Abstract
Neofusicoccum parvum and Rhizoctonia solani are fungal pathogens with an increasing incidence in young grapevine plants. In this study, the antagonistic potential of some strains of the genus Trichoderma isolated from grapevine against these pathogens was investigated at the laboratory and greenhouse levels. In-plate confrontation assays showed that the selected Trichoderma strains could inhibit the mycelial growth of both taxa, being more effective against N. parvum. In the in vivo assays, the biocontrol activity of the mentioned strains against the pathogens, when applied either simultaneously or successively, was tested on both grafted plants and seedlings germinated from seed. The effectiveness of the treatments was evaluated by comparing biomass weight and vascular rot lengths data. In seedling trials, successive treatments resulted in higher root development and a lower colonization rate of the pathogens, especially against R. solani. In grafted plants, some disparity was observed against N. parvum: simultaneous treatments resulted in higher aerial biomass, but successive treatments resulted in higher root biomass and lower necrosis. Against R. solani, simultaneous treatments were clearly more effective, with higher root and aerial length values and lower necrosis. The obtained data suggest that the use of Trichoderma spp. isolates can constitute an alternative to conventional fungicides to control certain grapevine wood diseases.
1. Introduction
One of the main challenges that modern viticulture faces is the control of fungal pathogens that cause grapevine trunk diseases (GTDs) [1]. These diseases decrease crop yields, limit grape and wine qualities, and reduce plant lifespan in many growing areas worldwide [2]. Moreover, they have a significant economic impact on the establishment of new plantations, as it has been estimated that the annual cost of replanting dead plants due to GTDs globally is 1132 M EUR [3]. The present study focuses on two pathogens associated with GTDs with a high incidence on young grapevine plants from nursery stock: Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L. Phillips and Rhizoctonia solani J.G. Kühn.
Neofusicoccumparvum is currently considered one of the most frequent and aggressive agents associated with the “black dead arm” or “Botryosphaeria dieback” disease, colonizing the woody tissue through wounds, being able to remain for a long time as a latent endophyte in these tissues, and eventually causing internal cankers, leaf chlorosis, and necrosis [4]. Rhizoctonia solani, a well-known polyphagous soil-borne pathogen, usually appears as an increasing problem in grapevine nurseries, mainly associated with basal root and stem base rots, either in rootstocks or grafted plants produced in these facilities [5,6].
Currently, there are no completely effective measures for the elimination and/or control of fungi associated with GTDs [3]. With the withdrawal from official registers of numerous active materials of chemical origin, usually effective against these etiological agents, the current context of Integrated Pest Management (IPM) requires joining research efforts in aspects such as improving the sanitary quality of the propagation material, the reduction of practices that favor the spread and extension of infections of these fungi in the vineyard through improvements in management techniques, and the development and increased use of biological control agents (BCAs) [7].
Some of the best known and commercialized BCAs, also used to control GTDs, are species of the genus Trichoderma Pers. (Hypocreales, Ascomycetes), widely cited as an antagonist of numerous plant pathogens [8]. Their biocontrol mechanism is considered to be mixed, and it is mainly based on an active hyperparasitism behavior, together with the production of lytic enzymes, antimicrobial substances, and other secondary metabolites with germicidal action [9]. However, it has been shown that the different species of Trichoderma also present other positive effects on their host, improving growth rates and inducing plant defense responses, being considered a very effective biofertilizer [10]. In this sense, it has been demonstrated that the enzymes produced by the species of this genus, cause the release of oligosaccharides of low molecular weight that induce resistance [11].
In the case of GTDs, most products made up of Trichoderma-based formulations for the control of wood diseases are usually applied—by spraying—for wound protection shortly after pruning, at the time of the vegetative arrest of the vine or bleeding. The major obstacle for the extension and massive adoption of this type of treatment has been its variable effectiveness in field conditions, attributable to various factors, such as the phenological stage at which the treatment is carried out, the mode of application, the time interval between pruning and treatment, the climatic conditions during and after application, the level of incidence of GTDs in the treated vineyard, or the geographical origin of the grapevine used [12]. In this sense, many authors have pointed out that it is essential to complement the use of Trichoderma spp. with good management practices in the vineyard (less invasive pruning methods, restriction and elimination of potential inoculum sources, good nutritional balance of the vine, etc.) [13]. Different Trichoderma species have been evaluated against grapevine wood pathogens such as Eutypa lata (Pers.) Tul. & C. Tul., Phaeomoniella chlamydospora (W. Gams, Crous, M.J. Wingf. & Mugnai) Crous & W. Gams, Phomopsis viticola (Sacc.) Sacc., and different species of the family Botryosphaeriaceae [11,14,15]. However, independently of the level of protection against these species, a variable control with Trichoderma spp. was demonstrated, which was notably increased when the pathogens were inoculated shortly after applying the biocontrol agent [16].
This work has aimed to evaluate the antagonistic potential of different isolates of the genus Trichoderma previously isolated as endophytes from grapevine plants against Neofusicoccum parvum and Rhizoctonia solani through in vitro and in vivo assays on young plants in the greenhouse, in order to provide an alternative, benign, and environmentally safe control method.
2. Materials and Methods
2.1. Fungal Isolates
The selected pathogens used in the study were Neofusicoccum parvum and Rhizoctonia solani, while the antagonistic microorganisms were five different strains of the species Trichoderma harzianum Rifai, all of them conserved at the mycotheca of the Mycology Lab of the Plant Protection Unit at Centro de Investigación y Tecnología Agroalimentaria de Aragón (CITA), previously isolated as endophytes and/or pathogens of grapevine plants coming from both healthy and diseased samples of vineyards of Aragón (NE Spain) and identified at the morphological and molecular level. They were recovered from −85 °C cryovials and refreshed in Petri dishes with potato dextrose agar (PDA) for subsequent use in the bioassays.
2.2. In Vitro Assays of Mycelial Growth Inhibition
To evaluate the antagonistic effects of the selected Trichoderma strains, confrontation assays in plates were carried out, determining the percentage of inhibition of the fungal growth exerted by the protective isolates against the aforementioned grapevine pathogens.
The confrontations of antagonistic isolates and pathogens were performed using the dual culture technique in 9 cm diameter Petri dishes containing PDA as a nutrient medium. A 4 mm diameter agar block with mycelium coming from the margin of a fresh colony (4–5 days) of each pathogen was placed at one end of the plate (at 1 cm from its margin), and at the opposite end, the same operation was performed with the Trichoderma antagonists under the same conditions. Three replicates were made for each confrontation. For the controls, 4 mm diameter discs of pathogens and antagonists were individually sowed in the center of plates with the mentioned PDA medium. They were incubated for ten days at 25 °C in the dark, measuring the growth radius of pathogens and biocontrol agents every 24 h with a precision digital caliper.
The evaluation of the in vitro antagonistic effect was calculated using the formula of the Percentage of Inhibition of Radial Growth (PIRG) [17]: PICR = ((R1 − R2)/R1) × 100, where R1 = radius of the pathogen in control plate and R2 = radius of the pathogen in confrontation plate.
2.3. Maintenance, Preservation, and Production of Fungal Inocula
Pure cultures of N. parvum, R. solani, and several strains of T. harzianum were maintained in Petri dishes of 12 cm diameter with PDA in a bacteriological oven at 25 °C in the dark, performing periodic replicates of each of the isolates to maintain fresh colonies.
Fungal inocula of both R. solani isolates employed in grafted plants and seedlings and N. parvum inocula in seedlings were made on a formulation based on cereal grains previously colonized by the aforementioned fungal taxa. For this purpose, organic wheat grains were used. Briefly, for pre-sterilization of the wheat grains, they were washed in a flask several times with sterilized bi-distilled water, then covered with the same type of water, incorporating streptomycin sulfate (0.3 g·L−1) to prevent the development of bacterial contaminants, and left overnight in a refrigerator at 4 °C. Then, water was removed, and the flask was autoclaved (120 °C, 20 min). Afterwards, it was rewashed and drained. The grains were autoclaved two more times before use. Finally, sterilized grains were placed on 12 cm diameter Petri dishes and inoculated with 10–15 agar plugs from a fresh culture of each fungus. The plates were incubated for 5–7 days at 25 °C in the darkness. After this period, the fungi could colonize the whole grain content uniformly.
On the other hand, to obtain conidial solutions of the different strains of T. harzianum employed, each of them was inoculated in quintuplicate (4 mm diameter agar plugs) in 12 cm diameter PDA plates and incubated at 25 °C in the darkness until a profuse production of conidia was observed on the surface of the colonies. To obtain the conidial solution, the propagules were harvested. For this, sterile bi-distilled water was poured into each plate, completely covering each colony, and plates were sealed with ParafilmTM. After this, the plates were shaken to detach the conidia, and the aqueous solution containing the spores was recovered with a syringe. Subsequently, the titration and adjustment of the different conidial solutions were carried out using a hematocytometer at a final concentration of 4.5 × 106 conidia·mL−1 for each isolate.
2.4. Greenhouse Bioassays with Plants
To scale up the results of the in vitro tests, in vivo trials were carried out on two types of plant material: seedlings produced from germinated seeds and grafted grapevine plants from a commercial nursery. Inoculation was performed on 105 one-year-old grafted plants of the variety ‘Tempranillo’ (Clone 151) grafted on rootstock 110-R, and on 102 seedlings of the same variety, obtained from an organic vineyard in Bespén (Huesca, Spain). The method used for seed germination consisted in soaking the seeds in commercial sodium hypochlorite diluted at 50% for 24 h, then rinsing them with sterile water and discarding those that floated. Then, the seeds were treated with gibberellic acid (1000 ppm), mixing it with 1 mL of 95% ethanol and distilled water for 24 h. The seeds were then rinsed with sterile water and allowed to dry on filter paper. After this, a seedbed was arranged.
The grafted plants and seedlings were planted in plastic pots of 3.5 and 0.3 L, respectively, containing a substrate of commercial peat and sterilized natural soil from a vineyard in Bespén (Huesca, Spain) in a 2:1 ratio.
For five months, plants were kept in the greenhouse with manual irrigation when needed (2–3 times per week). Three inoculation modalities were used according to the type of pathogen and/or the type of action of the biocontrol agent used, carrying out both successive (inoculating first the antagonist and five days later the pathogen) and simultaneous treatments (inoculating both types of fungi at the same time). Six replicates were arranged for each pathogen/antagonist combination, three of them being of the successive inoculation type and the other three of the simultaneous inoculation one, together with three positive controls inoculated only with pathogens, three negative controls that incorporated only the propagation material of the fungus according to the type of inoculation (agar block, wheat grain, and/or distilled water), and other three negative controls of each strain of Trichoderma (15 pots in total), as shown in Table 1.

Table 1.
Summary of the experimental assays carried out for each pathogen and T. harzianum strain.
2.4.1. Inoculation of Trichoderma harzianum against Neofusicoccum parvum in Grafted Grapevine Plants
Inoculations were performed on the stem at two separate points below the graft union. Agar plugs from fresh PDA cultures of each fungus were used as fungal inoculum. At the defined points of each grafted plant (2 per individual), slits were made (with a scalpel) about 5 mm in diameter and 5 mm deep. After this, 4 mm diameter agar discs previously colonized by each fungus were inoculated and placed in such a way that the mycelium was in contact with the incision of the stem. The discs were covered with sterile cotton wool moistened in sterile bi-distilled water and sealed with ParafilmTM.
2.4.2. Inoculation of Trichoderma harzianum against Rhizoctonia solani in Grafted Grapevine Plants
The inoculations were carried out at root level, in a different way according to the incorporated strain: (1) the treatment of the grafted plants with Trichoderma strains was carried out by root immersion, and (2) the artificial infection with R. solani was carried out inoculating the culture substrate of the grafted plants with the pathogen previously propagated in solid medium (colonized wheat grains). For the different strains of T. harzianum, the roots of the tested plants were immersed in a series of conidial solutions of the mentioned antagonistic fungi previously adjusted to 4.5 × 106 conidia·mL−1 for 2–2.5 h, before being transplanted to the pot. For the pathogen (R. solani), the grafted plants were infected upon contact with the potting substrate containing mixed wheat grains previously colonized by the fungus, at a dose of 15 g of colonized wheat per 2 L of the growing substrate.
2.4.3. Inoculation of Trichoderma harzianum against Neofusicoccum parvum and Rhizoctonia solani in Grapevine Seedlings Germinated from Seeds
The inoculations with the different strains of Trichoderma were carried out through root immersion of the seedlings in a conidial suspension of Trichoderma previously adjusted to 4.5 × 106 conidia·mL−1 for 3–5 min. In the case of the pathogens (R. solani and N. parvum), they infected the seedlings (previously protected or not with Trichoderma, depending on whether they were inoculated successively or simultaneously) by contact with the culture substrate containing wheat grains previously colonized by each pathogen strain in question, at a dose of 2.5 g per pot (330 mL) (grams of colonized wheat per liter of growth substrate).
Five months after inoculation, both types of grapevine plants were removed, and, in the case of grafted plants, each one was measured longitudinally (root, aerial, and total biomass). After this, sections of them were cut longitudinally, and the length of the lesions caused by the pathogens (tracheomycosis) was evaluated. For this purpose, the extent of the vascular lesions was measured longitudinally at both sides of the inoculation point in the case of artificial infection with N. parvum (analyzing lower, upper, and total necrosis). In contrast, in the R. solani trial, only the length of the basal necrosis (coming up from the root crown) was measured since this pathogen was inoculated by root infection. In this way, the extension of the different vascular lesions was analyzed and statistically compared as a function of if the grafted plants had been protected with Trichoderma in successive or simultaneous inoculation. All necrosis measurements were compared with those obtained for the controls. In the case of bioassays on seedlings, in addition to the longitudinal measurements of the root, aerial, and total biomass, the total fresh/dry weight of aerial and root biomass were analyzed. Finally, in grafted plants, after carrying out the above-mentioned measurements, pathogens previously inoculated were isolated (from over 87% of the inoculated plants) from the decayed vascular tissues in order to fulfil Koch’s postulates.
2.5. Statistical Analysis
When the requirements of homogeneity and homoscedasticity were met, the results of the in vitro mycelial growth inhibition tests were statistically analyzed using ANOVA analysis, followed by comparisons of the measurements by Tukey’s test (p < 0.05). For greenhouse bioassay results in which the requirements of normality and homoscedasticity were not met, the Kruskal–Wallis non-parametric test was used instead, accompanied by pairwise comparison using Dunn’s and Conover–Iman’s methods, applying the Bonferroni correction. IBM SPSS STATISTICS software was used for all statistical analyses.
3. Results
3.1. In Vitro Mycelial Growth Inhibition Tests
In general terms, the presence of high PIRG values (Table 2) was correlated with the ability of a given isolate to inhibit the development of a pathogen. The results showed that the different T. harzianum isolates used were more effective in inhibiting the mycelial growth of N. parvum (with PIRG values between 44.65 and 51.32%) than that of R. solani, for which PIRG values between 20.57 and 28.04% were recorded, and even negative PIRG percentages (−3.47%) were obtained in some cases (T. harzianum isolate 1).

Table 2.
PIRG values (mean ± standard deviation) obtained in each dual plate confrontation, for the two pathogens (N. parvum and R. solani) against 5 T. harzianum isolates, after 7 days of culture.
The data obtained for both pathogens suggest that the isolate with the best antagonistic behavior in plate assays against both pathogens would be the strain T. harzianum 2.
Along with the observed mycelial growth inhibition values, the presence of hyphal structures related to the presence of active hyperparasitism was verified. As a result of this, different types of interactions usually described in the confrontation of species of the genus Trichoderna against other fungal taxa were observed. Some of these included the formation of penetration structures, papilla-like bodies, appressoria, lysis of pathogen hyphae, profuse sporulation, intracellular growth, or the presence of typical coil hyphae (Figure 1 and Figure 2).
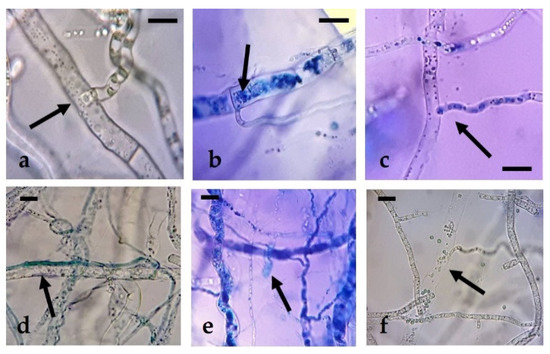
Figure 1.
Hyphal interactions of T. harzianum with N. parvum in dual plate confrontations: (a–c): penetration structures; (d) appressorium; (e) papilla-like structures; (f) hyphal lysis. Bars = 10 µm.
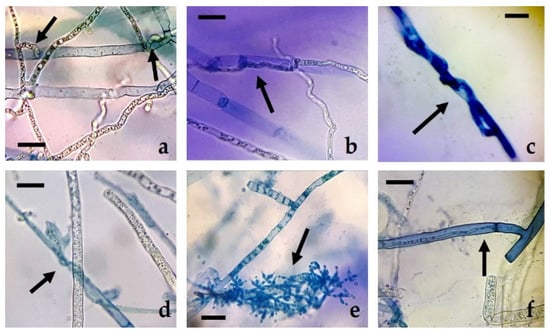
Figure 2.
Hyphal interactions of T. harzianum with R. solani in dual plate confrontations: (a) penetration structures; (b) appressorium; (c,d) coiling hyphae; (e) sporulation; (f) intracellular growth. Bars = 10 µm.
3.2. In Planta Bioassays
3.2.1. Control of Neofusicoccum parvum and Rhizoctonia solani with Trichoderma harzianum Isolates in Grapevine Seedlings Germinated from Seed
In biocontrol trials with seedlings, the protective effect of successive treatments with the T. harzianum antagonist strains resulted in higher root development and dry weight (compared to controls) and a lower colonization rate of the pathogen, especially against R. solani. The results suggest the convenience of incorporating the antagonist a few days before inoculation of the pathogen.
For N. parvum, in general, and regardless of the statistical significance, the highest dry weight values were obtained in all successive treatments with T. harzianum strains, except in the case of T. harzianum isolate 4 (which obtained better values in simultaneous inoculation). In the case of R. solani, it was also observed that the dry weight values of the successive treatments based on the different isolates of T. harzianum were higher than the rest, except for the controls (i.e., plants without inoculation of any microorganism) (Table 3).

Table 3.
Total dry weight of seedlings in grams (mean ± standard deviation) for each type of treatment (successive, simultaneous, and controls with T. harzianum, N. parvum, R. solani, and uninoculated wheat) obtained for each T. harzianum strain.
3.2.2. Control of Neofusicoccum parvum with Trichoderma harzianum in Grafted Grapevine Plants
The results of the biological control tests in grafted plants against N. parvum were shown to be heterogeneous, provided that—contrary to what was expected—no correlation was found between the increase (comparing with controls) of aerial biomass and the decrease of necrosis lengths in successive treatments versus simultaneous inoculations.
In this sense, it should also be noted that, regarding the length of the aerial biomass, in general, the simultaneous treatments led to higher aerial tissues lengths than the successive treatments, although none of the interactions was statistically significant (Table 4).

Table 4.
Aerial and root biomass lengths of grafted plants in cm (mean ± standard deviation) for each type of treatment (successive or simultaneous inoculation with N. parvum and T. harzianum, and controls with plant inoculated only with agar) as a function of the T. harzianum isolate used.
It was found that the only statistically significant differences in total necrosis values corresponded to those bioassays in which T. harzianum isolate 4 was employed. Nonetheless, when lower necrosis lengths were separately analyzed, statistically significant differences were also detected for T. harzianum isolate 2. The necrosis measurements (Table 5) obtained for the simultaneous treatments were higher than those of the successive treatments (except for the treatment with T. harzianum 3 in the lower necrosis), with a higher incidence of necrosis occurring when the antagonists were inoculated at the same time as N. parvum. As expected, thus validating the inoculation method, the highest necrosis length was found in the N. parvum pathogen control.

Table 5.
Total necrosis and lower necrosis of grafted plants in cm (mean ± standard deviation) for each type of treatment (successive or simultaneous inoculation with N. parvum and T. harzianum, and controls with plant inoculated only with agar) for each T. harzianum strain.
3.2.3. Control of Rhizoctonia solani with Trichoderma harzianum in Grafted Grapevine Plants
In these trials, it was found that the simultaneous treatments of both pathogen and protective isolates led to higher root and aerial biomass lengths than those of the controls, although these were not statistically significant (Table 6). Furthermore, in the aerial tissues length values, the treatments with T. harzianum isolates 3 and 4—both in simultaneous and successive inoculation—resulted in higher lengths than those observed for the R. solani control, slightly promoting the growth of the grafted plants.

Table 6.
Total aerial and root biomass length of grafted plants in cm (mean ± standard deviation) for each type of treatment (successive or simultaneous inoculation with R. solani and T. harzianum, and controls with plants inoculated only with wheat and dipped in distilled water) as a function of the T. harzianum isolate used.
When comparing internal vascular necrosis length measurements, simultaneous treatments seemed to work better against R. solani. In this sense, the best tracheomycosis inhibition data corresponded to T. harzianum isolate 4 (Table 7).

Table 7.
Basal vascular necrosis length of grafted plants in cm (mean ± standard deviation) for each type of treatment (successive or simultaneous inoculation with R. solani and T. harzianum, and controls with plants inoculated only with wheat and submerged in distilled water) as a function of the T. harzianum isolate used.
3.2.4. Trichoderma harzianum as a Growth Promoter
To evaluate the capacity as plant growth promoters of the T. harzianum isolates under study, the controls of these isolates (both in seedlings and in grafted plants inoculated with the five protective strains) were compared with the control plants without any treatment. This type of comparison was carried out because the potential of certain species of the genus Trichoderma as plant growth promoters has been widely demonstrated and documented, resulting in an increase in the size of the plants even in the absence of any pathogen [18].
In the case of seedlings, only T. harzianum isolate 5 significantly promoted root growth of treated plants compared to non-inoculated controls (Figure 3).

Figure 3.
Growth promotion effects of Trichoderma strains: (a) control seedling inoculated with T. harzianum 2; (b) control seedling inoculated with T. harzianum 1; (c) control seedling inoculated with T. harzianum 5; (d) control seedling not inoculated.
In grafted plants, an increase in plant growth rates was detected in the controls inoculated with T. harzianum, especially in the roots, regardless of the statistical significance (Table 8). Thus, the different isolates of T. harzianum employed promoted and increased growth (in biometric terms) compared with the control plants. In the case of total biomass length measurements, T. harzianum isolates 1 and 5 obtained the best values, which were also statistically significant. Regarding root length, T. harzianum isolates 1 and 4 (whose interaction was also statistically significant) stood out, followed by the values obtained with the application of T. harzianum isolate 5.

Table 8.
Growth promotion effects of Trichoderma strains. Total, aerial, and root biomass lengths in cm (mean ± standard deviation) of grafted plant controls treated with T. harzianum and untreated (agar only). Data are referred to the experiment of protection against N. parvum.
4. Discussion
4.1. In Vitro Mycelial Growth Inhibition
Plate confrontation assays demonstrated the ability of T. harzianum strains to inhibit the mycelial growth of the two selected grapevine pathogens. In general terms, the highest inhibition values were recorded against N. parvum, representing almost twice those recorded against R. solani. Numerous studies have evaluated in vitro the antagonistic potential of several Trichoderma species, a well-known group of hyperparasitic mitosporic fungi [19,20]. In this sense, the values obtained in the present study seem to be relatively discrete in comparison with PIRG data obtained from the confrontations of species of the mentioned genus against other plant pathogens [21,22,23], in which the average rates of inhibition of microbial growth in dual confrontations with T. harzianum were usually higher than 50%, sometimes reaching PIRG values above 99%.
One possible explanation is that both N. parvum and R. solani species have simple nutritional requirements and usually show high-speed growth rates. This is especially true for isolates of the genus Rhizoctonia, a polyphagous basidiomycete with great colonizing capacity and very high vegetative growth rates (with practically absent reproductive mechanisms) [24]. This would hinder its inhibition by other antagonists, especially by those that, like Trichoderma, base their mode of action mainly on the colonization and parasitism of the mycelium of the pathogen to be controlled, always accompanied by an effective occupation of the culture medium. Thus, in the case of R. solani, the presence of hyperparasitism phenomena (enzymatic lysis of the mycelium of R. solani) and sporulation of the different isolates of Trichoderma on the mycelium of the pathogen can be observed, but not beyond half of the Petri dish (Figure 4), due to the rapid growth and colonization capacity of R. solani, with rates similar to those of Trichoderma. Siameto et al. [25], in a study on the growth inhibition of selected soil pathogens in Africa, found that T. harzianum inhibited the growth of R. solani with the highest PIRG being 61.55%, while the lowest was 25.88%; and, according to Guedez et al. [26], in a study on the in vitro growth-inhibitory activity of different strains of T. harzianum against R. solani and two other tomato pathogens, rates between 62 and 72% were recorded for inhibition against this fungus, well above those obtained here. However, the aggressiveness, virulence, or speed of colonization of isolates of a species as biologically complex as R. solani largely depends on the type of anastomosis group and/or subgroup in question [24,26].
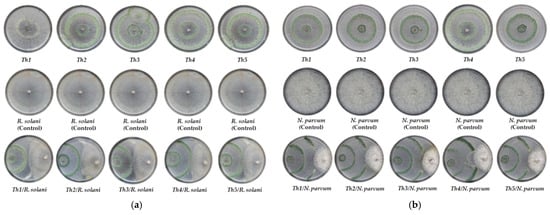
Figure 4.
Dual plate confrontations of Trichoderma harzianum strains against the selected pathogens. (a) Rhizoctonia solani. Top row: control plates of the different strains of T. harzianum; middle row; control plates of R. solani; bottom row: dual confrontations with each of the protective strains; (b) Neofusicoccum parvum. Top row: control plates of the different strains of T. harzianum; middle row; control plates of N. parvum; bottom row: dual confrontations with each of the isolates of T. harzianum.
In the case of N. parvum, as indicated above, PIRG values were shown to be clearly higher (Figure 4). These values seem to be in line with the mycelial growth inhibition rates observed in certain species of the grapevine pathogenic Botrysphaeriaceae family in plate confrontations with different isolates of the genus Trichoderma. Marraschi et al. [27] evaluated the potential of different BCAs and determined that the tested Trichoderma species and commercial formulations could inhibit the growth of a member of the mentioned ascomycetous family such as Lasiodiplodia theobromae (Pat.) Griffon & Maubl. at rates above 75%. Urbez-Torres et al. [28] studied different isolates of Trichoderma spp. that inhibited the growth of several species of Botryosphaeriaceae in a range from 44.5 to 74.3%, with T. atroviride being the most effective, close to T. harzianum. Plata-Caudillo [29] obtained mycelial growth inhibition values of N. parvum around 50% in direct plate confrontations with several isolates and formulations based on T. harzianum, in line with the values obtained in the present work. Mutawila et al. [30] observed mycelial growth inhibition rates against N. parvum of 45–50%, although using extracts containing a metabolite of interest previously extracted from different Trichoderma strains. Finally, Kotze et al. [31], in an in vitro and in vivo evaluation of the antagonistic activity of different microorganisms and formulations, reported, in the case of plate confrontations with T. harzianum versus N. parvum, the existence of hyperparasitism of the former towards the latter, based on the presence of hyphal interactions, enzymatic lysis, and occupation of space and nutrients in Petri dish. These hyphal interactions were also observed in the confrontations of the present study, although they were not particularly abundant along the whole contact surface between the colonies.
4.2. Bioassays of Trichoderma harzianum against Neofusicoccum parvum in Plants
Neofusicoccum parvum has been repeatedly isolated from nurseries throughout the world’s wine-growing areas in the last two decades and has been the subject of numerous investigations related to its epidemiology and characterization, as well as its control, including biological methods [12,32,33]. Most of the approaches related to the biocontrol of N. parvum and related Botryosphaeriaceae species have been based either on the protection of pruning wounds with antagonist-based formulations or other alternative methods to chemical fungicides [31,34,35], or with the application of these biocontrol agents in the different stages of production of grafted plants in nurseries [9,12,36].
In this sense, most of the investigations on the control of this and other pathologies of young grapevine plants, have been focused on grafted plants in the nursery and/or adult plants in the field, so the methodology and biometric results obtained after the treatment of grapevine plants germinated from seeds with different isolates of T. harzianum in the present study represents a novelty in this type of research. Nevertheless, the biometric data did not provide conclusive results regarding the protective effect of the different antagonistic strains used, given that a disparate behavior was observed among isolates, type of application, or measured parameter. Nevertheless, the reported data indicate, in agreement with the majority of studies based on the application of microbial antagonists at the root level, that the parameters most related to the development and activity of this type of tissue (root length and total weight) were the ones that offered better values compared to control plants inoculated with the pathogen.
Regarding the protection tests on grafted plants, successive treatments showed good root biomass lengths and lower necrosis (compared to controls). Several researchers claim that successive treatments with antagonists reduce the incidence of N. parvum. In this sense, Pintos et al. [37] concluded that inoculating Trichoderma in plants three days before the pathogen reduced more than twice the length of necrosis caused by the latter, being more effective than inoculating three days after the pathogen. Similarly, Kotze et al. [31] demonstrated a reduction of N. parvum damage in pruning wounds by applying Trichoderma products seven days before exposure, when the plant was healthy.
4.3. Bioassays of Trichoderma harzianum against Rhizoctonia solani in Plants
Members of the so-called Rhizoctonia species complex are considered a mixture of filamentous fungi, having in common the possession of a non-spored imperfect state, usually referred to as the Rhizoctonia anamorph [5,6,38,39,40]. Among them, Rhizoctonia solani is considered a very destructive plant pathogen, having a broad host range and causing diseases in a great variety of crops. Although R. solani has long been known as one of the main pathogens associated with grapevine wood in young plants in greenhouses [5,6,38,39,40], to date, there have been few studies on the biological protection of grapevine plants with microbial antagonists or other alternative methods against it. Crous et al. [39] examined the effects of thermotherapy treatments on the prevalence and incidence of a number of pathogens associated with apparently healthy grapevine seedlings in nurseries, including R. solani, concluding that hot water treatments did not significantly reduce its presence and the presence of other associated fungal pathogens. On the other hand, Ziedan et al. [41] tested the efficacy of a series of bacterial and fungal antagonistic strains such as T. harzianum against infection by Fusarium oxysporum E.F.Sm. & Swingle and R. solani in grapevine plants of the variety ‘Thompson Seedless’, concluding that both the application of these antagonists by immersion of the root system of the plants and the incorporation of the same to the culture substrate managed to reduce the incidence of root rot or the colonization of the roots by the pathogens. The results obtained in the present study on seedlings from germinated seed, although they cannot be compared with those reported in the studies mentioned above based on grafted plants, seem to agree with those previously cited on the fact that the protective effects of the antagonistic strains of T. harzianum are basically associated to the root system of the treated plants, where successive treatments with the different strains of T. harzianum reflected higher root development associated to lower colonization rates by the pathogen, compared to control plants artificially infected only with R. solani.
Regarding biological control trials on grafted plants, it was shown that simultaneous applications of certain strains of T. harzianum obtained higher values of root and aerial biomass length than controls with R. solani. This is consistent with the findings of authors such as Marais [38], Walker [42], and Hemida et al. [43], who stated that R. solani infections in young grapevine plants are associated with a reduction in root biomass and the existence of active root rot, in addition to general retardation of plant growth.
4.4. Trichoderma harzianum as a Growth Promoter
Along with the protective effects against specific plant pathogens, another important aspect related to biocontrol trials with Trichoderma is the ability of the different species of this genus to promote and stimulate plant growth, widely studied in different plant species [19], including grapevine [44,45,46,47].
In the present study, the inoculation of Trichoderma protective strains was carried out at two levels: (1) on grapevine seedlings germinated from seed and (2) on grafted plants coming from the protection experiments against N. parvum and R. solani.
In the case of seedlings, the results obtained suggested that the different strains of T. harzianum were not able to increase the different biometric parameters analyzed compared to control plants without any microbial inoculant, except for T. harzianum 5, which promoted root development. A possible explanation for this could be the scarce development of the root systems of this type of plant, where the culture system based on a small container with inert substrate could be a limitation for the incorporation and colonization of the plant by the mentioned isolates of T. harzianum.
Regarding T. harzianum growth promotion assays on grafted grapevine plants, biometric increases were recorded in plants from T. harzianum control experiments with the two mentioned pathogens. This increase was especially significant in root biomass, regardless of the statistical significance of this type of interaction. This type of beneficial effects, especially at the root system level, has been previously reported for this antagonistic genus in numerous plant hosts, including grapevine, where some authors (e.g., Di Marco and Osti [44]) observed significant increases in root biomass in grapevine plants treated with Trichoderma, especially at the time of rooting, suggesting another relevant feature of this genus from the biotechnological point of view: its potential use as a microbial fertilizer [48,49]. In this sense, in the study of Di Marco et al. [12], it was observed that grapevine roots developed four times more compared to controls when treated with Trichoderma. The mentioned authors concluded that the grapevines treated with the mentioned genus presented a more developed root system, which improved the absorption of water and nutrients and endowed them with a greater tolerance to stress-related diseases. Similarly, Fourie et al. [47] observed that the early shoot growth of Trichoderma treated grapevine plants was visibly better than that of untreated control plants. Regarding the effect of Trichoderma on rooting, these authors found that the fresh root weight of grapevine increased by 41.7% after monthly treatments with Trichoderma in the greenhouse soil.
5. Conclusions
In dual plate confrontations, it was found that the selected native T. harzianum strains were able to inhibit the mycelial growth of two grapevine pathogens, although the inhibition of N. parvum was much more effective than that of R. solani. In trials conducted on seedlings, the protective effects of successive treatments with the T. harzianum antagonist strains resulted in higher root development and dry weight, and a lower colonization rate of the pathogen, especially against R. solani. Concerning the tests carried out on grafted plants, in the case of N. parvum, the results were disparate, while simultaneous treatments led to higher aerial biomass lengths and higher necrosis than successive treatments, root lengths showed the opposite behavior. In the protection against R. solani tests, simultaneous treatments were more effective in several cases, resulting in higher values of root and aerial biomass length and lower rates of vascular necrosis. The T. harzianum strains tested herein offer a promising alternative as BCAs to traditional chemical fungicides, in addition to their ability to promote the growth of grafted plants, especially root biomass.
Author Contributions
Conceptualization, V.G.-G. and N.L.-L.; methodology, V.G.-G., N.L.-L., J.C.-G. and C.J.-L.; software, P.M.-R.; data curation, N.L.-L. and C.J.-L.; validation, V.G.-G., N.L.-L., J.C.-G. and P.M.-R.; investigation, V.G.-G., N.L.-L. and C.J.-L.; writing—original draft preparation, V.G.-G., N.L.-L., J.C.-G. and P.M.-R.; writing—review and editing, V.G.-G., N.L.-L. and P.M.-R.; supervision, V.G.-G. and P.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to their relevance to be part of an ongoing Ph.D. Thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clement, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezgui, A.; Vallance, J.; Ben Ghnaya-Chakroun, A.; Bruez, E.; Dridi, M.; Demasse, R.D.; Rey, P.; Sadfi-Zouaoui, N. Study of Lasidiodiplodia pseudotheobromae, Neofusicoccum parvum and Schizophyllum commune, three pathogenic fungi associated with the grapevine trunk diseases in the North of Tunisia. Eur. J. Plant Pathol. 2018, 152, 127–142. [Google Scholar] [CrossRef]
- Gramaje, D. Enfermedades de la madera de la vid: Situación actual y estrategias de control. In Proceedings of the Jornadas Vitivinícolas, Logroño, Spain, 1 December 2017; pp. 1–54. [Google Scholar]
- Massonnet, M.; Figueroa-Balderas, R.; Galarneau, E.R.; Miki, S.; Lawrence, D.P.; Sun, Q.; Wallis, C.M.; Baumgartner, K.; Cantu, D. Neofusicoccum parvum colonization of the grapevine woody stem triggers asynchronous host responses at the site of infection and in the leaves. Front. Plant Sci. 2017, 8, 1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, G.E. Root rot of grapevine rootlings in South Australia caused by Rhizoctonia solani. Australas. Plant Pathol. 1992, 21, 58–60. [Google Scholar] [CrossRef]
- Halleen, F.; Crous, R.W.; Petrin, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codon, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar]
- Pertot, I.; Prodorutti, D.; Colombini, A.; Pasini, L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Monte, E.; Liobell, A. Trichoderma in organic agriculture. In Proceedings of the V World Avocado Congress (Actas V Congreso Mundial del Aguacate), Granada-Málaga, Spain, 19–24 October 2003; pp. 725–733. [Google Scholar]
- Compant, S.; Mathieu, F. Biocontrol of Major Grapevine Diseases: Leading Research; CABI International: Wallingford, UK, 2016; p. 256. [Google Scholar]
- Di Marco, S.; Osti, F.; Cesari, A. Experiments on the control of esca by Trichoderma. Phytopathol. Mediterr. 2004, 43, 108–115. [Google Scholar]
- Fanjul, M.J. Use of Trichoderma spp. in the Management of Grapevine Trunk Diseases in Europe; Winetwork European Knowledge Transfer: Lisle-sur-Tarn, France, 2017; pp. 1–5. [Google Scholar]
- Wallis, C.M. Nutritional niche overlap analysis as a method to identify potential biocontrol fungi against trunk pathogens. Biocontrol 2021, 66, 559–571. [Google Scholar] [CrossRef]
- Silva-Valderrama, I.; Toapanta, D.; Miccono, M.D.; Lolas, M.; Diaz, G.A.; Cantu, D.; Castro, A. Biocontrol potential of grapevine endophytic and rhizospheric fungi against trunk pathogens. Front. Microbiol. 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Mutawila, C. Improving Pruning Wound Protection Against Grapevine Trunk Disease Pathogens; Stellenbosch University: Stellenbosch, South Africa, 2014. [Google Scholar]
- Ezziyyani, M.; Sánchez, C.P.; Ahmed, A.S.; Requena, M.E.; Castillo, M.E.C. Trichoderma harzianum como biofungicida para el biocontrol de Phytophthora capsici en plantas de pimiento (Capsicum annuum L.). An. Biol. 2004, 26, 35–45. [Google Scholar]
- Monte, E. Understanding Trichoderma: Between biotechnology and microbial ecology. Int. Microbiol. 2001, 4, 1–4. [Google Scholar] [PubMed]
- Gupta, V.G.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.; Druzhinina, I.; Tuohy, M. Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bastakoti, S.; Belbase, S.; Manandhar, S.; Arjyal, C. Trichoderma species as biocontrol agent against soil borne fungal pathogens. Nepal J. Biotechnol. 2017, 5, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Aly, A.; Abdel-Sattar, M.; Omar, M.; Abd-Elsalam, K. Differential antagonism of Trichoderma sp. against Macrophomina phaseolina. J. Plant Prot. Res. 2007, 47, 91–102. [Google Scholar]
- Boughalleb, N.; Ben-Salem, I.; M’Hamdi, M. Evaluation of the efficiency of Trichoderma, Penicillium, and Aspergillus species as biological control agents against four soil-borne fungi of melon and watermelon. Egypt. J. Biol. Pest Control 2018, 28, 25. [Google Scholar] [CrossRef] [Green Version]
- Fernández, R.J.; Suárez, C.L. Antagonismo in vitro de Trichoderma harzianum Rifai sobre Fusarium oxysporum Schlecht f. sp. passiflorae en maracuyá (Passiflora edulis Sims var. flavicarpa) del municipio zona bananera colombiana. Rev. Fac. Nac. Agron.-Medellín 2009, 62, 4743–4748. [Google Scholar]
- González, V.; Portal, M.; Rubio, V. Biology and systematics of the form genus Rhizoctonia. Span. J. Agric. Res. 2006, 4, 55–79. [Google Scholar] [CrossRef] [Green Version]
- Siameto, E.; Okoth, S.; Amugune, N.; Chege, N. Antagonism of Trichoderma harzianum isolates on soil borne plant pathogenic fungi from Embu District, Kenya. J. Yeast Fungal Res. 2010, 1, 47–54. [Google Scholar]
- Guedez, C.; Cañizalez, L.; Castillo, C.; Olivar, R. Evaluación in vitro de aislamientos de Trichoderma harzianum para el control de Rhizoctonia solani, Sclerotium rolfsii y Fusarium oxysporum en plantas de tomate. Rev. Soc. Venez. Microbiol. 2012, 32, 44–49. [Google Scholar]
- Marraschi, R.; Ferreira, A.; da Silva Bueno, R.N.; Leite, J.; Lucon, C.M.; Harakava, R.; Leite, L.G.; Padovani, C.R.; Bueno, C.J. A protocol for selection of Trichoderma spp. to protect grapevine pruning wounds against Lasiodiplodia theobromae. Braz. J. Microbiol. 2019, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Urbez-Torres, J.R.; Tomaselli, E.; Pollard-Flamand, J.; Boule, J.; Gerin, D.; Pollastro, S. Characterization of Trichoderma isolates from southern Italy, and their potential biocontrol activity against grapevine trunk disease fungi. Phytopathol. Mediterr. 2020, 59, 425–439. [Google Scholar] [CrossRef]
- Plata-Caudillo, J.A. Aislamiento y Evaluación In Vitro del Efecto de Trichoderma spp. Nativas Sobre los Hongos Patógenos de la Madera de Vid Aislados en la Región Vitivinícola de Ensenada, Baja California; Centro de Investigación Científica y de Educación Superior de Ensenada: Ensenada, Mexico, 2010. [Google Scholar]
- Mutawila, C.; Vinale, F.; Halleen, F.; Lorito, M.; Mostert, L. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2016, 65, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Kotze, C.; Van Niekerk, J.; Mostert, L.; Halleen, F.; Fourie, P.H. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, S247–S263. [Google Scholar]
- D’Enjoy, G.; Nesler, A.; Frati, S. Trichoderma atroviride SC1 is a tool for life-long protection of grape against trunk diseases. Nat. Prod. Biocontrol. 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Hunt, J.S.; Gale, D.S.J.; Harvey, I.C. Evaluation of Trichoderma as bio-control for protection against wood-invading fungi implicated in grapevine trunk diseases. Phytopathol. Mediterr. 2001, 40, 485–486. [Google Scholar]
- Halleen, F.; Fourie, P.H.; Lombard, P.J. Protection of grapevine pruning wounds against Eutypa lata by biological and chemical methods. Enol. Vitic. 2010, 31, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Harvey, I.; Hunt, J. Penetration of Trichoderma harzianum into grapevine wood from treated pruning wounds. N. Z. Plant Prot. 2006, 59, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Halleen, F.; Fourie, P.H. An integrated strategy for the proactive management of grapevine trunk disease pathogen infections in grapevine nurseries. S. Afr. J. Enol. 2016, 37, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Pintos, C.; Redondo, V.; Aguín, O.; Chaves, M.; Rial, C.; Mansilla, J. Evaluation of Trichoderma atroviride as biocontrol agent against five Botryosphaeriaceae grapevine trunk pathogens. Phytopathol. Mediterr. 2012, 51, 450. [Google Scholar]
- Marais, P. Fungi associated with root rot in vineyards in the Western Cape. Phytophylactica 1979, 11, 65–68. [Google Scholar]
- Crous, P.W.; Swart, L.; Coertze, S. The effect of hot-water treatment on fungi occurring in apparently healthy grapevine cuttings. Phytopathol. Mediterr. 2001, 40, S464–S466. [Google Scholar]
- Mahrous, H. Reaction of different grapevine cultivars to infection with root rotting fungi and its control. Phytopathol. Mediterr. 2001, 40, S479–S486. [Google Scholar]
- Ziedan, E.; Moataza, M.; Eman, S. Biological control of grapevine root-rot by antagonistic microorganisms. Afr. J. Mycol. Biotechnol. 2005, 13, 19–36. [Google Scholar]
- Walker, G.E. Effects of Meloidogyne spp. and Rhizoctonia solani on the growth of grapevine rootings. J. Nematol. 1997, 29, 190. [Google Scholar] [PubMed]
- Hemida, K.; Ziedan, E.H.; El-Saman, M.; El-Naggar, M.; Mostafa, H. Etiology of fungi associated with grapevine decline and their pathological potential. Arab. Univ. J. Agric. Sci. 2017, 25, 355–365. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F. Applications of Trichoderma to prevent Phaeomoniella chlamydospora infections in organic nurseries. Phytopathol. Mediterr. 2007, 46, 73–83. [Google Scholar]
- Ahmed, M. Evaluation of some biocontrol agents to control Thompson seedless grapevine powdery mildew disease. Egypt. J. Biol. Pest Control 2018, 28, 93. [Google Scholar] [CrossRef] [Green Version]
- Fourie, P.H.; Halleen, F. Proactive control of Petri disease of grapevine through treatment of propagation material. Plant Dis. 2004, 88, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Fourie, P.H.; Halleen, F.; Vyver, J.; Schreuder, W. Effect of Trichoderma treatments on the occurrence of decline pathogens in the roots and rootstocks of nursery grapevines. Phytopathol. Mediterr. 2001, 40, 473–478. [Google Scholar] [CrossRef]
- Kamal, R.K.; Athisayam, V.; Gusain, Y.S.; Kumar, V. Trichoderma: A most common biofertilizer with multiple roles in agriculture. Biomed. J. Sci. Tech. Res. 2018, 4, 4136–4137. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).