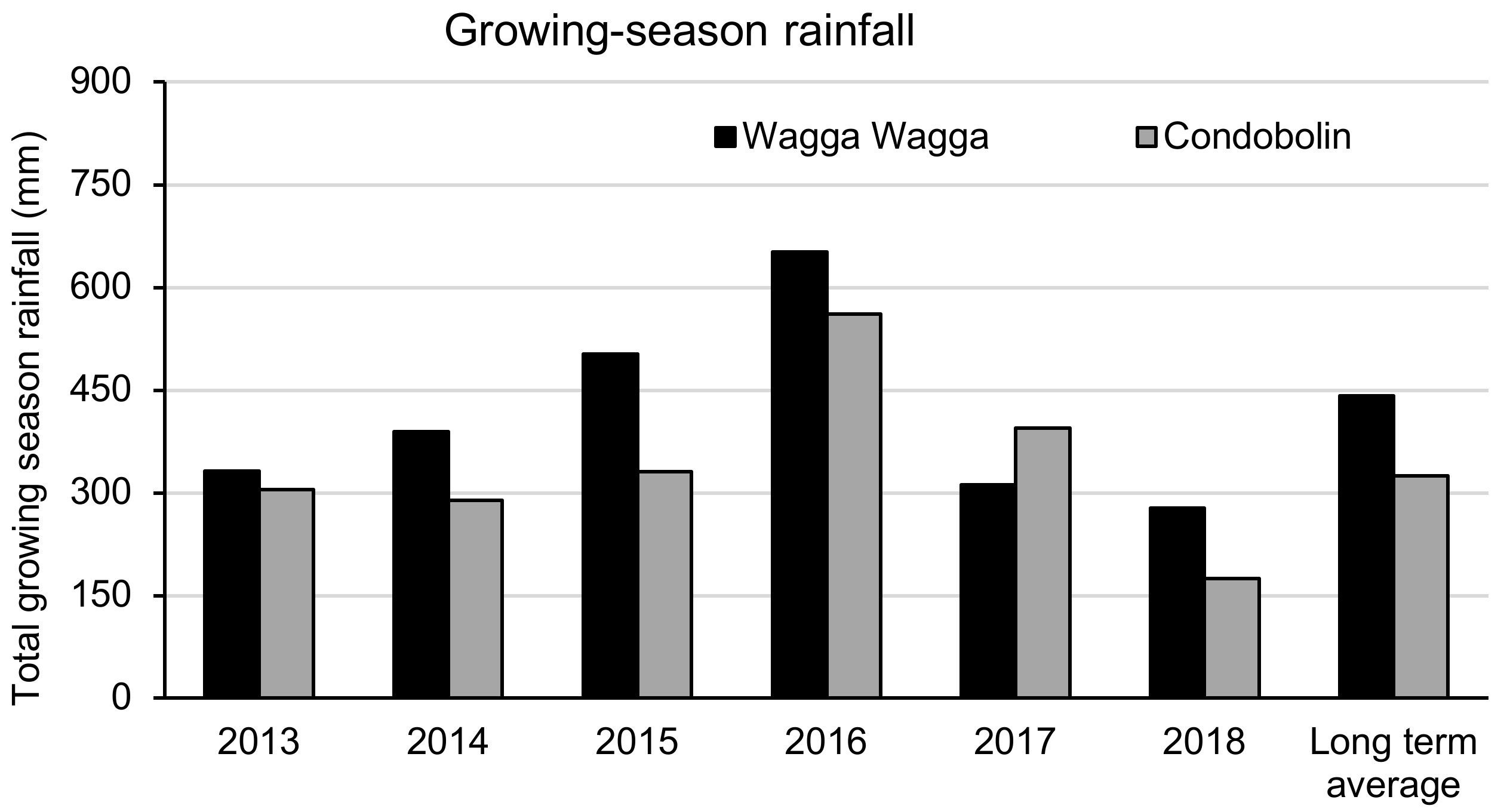
3.1. Climatic Conditions
3.2. The Impact of Crop Rotational Sequences on the Abundance and Diversity of Key Annual Weeds
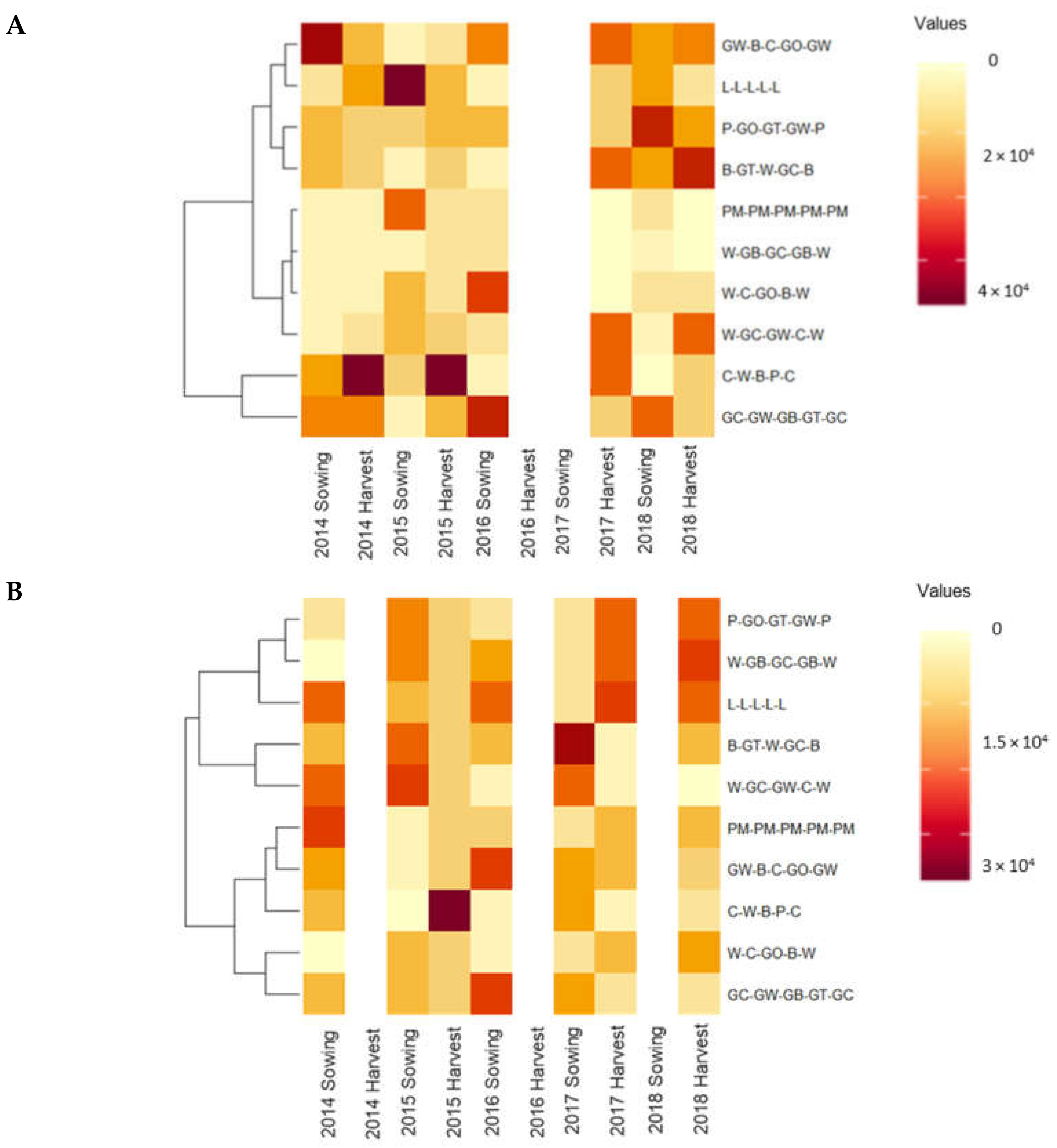
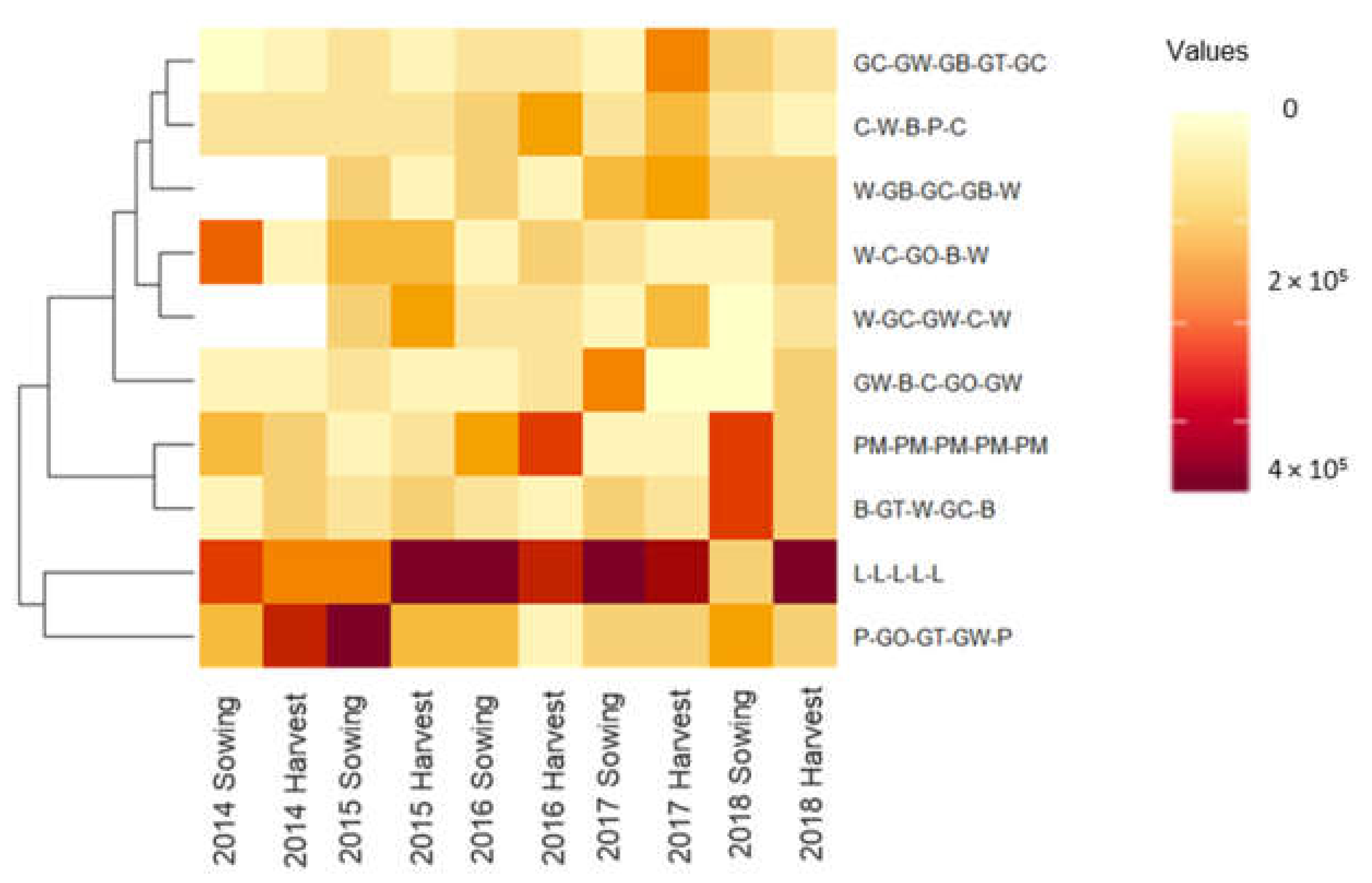
3.3. The Impact of Selected Crop Rotational Sequences on the Soil Weed Seedbank Density in Wagga Wagga
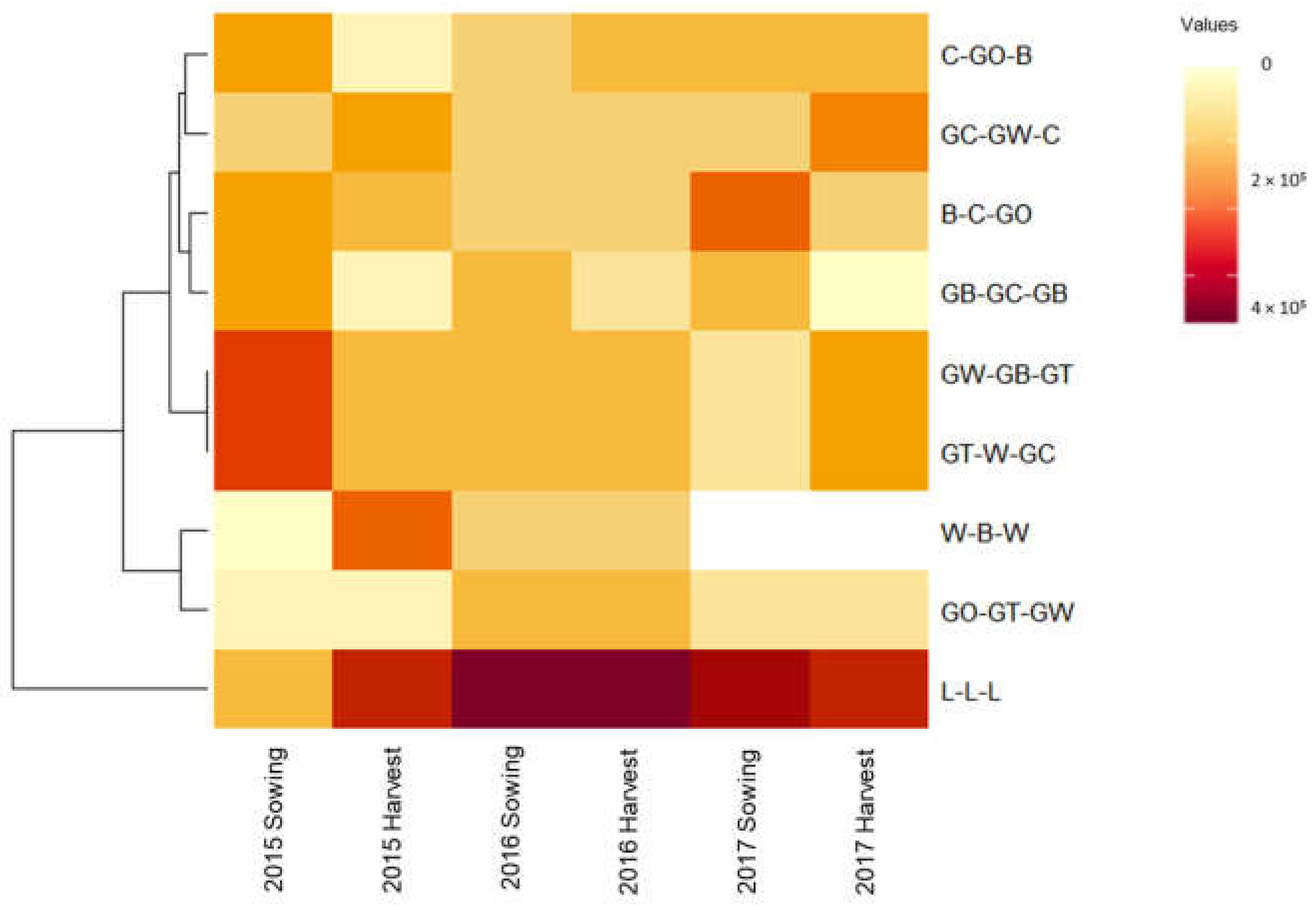
3.4. The Impact of Selected Crop Rotational Sequences on the Soil Weed Seedbank Density in Condobolin
3.5. Crop Yield
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, M.J.; Holland, J.F.; Bambach, R. Response of canola and Indian mustard to sowing date in the grain belt of north-eastern Australia. Aust. J. Exp. Agric. 2004, 44, 43–52. [Google Scholar] [CrossRef]
- Value of Agricultural Commodities Produced, Australia, 2019–2020 Financial Year | Australian Bureau of Statistics. Available online: https://www.abs.gov.au/statistics/industry/agriculture/value-agricultural-commodities-produced-australia/latest-release (accessed on 22 November 2021).
- Peters, R.; Sturz, A.; Carter, M.; Sanderson, J. Developing disease-suppressive soils through crop rotation and tillage management practices. Soil Tillage Res. 2003, 72, 181–192. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. In Legumes for Soil Health and Sustainable Management; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 487–510. [Google Scholar]
- Hufnagel, J.; Reckling, M.; Ewert, F. Diverse approaches to crop diversification in agricultural research. A review. Agron. Sustain. Dev. 2020, 40, 14. [Google Scholar] [CrossRef]
- Bell, M.; Seymour, N.; Stirling, G.R.; Stirling, A.M.; Van Zwieten, L.; Vancov, T.; Sutton, G.; Moody, P. Impacts of management on soil biota in Vertosols supporting the broadacre grains industry in northern Australia. Soil Res. 2006, 44, 433–451. [Google Scholar] [CrossRef][Green Version]
- Seymour, M.; Kirkegaard, J.; Peoples, M.B.; White, P.F.; French, R.J. Break-crop benefits to wheat in Western Australia—Insights from over three decades of research. Crop Pasture Sci. 2012, 63, 1–16. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef] [PubMed]
- Broster, J.C.; Pratley, J.E.; Ip, R.H.L.; Ang, L.-M.; Seng, K.P. A quarter of a century of monitoring herbicide resistance in Lolium rigidum in Australia. Crop Pasture Sci. 2019, 70, 283–293. [Google Scholar] [CrossRef]
- Owen, M.J.; Walsh, M.J.; Llewellyn, R.S.; Powles, S.B. Widespread occurrence of multiple herbicide resistance in Western Australian annual ryegrass (Lolium rigidum) populations. Aust. J. Agric. Res. 2007, 58, 711–718. [Google Scholar] [CrossRef]
- Bajwa, A.; Latif, S.; Borger, C.; Iqbal, N.; Asaduzzaman, M.; Wu, H.; Walsh, M. The Remarkable Journey of a Weed: Biology and Management of Annual Ryegrass (Lolium rigidum) in Conservation Cropping Systems of Australia. Plants 2021, 10, 1505. [Google Scholar] [CrossRef] [PubMed]
- Mwendwa, J.M.; Jeffrey, D.W.; Weston, L.A. The use of allelopathy and competitive crop cultivars for weed suppression in cereal crops. In Integrated Weed Management for Sustainable Agriculture; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; pp. 361–388. [Google Scholar]
- Mwendwa, J.; Brown, W.B.; Wu, H.; Weston, P.A.; Weidenhamer, J.D.; Quinn, J.; Weston, L. The weed suppressive ability of selected Australian grain crops; case studies from the Riverina region in New South Wales. Crop. Prot. 2018, 103, 9–19. [Google Scholar] [CrossRef]
- Schuster, M.Z.; Harrison, S.K.; de Moraes, A.; Sulc, R.M.; Carvalho, P.C.F.; Lang, C.R.; Anghinoni, I.; Lustosa, S.B.C.; Gastal, F. Effects of crop rotation and sheep grazing management on the seedbank and emerged weed flora under a no-tillage integrated crop-livestock system. J. Agric. Sci. 2018, 156, 810–820. [Google Scholar] [CrossRef]
- Broster, J.; Rayner, A.; Ruttledge, A.; Walsh, M.J. Impact of stripper fronts and chaff lining on harvest weed seed control. In Proceedings of the GRDC Grains Research Update, West Wyalong, Australia, 25 July 2018; pp. 55–61. [Google Scholar]
- Walsh, M.J.; Harrington, R.B.; Powles, S.B. Harrington Seed Destructor: A New Nonchemical Weed Control Tool for Global Grain Crops. Crop Sci. 2012, 52, 1343–1347. [Google Scholar] [CrossRef]
- Chauhan, B.; Singh, R.G.; Mahajan, G. Ecology and management of weeds under conservation agriculture: A review. Crop Prot. 2012, 38, 57–65. [Google Scholar] [CrossRef]
- Bertin, C.; Yang, X.; Weston, L.A. The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 2003, 256, 67–83. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Van der Meulen, A.; Chauhan, B. A review of weed management in wheat using crop competition. Crop. Prot. 2017, 95, 38–44. [Google Scholar] [CrossRef]
- Mwendwa, J.M.; Brown, W.B.; Weston, P.A.; Haque, K.M.S.; Preston, C.; Weston, L.A. Evaluation of selected commercial oilseed rape cultivars for early vigour, weed suppression and yield in southern New South Wales. Weed Res. 2020, 60, 450–463. [Google Scholar] [CrossRef]
- Weisberger, D.; Nichols, V.; Liebman, M. Does diversifying crop rotations suppress weeds? A meta-analysis. PLoS ONE 2019, 14, e0219847. [Google Scholar] [CrossRef]
- Latif, S.; Gurusinghe, S.; Weston, P.A.; Brown, W.B.; Quinn, J.C.; Piltz, J.W.; Weston, L.A. Performance and weed-suppressive potential of selected pasture legumes against annual weeds in south-eastern Australia. Crop Pasture Sci. 2019, 70, 147–158. [Google Scholar] [CrossRef]
- Kleemann, S.G.L.; Preston, C.; Gill, G.S. Influence of Management on Long-Term Seedbank Dynamics of Rigid Ryegrass (Lolium rigidum) in Cropping Systems of Southern Australia. Weed Sci. 2016, 64, 303–311. [Google Scholar] [CrossRef]
- Butkevičienė, L.; Skinulienė, L.; Auželienė, I.; Bogužas, V.; Pupalienė, R.; Steponavičienė, V. The Influence of Long-Term Different Crop Rotations and Monoculture on Weed Prevalence and Weed Seed Content in the Soil. Agronomy 2021, 11, 1367. [Google Scholar] [CrossRef]
- Adeux, G.; Munier-Jolain, N.; Meunier, D.; Farcy, P.; Carlesi, S.; Barberi, P.; Cordeau, S. Diversified grain-based cropping systems provide long-term weed control while limiting herbicide use and yield losses. Agron. Sustain. Dev. 2019, 39, 42. [Google Scholar] [CrossRef]
- Cordeau, S.; Baudron, A.; Farcy, P.; Vieren, E.; Smith, R.; Munier-Jolain, N.; Adeux, G. Legacy effects of contrasting long-term integrated weed management systems. Front. Agron. 2021, 3, 111. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 13 December 2021).
- Konopiński, M.K. Supplemental Information 7: R code. PeerJ 2020, 8, e9391. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Bureau of Meteorology. Available online: http://www.bom.gov.au/climate/data/index.shtml?bookmark=136&zoom=3&lat=-32.5355&lon=147.74&layers=B00000TFFFFFFFTFFFFFFFFFFFFFFFFFFFFTTT&dp=IDC10002-d (accessed on 13 December 2021).
- Mwendwa, J.M.; Brown, W.B.; Weidenhamer, J.D.; Weston, P.A.; Quinn, J.C.; Wu, H.; Weston, L.A. Evaluation of Commercial Wheat Cultivars for Canopy Architecture, Early Vigour, Weed Suppression, and Yield. Agronomy 2020, 10, 983. [Google Scholar] [CrossRef]
- Murphy, S.D.; Clements, D.; Belaoussoff, S.; Kevan, P.G.; Swanton, C.J. Promotion of weed species diversity and reduction of weed seedbanks with conservation tillage and crop rotation. Weed Sci. 2006, 54, 69–77. [Google Scholar] [CrossRef]
- Shah, K.K.; Modi, B.; Pandey, H.P.; Subedi, A.; Aryal, G.; Pandey, M.; Shrestha, J. Diversified Crop Rotation: An Approach for Sustainable Agriculture Production. Adv. Agric. 2021, 2021, 8924087. [Google Scholar] [CrossRef]
- Angus, J.F.; Kirkegaard, J.; Hunt, J.R.; Ryan, M.; Ohlander, L.; Peoples, M.B. Break crops and rotations for wheat. Crop. Pasture Sci. 2015, 66, 523–552. [Google Scholar] [CrossRef]
- Mahajan, G.; Walsh, M.; Chauhan, B.S. Junglerice (Echinochloa colona) and feather fingergrass (Chloris virgata) seed production and retention at sorghum maturity. Weed Technol. 2020, 34, 272–276. [Google Scholar] [CrossRef]
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, G.K. Do microorganisms influence seed-bank dynamics? Weed Sci. 2006, 54, 575–587. [Google Scholar] [CrossRef]
- Jourdan, M.; Vitou, J.; Thomann, T.; Maxwell, A.; Scott, J.K. Potential biological control agents for fumitory (Fumaria spp.) in Australia. In Proceedings of the XII International Symposium on Biological Control of Weeds, La Grande Motte, France, 22–27 April 2007; CABI: Wallingford, UK, 2008; pp. 160–164. [Google Scholar]
- Norton, G.M. Understanding the Success of Fumitory as a Weed in Australia. Charles Sturt University: Wagga Wagga, Australia, 2003. [Google Scholar]
- Chen, Y.; Zhu, X.; Loukopoulos, P.; Weston, L.A.; Albrecht, D.E.; Quinn, J.C. Genotypic identification of Panicum spp. in New South Wales, Australia using DNA barcoding. Sci. Rep. 2021, 11, 16055. [Google Scholar] [CrossRef] [PubMed]
- Gurusinghe, S.; Latif, S.; Brown, W.B.; Weston, P.A.; Weston, L.A. A useful in vitro bioassay for evaluation of weed suppression by legume cover crop residues. In Proceedings of the 21st Australasian Weeds Society, Manly, Australia, 9–13 September 2018. [Google Scholar]
- Schwartz-Lazaro, L.M.; Copes, J.T. A Review of the Soil Seedbank from a Weed Scientists Perspective. Agronomy 2019, 9, 369. [Google Scholar] [CrossRef]
- Nikolić, N.; Loddo, D.; Masin, R. Effect of Crop Residues on Weed Emergence. Agronomy 2021, 11, 163. [Google Scholar] [CrossRef]
- Weston, L.A.; Stanton, R.; Wu, H.; Mwendwa, J.; Weston, P.A.; Weidenhamer, J.; Brown, W.B. Comparison of grain crops and their associated residues for weed suppression in the southern Australian mixed farming zone. In Proceedings of the 19th Australasian Weeds Conference, Hobart, Australia, 1–4 September 2014; pp. 296–299. [Google Scholar]
- Piltz, J.W.; Morris, S.G.; Weston, L.A. Winter Forage Crop Harvest Time Impacts Regeneration of the Annual Weeds Barley Grass, Annual Ryegrass and Wild Radish. Agronomy 2021, 11, 1700. [Google Scholar] [CrossRef]
| Crop/pasture | Cultivar | Scientific name | Description | Identification key |
| Wheat | Suntop | Triticum aestivum L. | Grain | W |
| Wedgetail | Dual-purpose | GW | ||
| Barley | Hindmarsh | Hordeum vulgare L. | Grain | B |
| Urambie | Dual-purpose | GB | ||
| Oats | Echidna | Avena sativa L. | Dual-purpose | GO |
| Triticale | Endeavour | ×Triticosecale Wittmack | Dual-purpose | GT |
| Canola | GT-50 | Brassica napus L. | Grain | C |
| 971-CL | Dual- purpose | GC | ||
| Lucerne | SARDI | Medicago sativa L. | Grazing | L |
| Field pea | Twilight | Pisum sativum L. | Seed | P |
| Faba bean | IMI-3 | Vicia faba L. | Seed | F |
| Phalaris/ | Holdfast/ | Phalaris aquatic L. | Grazing | PM |
| Subterranean clover | Mintaro | Trifolium subterraneum L. | Grazing |
| Scientific name | Common name |
| Arctotheca calendula (L.) Levyns | capeweed |
| Atriplex spp. | saltbush |
| Bellis perennis L. | daisy |
| Billardiera fusiformis Labill. | bluebell |
| Bromus diandrus Roth. | brome grass |
| Capsella bursa-pastoris L. | shepherd’s purse |
| Cardamine hirsuta L. | bittercress |
| Chenopodium album L. | Lamb’s quarters |
| Conyza bonariensis L. | flaxleaf fleabane |
| Crassula sieberiana Domin. | stonecrop |
| Dysphania pumilio (R.Br.) Mosyakin & Clemants | crumb weed |
| Echium plantagineum L. | Paterson’s curse |
| Fumaria officinalis L. | fumitory |
| Gamochaeta calviceps (Fernald) Cabrera | cudweed |
| Hordeum leporinum Link | barley grass |
| Lolium rigidum Gaudin | annual ryegrass |
| Medicago lupulina L. | black medic |
| Panicum spp. | panic grass |
| Papaver rhoeas L. | poppy |
| Polygonum aviculare L. | wireweed |
| Raphanus raphanistrum L. | wild radish |
| Rumex brownii Campd. | rumex |
| Sedum acre L. | stone crop |
| Sisymbrium orientale L. | Indian hedge mustard |
| Sonchus oleraceus L. | sowthistle |
| Stellaria media (L.) Vill. | chickweed |
| Trifolium subterraneum L. | Subterranean clover |
| Vulpia myuros Rchb. | silver grass |
| Rotational crop sequence | 2014 | 2015 | 2016 | 2017 | 2018 | |||||
| Grass weed propagules/m3 | ||||||||||
| L-L-L-L-L | 25,431 | A | 19,540 | A | 30,460 | A | 57,471 | A | 17,816 | A |
| PM-PM-PM-PM-PM | 14,655 | A | 14,224 | A | 32,759 | AB | 8908 | C | 12,787 | AB |
| GC-GW-GB-GT-GC | 31,322 | A | 13,793 | A | 10,489 | ABC | 7328 | C | 13,506 | AB |
| GW-B-C-GO-GW | 23,994 | A | 12,213 | A | 6609 | ABC | 50,719 | AB | 14,512 | AB |
| W-C-GO-B-W | - | 15,948 | A | 8046 | ABC | 14,224 | BC | 12,787 | AB | |
| W-GC-GW-C-W | 17,960 | A | 28,879 | A | 4897 | ABC | 5604 | C | 6,465 | B |
| C-W-B-P-C | 41,523 | A | 12,213 | A | 18,247 | ABC | 6753 | C | 7,471 | B |
| B-GT-W-GC-B | 14,655 | A | 29,742 | A | 4885 | BC | 26,437 | ABC | 15,373 | AB |
| P-GO-GT-GW-P | 14,081 | A | 28,305 | A | 3592 | C | 11,925 | BC | 22,126 | A |
| W-GB-GC-GB-W | - | 17,816 | A | 1868 | C | 31,322 | ABC | 15,948 | AB | |
| LSD (p = 0.05) between rotational treatments in each year | ns | ns | 9321 | 7980 | 16,662 | |||||
| Broadleaf weed propagules/m3 | ||||||||||
| L-L-L-L-L | 147,270 | A | 181,322 | A | 251,494 | A | 235,201 | A | 193,678 | A |
| PM-PM-PM-PM-PM | 105,747 | A | 60,058 | B | 160,632 | ABC | 60,345 | B | 245,402 | A |
| GC-GW-GB-GT-GC | 64,081 | A | 65,517 | B | 59,310 | BC | 151,006 | AB | 98,850 | AB |
| GW-B-C-GO-GW | 73,276 | A | 67,098 | B | 47,126 | BC | 78,017 | AB | 60,632 | B |
| P-GO-GT-GW-P | 160,506 | A | 237,500 | A | 143,391 | AB | 126,581 | AB | 128,736 | AB |
| W-C-GO-B-W | - | 110,920 | AB | 30,172 | C | 100,719 | AB | 76,150 | AB | |
| W-GB-GC-GB-W | - | 88,075 | AB | 51,868 | C | 135,920 | AB | 116,810 | AB | |
| B-GT-W-GC-B | 98,563 | A | 58,764 | B | 71,034 | ABC | 100,011 | AB | 185,776 | A |
| W-GC-GW-C-W | 117,385 | A | 87,357 | AB | 40,086 | C | 131,466 | AB | 75,144 | B |
| C-W-B-P-C | 62,356 | A | 69,540 | B | 116,092 | ABC | 129,598 | AB | 79,310 | B |
| LSD (p = 0.05) between rotational treatments in each year | ns | 25,332 | 26,082 | 44,944 | 28,777 | |||||
| Rotational crop sequence | 2015 | 2016 | 2017 | |||
| Grass weed propagules/m3 | ||||||
| B-C-GO | 8621 | A | 144 | A | 4023 | A |
| C-GO-B | 8046 | AB | 1149 | A | 3448 | A |
| GB-GC-GB | 5747 | AB | 0 | A | 2730 | A |
| GC-GW-C | 5316 | AB | 0 | A | 9626 | A |
| GO-GT-GW | 6035 | AB | 287 | A | 3305 | A |
| GT-W-GC | 7471 | AB | 1437 | A | 3592 | A |
| GW-GB-GT | 6609 | AB | 287 | A | 5029 | A |
| L-L-L | 3305 | B | 287 | A | 7471 | A |
| W-B-W | 5460 | AB | 144 | A | 5188 | A |
| LSD (p = 0.05) between rotational treatments in each year | 1354 | ns | ns | |||
| Broadleaf weed propagules/m3 | ||||||
| L-L-L | 191,092 | A | 413,362 | A | 96,552 | A |
| B-C-GO | 181,178 | A | 92,098 | B | 65,517 | AB |
| C-GO-B | 161,063 | A | 117,529 | B | 52,586 | AB |
| GB-GC-GB | 164,224 | A | 83,908 | B | 41,810 | B |
| GC-GW-C | 151,437 | A | 104,023 | B | 46,839 | B |
| GO-GT-GW | 106,465 | A | 126,868 | B | 36,207 | B |
| GT-W-GC | 167,960 | A | 124,466 | B | 33,477 | B |
| GW-GB-GT | 205,891 | A | 118,678 | B | 43,678 | B |
| W-B-W | 113,219 | A | 110,345 | B | - | |
| LSD (p = 0.05) between rotational treatments in each year | ns | 29,104 | 55,864 | |||
| Location | Crop rotational sequence | 2014 | 2015 | 2016 | 2017 | 2018 |
| Wagga Wagga | B-GT-W-GC-B | - | 2.5 | 4.6 | 0.0 | 1.1 |
| C-W-B-P-C | - | 1.1 | 5.1 | 0.4 | 1.6 | |
| GC-GW-GB-GT-GC | - | 0.9 | 5.0 | 1.3 | 0.8 | |
| GW-B-C-GO-GW | - | 1.7 | 1.3 | 0.6 | 0.1 | |
| L-L-L-L-L | NA | NA | NA | NA | NA | |
| P-GO-GT-GW-P | - | 3.4 | 1.6 | 0.9 | 1.5 | |
| PM-PM-PM-PM-PM | NA | NA | NA | NA | NA | |
| W-C-GO-B-W | - | 2.1 | 2.8 | 1.8 | 0.7 | |
| W-GB-GC-GB-W | - | 2.6 | 1.3 | 1.1 | 0.1 | |
| W-GC-GW-C-W | - | 2.0 | 4.6 | 0.0 | 0.7 | |
| Condobolin | B-C-GO | 1.8 | 1.6 | 0.1 | ||
| C-GO-B | - | 3.4 | 0.3 | |||
| GB-GC-GB | 1.7 | 0.1 | - | |||
| GC-GW-C | 0.0 | 3.5 | 0.1 | |||
| GO-GT-GW | 0.0 | 1.4 | 0.4 | |||
| GT-W-GC | 0.1 | 3.5 | - | |||
| GW-GB-GT | 0.3 | 3.4 | 0.2 | |||
| L-L-L | NA | NA | NA | |||
| W-B-W | 1.2 | 3.7 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurusinghe, S.; Haque, K.M.S.; Weston, P.A.; Brown, W.B.; Weston, L.A. Impact of Rotational Sequence Selection on Weed Seedbank Composition in Australian Broadacre Crops. Agronomy 2022, 12, 375. https://doi.org/10.3390/agronomy12020375
Gurusinghe S, Haque KMS, Weston PA, Brown WB, Weston LA. Impact of Rotational Sequence Selection on Weed Seedbank Composition in Australian Broadacre Crops. Agronomy. 2022; 12(2):375. https://doi.org/10.3390/agronomy12020375
Chicago/Turabian StyleGurusinghe, Saliya, K. M. Shamsul Haque, Paul A. Weston, William B. Brown, and Leslie A. Weston. 2022. "Impact of Rotational Sequence Selection on Weed Seedbank Composition in Australian Broadacre Crops" Agronomy 12, no. 2: 375. https://doi.org/10.3390/agronomy12020375
APA StyleGurusinghe, S., Haque, K. M. S., Weston, P. A., Brown, W. B., & Weston, L. A. (2022). Impact of Rotational Sequence Selection on Weed Seedbank Composition in Australian Broadacre Crops. Agronomy, 12(2), 375. https://doi.org/10.3390/agronomy12020375








